Abstract
Purpose
To determine the involvement of ZIC2 in oral squamous cell carcinoma (OSCC).
Methods
ZIC2 mRNA and protein expression in primary OSCCs (n = 74), oral premalignant lesions (OPLs, n = 20) and five OSCC-derived cell lines (HSC-2, HSC-3, OK-92, H1, and Sa3) were analyzed by quantitative reverse transcriptase-polymerase chain reaction, Western blot and immunohistochemistry (IHC). In addition, we evaluated the correlation between ZIC2 IHC scores in OSCCs and the clinicopathologic status.
Results
Significant up-regulation of ZIC2 was detected in OSCC-derived cell lines (P < 0.05), primary OSCCs (P < 0.05) and OPLs (P < 0.05) compared with normal counterparts. Among the clinical variables analyzed, ZIC2 expression was associated with the histopathologic types of OSCC. Furthermore, the survival rates differed significantly between ZIC2-positive cases and ZIC2-negative cases.
Conclusions
These results suggested that ZIC2 expression is correlated with the differentiation type of OSCC and diagnosis and might be a potential prognostic indicator and therapeutic target for OSCCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinomas (OSCCs), the most common cancer of the head and neck, accounts for over 300,000 new cancer cases worldwide annually (Lippman et al. 2005). In addition, the 5-year survival rate for OSCC remains about 50% (Okamoto et al. 2002; Weinberg and Estefan 2002). A number of etiologic factors have been implicated in the development of OSCCs, such as the use of tobacco, alcohol, or the presence of incompatible prosthetic materials (Mashberg et al. 1993; Fearon and Vogelstein 1990; Macfarlane et al. 1995). However, some patients develop OSCC without risk factors, suggesting that host susceptibility might play an important role. Therefore, we have continued efforts to discover suitable biomarkers for early diagnosis of OSCCs and to understand the disease pathogenesis as a first step toward improving treatment for OSCCs.
Microarray technologies have been helpful to analyze changes in thousands of genes and identify significant patterns. We previously reported the gene expression profiling of OSCC to identify genes associated with oral carcinogenesis (Yamano et al. 2008). Among the genes identified using microarray analysis, ZIC2 expression was found to be significantly up-regulated.
The ZIC genes have encoded zinc-finger transcription factors and are comprised of five Cys2His2 zinc-finger domains (Grinberg and Millen 2005; Nagai et al. 1997; Mizugishi et al. 2004; Yang et al. 2000). Among these families, ZIC2 regulates the tissue-specific expression of dopamine receptor D1, the G-protein coupled receptor, which is the most abundant dopamine receptor in the central nervous system (Yang et al. 2000). Dopamine receptor D1 stimulates adenylyl cyclase and cyclic AMP-dependent protein kinases and regulates neuronal growth, development, and dopamine receptor D2-mediated events (Missale et al. 1998). Up-regulated ZIC2 has been reported in invasive human tumors, such as lung cancers, endometrial cancers, pediatric medulloblastoma and synovial sarcoma (Gure et al. 2000; Bidus et al. 2006; Pfister et al. 2007; Fernebro et al. 2006). In addition, ZIC2 antibody was isolated in sera obtained from patients with small cell lung cancer (Gure et al. 2000; Sabater et al. 2008; Vural et al. 2005).
In the current study, ZIC2 expression increased in OSCCs and oral premalignant lesions (OPLs) compared with normal oral tissues. Based on these data, we propose that ZIC2 might be a diagnostic marker and a key regulator of prognosis in OSCCs.
Materials and methods
Tissue specimens and OSCC-derived cell lines
OSCCs, OPLs and patient-matched normal oral epithelium were obtained during surgical resection at Chiba University Hospital after the patients provided informed consent for use of a protocol reviewed and approved by the institutional review board of Chiba University. The resected tissues were divided into two parts, one of which was frozen immediately and stored at –80°C until RNA isolation, and the second of which was fixed in 10% buffered formaldehyde solution for pathologic diagnosis and immunohistochemistry (IHC). Histopathologic diagnosis of each neoplastic tissue was performed according to the World Health Organization criteria by the Department of Pathology, Chiba University Hospital. Clinicopathologic staging was determined by the TNM classification of the International Union against Cancer. All patients had SCC that was histologically confirmed, and tumor samples were checked to ensure that tumor tissue was present in more than 80% of the specimens.
HSC-2 and HSC-3 cell lines, derived from human OSCCs, were purchased from the Human Science Research Resources Bank (Osaka, Japan). The OK92 cell line was established in our department. H1 and Sa3 cell lines were kindly provided from Dr. Fujita at Wakayama Medical University (Wakayama, Japan). Primary cultured human normal oral keratinocytes (HNOKs) were used as a normal control (Kasamatsu et al. 2005, Endo et al. 2004). All cells were grown in Dulbecco’s modified Eagle medium/F-12 HAM (Sigma–Aldrich Co, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma) and 50 units/ml penicillin and streptomycin (Sigma).
Isolation of RNA
Total RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The extracted RNA samples were stored separately at –80°C until use.
Preparation of cDNA
cDNA was generated using 5 μg of total RNAs from OSCC-derived cell lines, using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare, Buckinghamshire, UK) and oligo (dT) primer (Sigma Genosys, Ishikari, Japan), according to the manufacturer’s instructions.
mRNA expression analysis
Real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed to evaluate the expression level of ZIC2 mRNA in the OSCC-derived cell lines (HSC-2, HSC-3, OK-92, H1, and Sa3) and HNOKs. In addition, primary tumors and paired specimens of normal oral tissues from 74 patients also were evaluated. qRT-PCR was performed with a single method using a LightCycler FastStart DNA Master SYBR Green 1 Kit (Roche Diagnostics GmbH, Mannheim, Germany). The primer sequences used to analyze ZIC2 mRNA expression were forward 5′-CTCCCTGCTAATCTCCATGC-3′ and reverse 5′-GAACGCAATCCGGAGTTTTA-3′. Amplified products were analyzed by 3% agarose gel electrophoresis to ascertain size and purity. The PCR reactions using the LightCycler (Roche) apparatus were carried out in a final volume of 20 μl of a reaction mixture consisting of 2 μl of FirstStart DNA Master SYBR Green I mix, 3 mM MgCl2, and l μM of the primers, according to the manufacturer’s instructions. The reaction mixture then was loaded into glass capillary tubes and subjected to an initial denaturation at 95°C for 10 min, followed by 45 rounds of amplification at 95°C (10 s) for denaturation, 62°C (10 s) for annealing, and 72°C (10 s) for extension, with a temperature slope of 20°C/s. The transcript amount for the ZIC2 gene was estimated from the respective standard curves and normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward 5′-CATCTCTGCCCCCTCTGCTGA-3′ and reverse 5′-GGATGACCTTGCCCACAGCCT-3′) transcript amount determined in corresponding samples.
Protein extraction
The cells were washed twice with cold phosphate-buffered saline, and were centrifuged briefly. The cell pellets were incubated at 4°C for 30 min in a lysis buffer (7 M urea, 2 M thiourea, 4% w/v CHAPS and 10 mM Tris pH 8.0) with proteinase inhibitor cocktail (Roche Mannheim, Germany). The protein concentration was measured with BCA (Thermo, Rockford, IL, USA).
Western blot analysis
Samples (50 μg) of proteins were separated by SDS/PAGE in 4–12% gel and transferred to nitrocellulose membranes for reaction with antibodies against ZIC2 and β-actin. Secondary antibodies, horseradish peroxidase-conjugated rabbit anti-goat and rabbit anti-mouse IgG, were detected by using SuperSignal Chemiluminescent substrate (Pierce Biotechnology, Rockford, IL, USA).
IHC
IHC of 4-μm sections of paraffin-embedded specimens was performed using goat anti-ZIC2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Briefly, after deparaffinization and hydration, The endogeneous peroxidase activity was quenched by 30 min incubation in a mixture of 0.3% hydrogen peroxide solution in 100% methanol, after which the sections were blocked for 2 h at room temperature with 1.5% blocking serum (Santa Cruz) in phosphate buffered saline (PBS) before reacting with anti-ZIC2 antibody (1:100 dilution) at 4°C in a moist chamber overnight. Upon incubation with the primary antibody, the specimens were washed three times in PBS and treated with Histofine Simplestain Max-PO (G) (Nichirei, Tokyo, Japan) followed by color development in 3,3′-diaminobenzidine tetrahydrochloride (DAKO, Carpinteria, CA, USA). Finally, the slides were lightly counterstained with hematoxylin, dehydrated with ethanol, cleaned with xylene, and mounted. Non-specific binding of an antibody to proteins other than the antigen sometimes occurred. To avoid non-specific binding, an immunizing peptide blocking experiment was performed. As a negative control, triplicate sections were immunostained without exposure to primary antibodies, which confirmed the staining specificity. To quantify the state of ZIC2 protein expression in those components, we used IHC score systems described previously (Endo et al. 2004; Shimada et al. 2005; Saito et al. 2006; Onda et al. 2006; Kouzu et al. 2006; Kato et al. 2007; Nomura et al. 2009; Sakuma et al. 2006; Tanaka et al. 2003; Yamano et al. 2008). Briefly, the stained cells were determined in at least five random fields at 400× magnification in each section. The intensity of the ZIC2 immunoreaction in the cell was scored as follows: 1+, weak; 2+, moderate; and 3+, intense. The cell number and the staining intensity then were multiplied to produce a ZIC2 IHC score. Cases with a score exceeding 64.6 (the highest score for normal tissue) were defined as ZIC2-positive. These judgments were made by two independent pathologists, neither of whom had any knowledge or information of the patients’ clinical status.
Statistical analysis
The statistical significance of the ZIC2 expression levels was evaluated using the Mann–Whitney U test, with P < 0.05 considered statistically significant. The data are expressed as the mean ± SE. Disease-free survival time was defined as the time between tumor treatment and detection of the first locoregional recurrence, distant metastasis, or both, or the date of the last follow-up, whichever occurred first. The overall survival time was defined as the interval between the date of treatment and death or until the last objective follow-up information was obtained. Patients without evidence of disease (local recurrence or metastasis) during follow-up were considered to have a good prognosis; patients with local recurrence or distant metastasis during follow-up were considered to have a poor prognosis. Survival curves were obtained using the Kaplan–Meier method and differences in survival rates between ZIC2-positive and ZIC2-negative cases were compared by log-rank tests with 95% significance.
Results
Evaluation of ZIC2 mRNA expression in OSCC-derived cell lines
We measured ZIC2 mRNA expression in five OSCC-derived cell lines (HSC-2, HSC-3, OK92, H1, and Sa3) and HNOKs using qRT-PCR analysis. ZIC2 mRNA was up-regulated significantly in all OSCC cell lines examined compared with the HNOKs (Fig. 1; *P < 0.05). These data were consistent with previous microarray data (Yamano et al. 2008).
a Quantification of ZIC2 mRNA and protein levels in OSCC-derived cell lines by qRT-PCR and Western blot analyses. Significant up-regulation of ZIC2 mRNA is seen in five OSCC-derived cell lines compared with that in the HNOKs. Data are expressed as means ± SEM of values from three assays (* P < 0.05; Mann–Whitney’s U test). b Western blot analysis of ZIC2 protein in the OSCC-derived cell lines and HNOKs. ZIC2 protein expression is up-regulated in OSCC-derived cell lines compared with HNOKs
Evaluation of ZIC2 protein expression in OSCC-derived cell lines
To investigate ZIC2 protein expression status in the OSCC-derived cell lines and the HNOKs, we performed Western blot analysis. Figure 1b shows representative results of the Western blot analysis. The molecular weight of the ZIC2 was 70 kDa. A significant increase of ZIC2 expression was observed in all OSCC cell lines compared with the HNOKs used as controls.
Evaluation of ZIC2 expression in primary OSCCs
We measured ZIC2 mRNA expression in primary OSCCs and paired normal oral tissues from 74 patients. Similar to the data from the OSCC-derived cell lines, qRT-PCR analysis showed that ZIC2 mRNA expression was up-regulated in 49 (66%) of 74 primary OSCCs compared with the matched normal oral tissues. The relative mRNA expression levels in the normal oral tissues and primary OSCCs ranged from 0.006 to 5.04 (median 0.172) and 0.01–8.69 (median 1.09), respectively (Fig. 2a, P < 0.05).
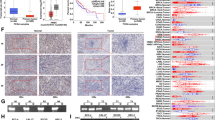
ZIC2 expression status in primary OSCCs and representative IHC staining of ZIC2 in normal oral tissue, OPLs and primary OSCC. a Comparison of ZIC2 mRNA expression levels between primary OSCCs and matched normal oral tissues. The relative mRNA expression levels in primary OSCCs (n = 74) and the matched oral tissues (n = 74) range from 0.01 to 8.69 (median 1.09) and 0.006 to 5.04 (median 0.172), respectively. Significantly higher ZIC2 expression is found in primary OSCCs than matched normal oral tissues (P < 0.05; Mann–Whitney’s U test). Data are expressed as the mean values ± SEM of two independent experiments with samples in triplicate. b The state of ZIC2 protein expression in normal oral tissues (n = 74), OPLs (n = 20) and primary OSCCs (n = 74). The ZIC2 IHC scores are calculated as follows: IHC score = 1 × (number of weak stained cells in the field) + 2 × (number of moderately stained cells in the field) + 3 × (number of intense stained cells in the field). The ZIC2 IHC scores for OSCCs, OPLs and normal oral tissues range from 14.63 to 140.1 (median 64.48), from 10.29 to 130.9 (median 55.01) and from 3.07 to 64.55 (median 30.12), respectively. ZIC2 protein expression levels in OSCCs and OPLs are significantly higher than that in normal oral tissues (P < 0.05; Mann–Whitney’s U test). c–e Representative IHC results of ZIC2 in normal oral tissue, OPLs, and primary OSCC. c Normal oral tissue exhibits negative ZIC2 protein expression. Original magnification, ×100. d A ZIC2-positive case of OPL. The immunoreaction is enhanced in the spinous layer. Original magnification, ×100. e A ZIC2-positive case of OSCC. Positive immunoreaction for ZIC2 is detected in the nucleus and cytoplasm. Original magnification, ×100. Negative control staining by ZIC2 peptide blocking primary antibody (f) and without primary antibody (g). Original magnification ×100
In addition to mRNA expression in the primary OSCCs, we measured ZIC2 protein expression by IHC. Representative results for ZIC2 protein expression in normal oral tissue, OPLs and primary OSCC were shown in Fig. 2c–e, respectively. Strong ZIC2 immunoreactions were detected in OSCCs and OPLs, whereas normal oral tissues showed negative immunostaining. The ZIC2 IHC scores for OSCCs and normal oral tissues ranged from 14.63 to 140.1 (median 64.48) and 3.07 to 64.55 (median 30.12), respectively. The ZIC2 IHC scores in primary OSCC cells were significantly higher than those of normal tissues (Fig. 2b, P < 0.05). In addition, ZIC2 IHC scores in OPLs ranged from 10.29 to 130.9 (median 55.01) and were also higher than those of normal oral tissues (Fig. 2b, P < 0.05). The correlation between the clinicopathologic characteristics of the patients with OSCC and the status of ZIC2 protein expression using the IHC scoring were shown in Table 1. Among the clinical classifications, ZIC2-positive OSCCs were correlated with the histopathologic types of OSCC (well differentiated, moderately differentiated, and poorly differentiated) (Table 1) (P < 0.032).
Effect of ZIC2 expression on patient survival
Survival analysis using the Kaplan–Meier method showed that ZIC2 up-regulation was a significant factor in disease-free survival (Fig. 3a; log-rank test, P = 0.038) and overall survival (Fig. 3b; log-rank test, P = 0.034). The disease-free survival rates in ZIC2 up-regulated cases and down-regulated cases were 66.71 and 87.84%, respectively. The overall survival rates in cases with up-regulated and down-regulated ZIC2 were 57.31 and 85.51%, respectively.
Kaplan–Meier survival curve for disease-free survival and overall survival rate of patients with OSCC. High levels of ZIC2 expression are significantly associated with a lower disease-free survival and b overall survival rates (a P = 0.038; b P = 0.034, respectively). The log-rank statistic is used to test the difference in survival times between the groups. ZIC2(+) up-regulated ZIC2, ZIC2(−) down-regulated ZIC2
Discussion
We previously reported the gene expression profiling of OSCC to identify genes associated with oral carcinogenesis (Yamano et al. 2008). Using microarray analysis, ZIC2 was one of the most significantly up-regulated genes in OSCC-derived cell lines. In this present study, we investigated the relationship between ZIC2 expression and clinical behaviors of patients with OSCC.
ZIC2 is widely known to be a transcriptional regulator (Brown and Brown 2009), and controlled dopamine receptor D1 is the most abundant dopamine receptor in the central nervous system (Yang et al. 2000), which regulates neuronal growth, development, and dopamine receptor D2-mediated events (Missale et al. 1998). Recent studies have reported the relationship between ZIC2 expression and malignant tumors, such as lung cancer, endometrial cancer, pediatric medulloblastoma and synovial sarcoma (Gure et al. 2000; Bidus et al. 2006; Pfister et al. 2007; Fernebro et al. 2006). However, detail mechanisms of ZIC2 in those cancers were unclear. Therefore, studies of ZIC2 function in OSCCs are important.
In this study, significant up-regulation of ZIC2 mRNA was observed in OSCCs and in five OSCC-derived cell lines, compared with the matched normal counterparts. These results were consistent with previous report of endometrial cancer (Bidus et al. 2006). By Western blot and IHC, in addition to ZIC2 mRNA expression, ZIC2 protein level was significantly up-regulated in primary OSCCs and OSCC-derived cell lines compared with matched normal counterparts. Up-regulation of ZIC2 was also detected even in the OPLs, suggesting that dysregulation of protein expression was seen in the premalignant stage. Futhermore, this study showed that high levels of ZIC2 expression were associated significantly with lower disease-free survival (Fig. 3a) and overall survival (Fig. 3b) rates. Therefore, these results indicated that ZIC2 might have the function as a prognosis indicator for OSCC. In contrast, ZIC2 protein expression was correlated with the degree of differentiation in OSCCs. Generally, prognosis of well-differentiated cancers is better than that of poor-differentiated cancers (Nishida et al. 1999). However, prognosis is associated with not only differentiation type but also tumor size and status of metastasis. Therefore, further studies with more clinical samples are needed to address in greater detail the status of ZIC2 in oral carcinogenesis and prognosis.
In conclusion, our results indicated that ZIC2 is frequently overexpressed not only in OSCCs but also in OPLs. In addition, up-regulated ZIC2 is associated with 5-year survival rate. Thus, we suggest here that ZIC2 might play an important role in the course of oral tumorigenesis and the prognosis of OSCC.
References
Bidus MA, Risinger JI, Chandramouli GV, Dainty LA, Litzi TJ, Berchuck A, Barrett JC, Maxwell GL (2006) Prediction of lymph node metastasis in patients with endometrioid endometrial cancer using expression microarray. Clin Cancer Res 12:83–88. doi:10.1158/1078-0432.CCR-05-0835
Brown L, Brown S (2009) Zic2 is expressed in pluripotent cells in the blastocyst and adult brain expression overlaps with makers of neurogenesis. Gene Expr Patterns 9:43–49. doi:10.1016/j.gep.2008.08.002
Endo Y, Uzawa K, Mochida Y, Shiiba M, Bukawa H, Yokoe H, Tanzawa H (2004) Sarcoendoplasmic reticulum Ca2 + ATPase type 2 downregulated in human oral squamous cell carcinoma. Int J Cancer 110:225–231. doi:10.1002/ijc.20118
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Fernebro J, Francis P, Eden P, Borg A, Panagopoulos I, Mertens F, Vallon-Christersson J, Akerman M, Rydholm A, Bauer HC, Mandahl N, Nilbert M (2006) Gene expression profiles relate to SS18/SSX fusion type in synovial sarcoma. Int J Cancer 118:1165–1172. doi:10.1002/ijc.21475
Grinberg I, Millen KJ (2005) The ZIC gene family in development and disease. Clin Genet 67:290–296. doi:10.1111/j.1399-0004.2005.00418.x
Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Old LJ, Chen YT (2000) Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA 97:4198–4203
Kasamatsu A, Uzawa K, Nakashima D, Koike H, Shiiba M, Bukawa H, Yokoe H, Tanzawa H (2005) Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int J Mol Med 16:269–273
Kato Y, Uzawa K, Yamamoto N, Kouzu Y, Koike H, Shiiba M, Bukawa H, Yokoe H, Shibahara T, Tanzawa H (2007) Overexpression of Septin1: possible contribution to the development of oral cancer. Int J Oncol 31:1021–1028
Kouzu Y, Uzawa K, Koike H, Saito K, Nakashima D, Higo M, Endo Y, Kasamatsu A, Shiiba M, Bukawa H et al (2006) Overexpression of stathmin in oral squamous-cell carcinoma: correlation with tumour progression and poor prognosis. Br J Cancer 94:717–723
Lippman SM, Sudbo J, Hong WK (2005) Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol 23:346–356
Macfarlane GJ, Zheng T, Marshall JR, Boffetta P, Niu S, Brasure J, Merletti F, Boyle P (1995) Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case–control studies. Eur J Cancer B Oral Oncol 31:181–187
Mashberg A, Boffetta P, Winkelman R, Garfinkel L (1993) Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer 72:1369–1375
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225
Mizugishi K, Hatayama M, Tohmonda T, Ogawa M, Inoue T, Mikoshiba K, Aruga J (2004) Myogenic repressor I-mfa interferes with the function of zic family proteins. Biochem Biophys Res Commun 320:233–240. doi:10.1016/j.bbrc.2004.05.158
Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K (1997) The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for zic genes in body pattern formation. Dev Biol 182:299–313. doi:10.1006/dbio.1996.8449
Nishida T, Katayama S, Tsujimoto M, Nakamura J, Matsuda H (1999) Clinicopathological significance of poorly differentiated thyroid carcinoma. Am J Surg Pathol 23:205–211
Nomura H, Uzawa K, Yamano Y, Fushimi K, Ishigami T, Kouzu Y, Koike H, Siiba M, Bukawa H, Yokoe H et al (2009) Overexpression and altered subcellular localization of autophagy-related 16-like 1 in human oral squamous-cell carcinoma: correlation with lymphovascular invasion and lymph-node metastasis. Hum Pathol 40:83–91
Okamoto M, Nishimine M, Kishi M, Kirita T, Sugimura M, Nakamura M, Konishi N (2002) Prediction of delayed neck metastasis in patients with stage I/II squamous cell carcinoma of the tongue. J Oral Pathol Med 31:227–233
Onda T, Uzawa K, Endo Y, Bukawa H, Yokoe H, Shibahara T, Tanzawa H (2006) Ubiquitous mitochondrial creatine kinase downregulated in oral squamous cell carcinoma. Br J Cancer 94:698–709
Pfister S, Schlaeger C, Mendrzyk F, Wittmann A, Benner A, Kulozik A, Scheurlen W, Radlwimmer B, Lichter P (2007) Array-based profiling of reference-independent methylation status (aPRIMES) identifies frequent promoter methylation and consecutive downregulation of ZIC2 in pediatric medulloblastoma. Nucleic Acids Res 35:e51. doi:10.1093/nar/gkm094
Sabater L, Bataller L, Suarez-Calvet M, Saiz A, Dalmau J, Graus F (2008) ZIC antibodies in paraneoplastic cerebellar degeneration and small cell lung cancer. J Neuroimmunol 201–202:163–165. doi:10.1016/j.jneuroim.2008.01.018
Saito K, Uzawa K, Endo Y, Kato Y, Nakashima D, Ogawara K, Shiba M, Bukawa H, Yokoe H, Tanzawa H (2006) Plasma membrane Ca2 + ATPase isoform 1 down-regulated in human oral cancer. Oncol Rep 15:49–55
Sakuma T, Uzawa K, Onda T, Shiiba M, Yokoe H, Shibahara T, Tanzawa H (2006) Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol 29:117–124
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba M, Bukawa H, Yokoe H, Seki N, Tanzawa H (2005) Aberrant expression of RAB1A in human tongue cancer. Br J Cancer 92:1915–1921
Tanaka C, Uzawa K, Shibahara T, Yokoe H, Noma H, Tanzawa H (2003) Expression of an inhibitor of apoptosis, survivin, in oral carcinogenesis. J Dent Res 82:607–611
Vural B, Chen LC, Saip P, Chen YT, Ustuner Z, Gonen M, Simpson AJ, Old LJ, Ozbek U, Gure AO (2005) Frequency of SOX group B (SOX1, 2, 3) and ZIC2 antibodies in turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer 103:2575–2583. doi:10.1002/cncr.21088
Weinberg MA, Estefan DJ (2002) Assessing oral malignancies. Am Fam Physician 65:1379–1384
Yamano Y, Uzawa K, Shinozuka K, Fushimi K, Ishigami T, Nomura H, Ogawara K, Shiiba M, Yokoe H, Tanzawa H (2008) Hyaluronan-mediated motility: a target in oral squamous cell carcinoma. Int J Oncol 32:1001–1009
Yang Y, Hwang CK, Junn E, Lee G, Mouradian MM (2000) ZIC2 and Sp3 repress Sp1-induced activation of the human D1A dopamine receptor gene. J Biol Chem 275:38863–38869. doi:10.1074/jbc.M007906200
Acknowledgments
This study was supported by a Grant-in-Aid Scientific Research (No. 20592353) from Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Lynda C. Charters for editing this manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakuma, K., Kasamatsu, A., Yamatoji, M. et al. Expression status of Zic family member 2 as a prognostic marker for oral squamous cell carcinoma. J Cancer Res Clin Oncol 136, 553–559 (2010). https://doi.org/10.1007/s00432-009-0689-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0689-y