Abstract
Aim
The purpose of this study was to investigate the co-expression of survivin, c-erbB2, and COX-2 in endometrial cancer tissues and evaluate its prognostic significance in endometrial cancer
Methods
Tumor tissue biopsies from 110 patients with primary untreated endometrial carcinomas were studied by immunohistochemistry. Statistical analysis evaluated correlation of antigen expression with tumor stage, grade, myometrial invasion, and histologic type. Association with disease outcome was also investigated
Results
The results showed that expression of the three antigens was independently associated with histological grade, disease stage, and myometrial invasion. Clinicopathological parameters were also associated with the number of antigens expressed by each tumor, the expression of more antigens correlating with advanced stage disease and deep myometrial invasion. In a 10-year follow-up, patients with tumors expressing more of these three antigens had significantly lower survival rate that those with smaller expression score
Conclusions
Our results indicate that the co-expression score has independent prognostic value for endometrial cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenocarcinoma of the endometrium remains fourth in incidence among invasive tumors in women (Hernandez 2001). Several surgical-pathologic characteristics such as histological grade, depth of myometrial invasion, cervical extension, and the presence of metastatic disease have significant prognostic value, and have been traditionally used to determine whether hysterectomy alone is likely to be curative or additional postoperative therapy is needed to prevent recurrence (Prat et al. 1994). Despite the generally good outcome when compared to other gynecological malignancies, a lot of effort is put today into defining better prognostic indicators that would allow a more precise strategy of treatment based upon the subgrouping of patients. Multiparametric methods using DNA array technologies and proteomics have been suggested to be applied (Smid-Koopman et al. 2004), which, however, remain expensive and technically demanding. However, it is well understood that multiple marker investigation rather than a single tumor marker would be of benefit towards this direction (Pappa and Anagnou 2005).
In the present study, we examine the prognostic value of the co-expressing pattern of three antigens that have been previously shown to be independent prognostic indicators of endometrial cancer outcome and survival. The c-erbB2 oncogene has been established to have close relation with endometrial adenocarcinoma outcome, being regulated by estrogens and glucocorticoids (Markogiannakis et al. 1997). Overexpression of this gene product has been related to cancers leading to increased fatality (Berchuck et al. 1991) and is associated with the presence of intraperitoneal metastatic disease (Bezwoda 2000; Cherchi et al. 2001). We have recently shown that c-erbB2 is an independent prognostic indicator of poor outcome, assessing separately cytoplasmic and membrane immunohistochemical staining, and confirming that cytoplasmic expression is as important as membrane and a specific finding rather than an artifact in endometrial adenocarcinomas (Lambropoulou et al. 2007). Also, we and others have reported the prognostic significance of cyclooxigenase-2 (COX-2), one of the two enzyme isoforms of prostaglandin synthesis (Ferrandina et al. 2002; Fujiwaki et al. 2002; Karahan et al. 2007; Lambropoulou et al. 2005), which seems to be related with tumor angiogenesis, growth and apoptosis, metastasis, and local immunosupression (Ohno et al. 2005). However, these findings were not supported by other studies showing no prognostic significance for this tumor type (Erkanli et al. 2007; Ferrandina et al. 2005; Fowler et al. 2005). Finally, survivin, a member of the apoptosis inhibitor protein family (IAP), is a defining diagnostic marker for endometrial carcinomas that may also yield prognostic information (Takai et al. 2002). The prognostic value of the co-expression pattern of c-erbB2, COX-2, and survivin in endometrial cancer tissues was examined by correlation to other clinicopathological parameters and the survival rate of endometrial cancer patients.
Materials and methods
One hundred and ten patients—cases of endometrial carcinoma admitted at the Pathology Department, University General Hospital of Alexandroupolis were studied. Patient age ranged from 40 to 88 years. The presenting symptom of patients was postmenopausal or intermenstrual bleeding. Paraffin-embedded tissue samples from diagnostic curettage and hysterectomy were available for all patients. Tissue sections were subjected to conventional hematoxylin and eosin staining (H&E). Unstained slides were used to investigate expression of survivin, c-erbB2, and COX-2 by immunohistochemistry. The clinical data were obtained from the patient files, including follow-up information. The clinicopathological parameters evaluated were age, FIGO stage, type of carcinoma, depth of myometrial invasion (tumor depth), and lymphovascular space invasion (LVSI). A bias toward more aggressive cancers is represented in our sample, because tissue collection for research was permitted only from patients with a large enough tumor, while preserving adequate tissue for standard pathologic examination. As a result, small Stage IA lesions are underrepresented relatively to their expected frequency. Survival was also studied. The study had received approval by the local Human Investigations Committee and it conforms to the provisions of the Declaration of Helsinki. Written informed consent was obtained from all patients and the procedures were in accordance with the institutional guidelines.
Immunohistochemistry
Tissue specimens were fixed in formalin and embedded in paraffin according to standard procedures. Four-micron serial sections (4 μm) of representative blocks from each case were deparaffinized, rehydrated, and immunostained by the peroxidase method (Envision System, DAKO, Carpinteria, Calif., USA). Slides were then incubated with the primary antibody: 75 min with the survivin rabbit polyclonal antibody at a 1:50 dilution (Medical & Biological Laboratories co, LTD, Japan), 30 min with the c-erbB2 rabbit polyclonal anti-human antibody (DAKO, Carpinteria, Calif., USA) at a 1:250 dilution or 75 min with COX-2 rabbit polyclonal antibody (Assay Designs, Inc.) at a 1:40 dilution. Control slides were incubated for the same period with nonimmunized rabbit serum (negative control). Finally, bound antibody complexes were stained for 10 min with 0.05% diaminobenzidine. Sections then were briefly counterstained with Mayer’s hematoxylin, mounted, and examined under a Nikon Eclipse 50× microscope. Cell count was performed in 10 high power fields (40×) for each section. Sections with greater than 10% stained tumor cells were considered as being positive. Samples with complete absence of either membranous or cytoplasmic staining or with weak/incomplete staining (<10%) would be classified as a tumor negative for antigen expression. Staining intensity was equal between different samples and areas of the tissue sections, and therefore it was not included in the scoring.
Statistical analysis
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS), version 10.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables were expressed as frequencies (and percentages) and continuous variables were expressed as the mean ± standard deviation. The chi-square test was used to evaluate any potential association between survivin, c-erbB2, and COX-2 expression and the clinicopathological parameters, while odds ratios and their 95% confidence interval (CI) were calculated by means of simple logistic regression analysis. As indicator of survival, the disease-specific survival (including only death related to the disease as an event) was investigated. Survival rates were calculated with the Kaplan–Meier method and the statistical difference between survival curves was determined with the log-rank test. Multivariate logistic and COX proportional hazards regression analysis, using a backward selection approach, were performed to explore the independent effect of variables on co-expression of survivin, c-erbB2, COX-2, and survival, respectively. All tests were two tailed and statistical significance was considered for P values <0.05.
Results
Patient and tumor characteristics
One hundred and ten primary untreated endometrial cancer patients underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy at the Department of Obstetrics and Gynecology, University General Hospital of Alexandroupolis (Table 1). Patient’s age ranged from 40 to 88 years, with a mean age of 59.05 ± 8.57 years and 47 (42.7%) patients exceeding the age of 60-years-old. Regarding to surgical stage, 89 (80.9%) carcinomas were FIGO stage I–II and 21 (19.1%) FIGO stage III–IV, while regarding to histological type, 95 (86.4%) were endometrioid adenocarcinomas and 15 (13.6%) non-endometrioid carcinomas. In particular, 2 (2.1%) of 95 endometrioid adenocarcinomas showed focal squamous metaplasia and 8 (8.4%) papillary configuration. Four (26.7%) of 15 non-endometrioid carcinomas were adenosquamous, 4 (26.7%) serous-papillary, 5 (33.4%) clear cell carcinomas, 1 (6.6%) squamous, and 1 (6.6%) undifferentiated. Seventy-eight (70.9%) were well differentiated (G1), 21 (19.1%) moderately (G2) and 11 (10.0%) poorly differentiated. Myometrial invasion did not exceed the inner half of the myometrial wall in 63 (57.3%) cases, while cancer infiltrated the outer half of the myometrium in 47 (42.7%) cases.
Immunohistochemical detection of c-erbB2, survivin, and COX-2 expression in endometrial cancer tissues
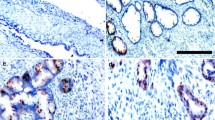
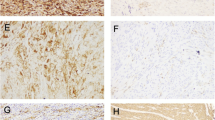
Antigen expression was analyzed by immunohistochemistry in all the above human endometrial tumors and the results are presented in Table 2. Membrane (m) and cytoplasmic (c) immunoreactivity for c-erbB2 was detected in the malignant cells and was assessed separately. Representative tissues are shown in Fig. 1. C-erbB2 membrane localization was found in 6 out of 110 cases (5.5%) that were all also positive for cytoplasmic c-erbB2, while, among cases with c-erbB2 membrane staining <10%, 70 cases (67.3%) showed cytoplasmic c-erbB2 (P = 0.092, χ2 test; P < 0.001, McNemar test). Survivin and COX-2 immunoreactivity were observed mainly in the malignant cell cytoplasm (Fig. 2). 47 (42.7%) and 28 (25.5%) of the tissues were found to express survivin and COX-2, respectively. With the exception of scattered lymphoid cells with variable intensities of COX-2 positivity, stromal cells did not show any immunoreactivity for all the antibodies.
Immunohistochemical staining of an endometrial cancer tissue for c-erbB2. Localization of antigen expression in the malignant cells can be observed in both the cell membrane (black arrow) and the cytoplasm (white arrow) (a) or only in the cytoplasm (b). The stroma is negative. Omission of the primary antibody resulted in abolishment of all staining (c, negative control). Original magnification ×200 (a), ×100 (b and c)
Immunohistochemical staining of an endometrial cancer tissue for survivin (a) and COX-2 (b). Immunoreactivity can be observed in the malignant cell cytoplasm. Scattered positive stromal lymphoid cells are shown with arrows. Omission of the primary antibody resulted in abolishment of all staining (c, negative control). Original magnification ×200 (a and b), ×100 (c)
Association of c-erbB2, survivin, and COX-2 expression with tumor clinicopathological parameters and antigen co-expression
All tumor antigen expression was analyzed in relation to the following parameters: patient’s age, surgical stage, histological type, histological grade, and depth of myometrial invasion as well as co-expression with the other antigens studied (Table 3). Expression of all tumor antigens showed statistically significant association with histological grade; well-differentiated tumors were more likely to express cytoplasmic c-erbB2 than moderately or poorly differentiated tumors (P = 0.020), whereas membrane c-erbB2, survivin, and COX-2 were more frequently expressed in less differentiated tumors (P = 0.037, P = 0.024 and P = 0.019, respectively). Survivin and COX-2 were also positively associated with advanced stage disease (P = 0.048 and P = 0.010, respectively) and deep myometrial invasion (P = 0.021 and P = 0.026, respectively). No association was found with other clinicopathological parameters. Between antigen expressing tissues, no association was detected between antigen positive tissues except between membrane c-erbB2 co-expressing with COX-2 (P = 0.017).
The clinicopathological parameters were also associated with the number of antigens expressed by each tumor (Table 4). The expression of more antigens was positively correlated with advanced stage disease (P = 0.049) and with deep myometrial invasion (P = 0.037), when cytoplasmic c-erbB2 and membrane c-erbB2 expression was estimated, respectively. In this regard, advanced surgical stage was more than three times (OR = 3.4, 95% CI = 1.2–9.5, P = 0.018) as likely to express two or more of the survivin, COX-2 and cytoplasmic c-erbB2 as surgical stages I or II, while tumors with deep myometrial invasion were almost four times (OR = 3.9, 95% CI = 1.3–12.2, P = 0.012) as likely to express two or more of the survivin, COX-2 and membrane c-erbB2 as tumors with low myometrial invasion.
Survival analysis in relation to tumor antigen co-expression
Follow-up was available for 101 patients, since 9 patients (8.2%) were lost during follow-up. Mean duration of follow-up was 76.58 ± 42.57 months (range 4–176 months, median 71 months). Twenty-five patients (24.8%) died during follow-up. The mean survival time was 136 ± 7 months (95% CI = 123–150 months). Patients were divided into three groups in relation to the number of tumor antigens found to be expressed: group A had no tumor antigen expression, in group B only one of the 3 antigens was found and group C expressed 2 or 3 antigens. Survival analysis in relation to survivin, COX-2, and cytoplasmic c-erbB2 co-expression (Kaplan–Meier) showed that the 1-, 5-, and 10-year survival of patients of group A (n = 19) was 100%, whereas the respective percentages for group B (n = 33) were 96.97 ± 2.98%, 84.85 ± 6.24% and 77.78 ± 8.86%, respectively, and for group C (n = 49) were 89.80 ± 4.32%, 70.24 ± 6.75%, and 51.40 ± 8.97% (Table 5A). Statistically significant differences were observed between the survival rates of these three groups of patients over time (P = 0.002, log-rank test), with group A having better prognosis (0.001) than group C and marginally better that group B (P = 0.071). Also, group B had better prognosis than group C (P = 0.038). During follow-up, mortality rate was 18.2 and 38.8% for groups B and C, respectively, whereas no death was recorded in group A. These differences were statistically significant (P = 0.002). COX regression analysis revealed that patients of group C were 3.85 times more likely to die of cancer than the rest of the patients (95% CI = 1.53–9.73, P = 0.004; Fig. 3a).
Similar results were found when analysis was done using the membrane c-erbB2 results (Table 5B). 1-, 5- and 10-year survival of patients of group A’ (n = 45) was 97.78 ± 2.20%, 88.89 ± 4.68%, and 85.33 ± 5.69%, whereas the respective percentages for group B’ (n = 39) were 89.74 ± 4.86%, 78.14 ± 6.99%, and 66.60 ± 9.71%, respectively, and for group C’ (n = 17) were 94.12 ± 5.71%, 64.71 ± 11.59%, and 41.83 ± 13.56%. Statistically significant differences were observed between the survival rates of these three groups of patients over time (P = 0.014, log-rank test), with group A’ having better prognosis (0.003) than group C’ and marginally better that group B’ (P = 0.054). Group B’ had marginally better prognosis than group C’ (P = 0.085). During follow-up, mortality rate was 13.3, 25.5, and 52.9% for groups A’, B’, and C’, respectively (P = 0.014). COX regression analysis revealed that patients of group C’ were 4.28 times more likely to die of cancer than those of group A’ (95% CI = 1.52–12.05, P = 0.006). In this respect, no statistically significant differences were found between groups B’ and C’ (RR = 2.06, 95% CI = 0.81–5.20, P = 0.127), or between groups A’ and B’ (RR = 2.04, 95% CI = 0.73–5.75, P = 0.176; Fig. 3b).
Investigation with multivariate Cox proportional hazards regression analysis revealed that older age (Hazard ratio (HR) = 6.10, 95% CI = 2.29–16.26, P < 0.001) non-endometrioid carcinomas (HR = 4.13, 95% CI = 1.57–10.84, P = 0.004), advanced stage (HR = 4.11, 95% CI = 1.80–9.40, P < 0.001) and cytoplasmic c-erbB2 overexpression (HR = 3.57, 95% CI = 1.17–10.89, P = 0.026) remained independent prognostic factors of worse overall survival. When the combination of cytoplasmic c-erbB2, survivn, and COX-2 was entered in the regression analysis, the simultaneous presence of the 2 or more antigens remained an independent determinant for poor survival (HR = 3.28, 95% CI = 1.28–8.41, P = 0.013 compared to 0 or 1). Furthermore, when the combination of membrane c-erbB2, survivin, and COX-2 was entered in the regression analysis, the simultaneous presence of the 2 or more antigens remained an independent determinant for poor survival (HR = 3.10, 95% CI = 1.08–8.92, P = 0.036 compared to 0 antigen). The independent impact of only one antigen (any of the three) on overall survival was of borderline statistical significance (HR = 2.52, 95% CI = 0.89–7.17, P = 0.082, compared to 0).
Discussion
In the present study, the prognostic value of the co-expression pattern of c-erbB2, COX-2, and survivin in endometrial cancer tissues was examined by correlation to traditional surgical pathologic prognostic factors and the survival rate of endometrial cancer patients. Our results showed that: (A) Expression of all the three antigens was independently associated with histological grade, whereas some of them were also associated with disease stage and myometrial invasion. Moreover, some of these clinicopathological parameters were also associated with the number of antigens expressed by each tumor, the expression of more antigens correlating with advanced stage disease and deep myometrial invasion. (B) Except in the case of the membrane c-erbB2 co-expressing with COX-2, no association was found between the expressions of tumor antigens, probably indicating that their role in the carcinogenetic procedure may be involved in different molecular events. (C) In a 10-year follow-up, patients with tumors positive for more of these three antigens had significantly lower survival rate that those with smaller expression score. Our results indicate that the expression score of these three antigens have independent prognostic value for endometrial cancer patient outcome.
The independent prognostic value of c-erbB2, COX-2, and survivin in endometrial cancer patients has been shown in numerous studies by our group and others. However, this is a first attempt to estimate the prognostic significance of their co-expression score. Erkanli et al. (2007) has recently shown that COX-2 and survivin are overexpressed in endometrial carcinomas when compared to hyperplastic and normal proliferative tissues, concluding that this seems to be an early event in the carcinogenetic process and suggesting that COX-2 and survivin may share a common molecular pathway since a correlation was found between their expression. In our study using a double in size tissue collection, we could not confirm this association. This difference in sample size may account for discrepancies found between our study, showing a significant correlation of COX-2 and survivin expression with myometrial invasion, histological grade, disease stage, and survival of endometrial cancer patients, to Erkanli et al., who showed no such correlation. On the other hand, Ferrandina et al. (2005), studied the expression of COX-2, steroid receptors, p53, Ki67, and c-erbB2 in endometrial cancer. In this study, COX-2 expression was not associated with c-erbB2, or with any clinicopathological features or survival rate. Reviewing this and other reports (Ferrandina et al. 2002; Fowler et al. 2005; Lambropoulou et al. 2005) shows that there is still controversy in the literature about the prognostic significance of COX-2. In our analysis, considering the three tumor markers in combination, c-erbB2, COX-2, and survivin, enhanced the prognostic value of this test and showed a clear correlation of antigen expression to the survival rate. More aggressive tumors had a higher antigen positive score and worst prognosis. These results indicate that such an analysis could have strong prognostic value for endometrial cancer patients.
In our data analysis, cytoplasmic and membrane c-erbB2 expression was considered separately. We have previously shown that cytoplasmic staining is as important as membranic and a specific finding in endometrial cancer tissues, rather than an artifact, as suggested by investigators working in other cancer types (Horvai et al. 2003). We have also shown that although c-erbB2 cytoplasmic expression correlated with G1 grade tumors (of better prognosis) is an indicator of a poorer prognosis sub-group (Horvai et al. 2003). Other reports have shown, however, that the c-erbB2 cytoplasmic staining in breast tumors correlates with c-erbB2 amplification (Bhatavdekar et al. 2000; Kuesters et al. 2006), in support to our findings. A small percentage of tissues (5.5%) presented membrane antigen localization and all of them showed cytoplasmic staining too. The percentage of cytoplasmic positive tissues was a lot higher (69.1%). However, survival analysis showed significant correlation to the co-expression score when either cytoplasmic or membrane staining were considered.
Studying 31 endometrial cancer cases, Takai et al. (2002) reported that survivin expression was significantly associated with proliferating cell nuclear antigen-labeling index, surgical stage, histological grade, the presence of invasion to >1/2 myometrium, surgical outcome, and survival rate. In addition, survivin mRNA levels were increased in correlation with ascending grade of endometrial adenocarcinoma (Lehner et al. 2002). However, other studies found no association of this gene product with classical prognostic factors (Erkanli et al. 2006, 2007; Pallares et al. 2005) showing that this issue needs further clarification. In our present study, we confirm the former data in a large sample size, showing survivin expression in 42.7% of the tumors and association with higher histological grade, stage, tumor depth, and LVSI. These results suggest that survivin may provide a defining diagnostic tool for endometrial carcinomas with possible prognostic information. Interestingly, survivin has been implicated to the malignant transformation process in this tissue by regulating apoptosis and cell proliferation (Ai et al. 2006; Pallares et al. 2005).
In conclusion, we showed that the co-expression score of c-erbB2, COX-2, and survivin in endometrial cancer tissues correlates significantly to classical clinicopathological parameters and most importantly to the survival rate of endometrial cancer patients, although there was not significant association between their expressions. Our results indicate that the expression score of these three antigens have independent prognostic value for endometrial cancer patient outcome and could be of clinical use. Finally, these data can contribute to the understanding of the tumorigenic process in this malignancy and aid the development of evidence-based therapeutic strategies such as Cox-2 inhibition or silencing of survivin.
References
Ai Z, Yin L, Zhou X et al (2006) Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer 107:746–756. doi:10.1002/cncr.22044
Berchuck A, Rodriguez G, Kinney RB et al (1991) Overexpression of HER-2/neu in endometrial cancer is associated with advanced stage disease. Am J Obstet Gynecol 164:15–21
Bezwoda WR (2000) c-erb-B2 expression and response to treatment in metastatic breast cancer. Med Oncol 17:22–28. doi:10.1007/BF02826212
Bhatavdekar JM, Patel DD, Shah NG et al (2000) Prognostic significance of immunohistochemically localized biomarkers in stage II and stage III breast cancer: a multivariate analysis. Ann Surg Oncol 7:305–311. doi:10.1007/s10434-000-0305-5
Cherchi PL, Marras V, Capobianco G et al (2001) Prognostic value of p53, c-erb-B2 and MIB-1 in endometrial carcinoma. Eur J Gynaecol Oncol 22:451–453
Erkanli S, Kayaselcuk F, Kuscu E et al (2006) Expression of survivin, PTEN and p27 in normal, hyperplastic, and carcinomatous endometrium. Int J Gynecol Cancer 16:1412–1418. doi:10.1111/j.1525-1438.2006.00541.x
Erkanli S, Bolat F, Kayaselcuk F, Demirhan B, Kuscu E (2007) COX-2 and survivin are overexpressed and positively correlated in endometrial carcinoma. Gynecol Oncol 104:320–325. doi:10.1016/j.ygyno.2006.08.044
Ferrandina G, Legge F, Ranelletti FO et al (2002) Cyclooxygenase-2 expression in endometrial carcinoma: correlation with clinicopathologic parameters and clinical outcome. Cancer 95:801–807. doi:10.1002/cncr.10736
Ferrandina G, Ranelletti FO, Gallotta V et al (2005) Expression of cyclooxygenase-2 (COX-2), receptors for estrogen (ER), and progesterone (PR), p53, ki67, and neu protein in endometrial cancer. Gynecol Oncol 98:383–389. doi:10.1016/j.ygyno.2005.04.024
Fowler JM, Ramirez N, DE Cohn et al (2005) Correlation of cyclooxygenase-2 (COX-2) and aromatase expression in human endometrial cancer: tissue microarray analysis. Am J Obstet Gynecol 192:1262–1271. doi:10.1016/j.ajog.2005.01.009 discussion 1271-1263
Fujiwaki R, Iida K, Kanasaki H et al (2002) Cyclooxygenase-2 expression in endometrial cancer: correlation with microvessel count and expression of vascular endothelial growth factor and thymidine phosphorylase. Hum Pathol 33:213–219. doi:10.1053/hupa.2002.31292
Hernandez E (2001) Endometrial adenocarcinoma: a primer for the generalist. Obstet Gynecol Clin North Am 28:743–757. doi:10.1016/S0889-8545(05)70233-3
Horvai AE, Li L, Xu Z et al (2003) Malignant mesothelioma does not demonstrate overexpression or gene amplification despite cytoplasmic immunohistochemical staining for c-Erb-B2. Arch Pathol Lab Med 127:465–469
Karahan N, Guney M, Baspinar S et al (2007) Expression of gelatinase (MMP-2 and MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J Gynaecol Oncol 28:184–188
Kuesters S, Maurer M, Burger AM, Metz T, Fiebig HH (2006) Correlation of ErbB2 gene status, mRNA and protein expression in a panel of >100 human tumor xenografts of different origin. Onkologie 29:249–256. doi:10.1159/000093048
Lambropoulou M, Alexiadis G, Limberis V, Nikolettos N, Tripsianis G (2005) Clinicopathologic and prognostic significance of cyclooxygenase-2 expression in endometrial carcinoma. Histol Histopathol 20:753–759
Lambropoulou M, Stefanou D, Alexiadis G et al (2007) Cytoplasmic expression of c-erb-B2 in endometrial carcinomas. Onkologie 30:495–500. doi:10.1159/000107734
Lehner R, Enomoto T, McGregor JA et al (2002) Correlation of survivin mRNA detection with histologic diagnosis in normal endometrium and endometrial carcinoma. Acta Obstet Gynecol Scand 81:162–167. doi:10.1034/j.1600-0412.2002.810213.x
Markogiannakis E, Georgoulias V, Margioris AN et al (1997) Estrogens and glucocorticoids induce the expression of c-erbB2/NEU receptor in Ishikawa human endometrial cells. Life Sci 61:1083–1095. doi:10.1016/S0024-3205(97)00617-6
Ohno S, Ohno Y, Suzuki N et al (2005) Multiple roles of cyclooxygenase-2 in endometrial cancer. Anticancer Res 25:3679–3687
Pallares J, Martinez-Guitarte JL, Dolcet X et al (2005) Survivin expression in endometrial carcinoma: a tissue microarray study with correlation with PTEN and STAT-3. Int J Gynecol Pathol 24:247–253. doi:10.1097/01.pgp.0000163849.37129.d4
Pappa KI, Anagnou NP (2005) Emerging issues of the expression profiling technologies for the study of gynecologic cancer. Am J Obstet Gynecol 193:908–918. doi:10.1016/j.ajog.2005.01.018
Prat J, Oliva E, Lerma E, Matias-Guiu X (1994) Uterine papillary serous adenocarcinoma. A 10-case study of p53 and c-erbB-2 expression and DNA content. Cancer 74:1778–1783. doi:10.1002/1097-0142(19940915)74:6<1778:AID-CNCR2820740621>3.0.CO;2-5
Smid-Koopman E, Blok LJ, Helmerhorst TJ et al (2004) Gene expression profiling in human endometrial cancer tissue samples: utility and diagnostic value. Gynecol Oncol 93:292–300. doi:10.1016/j.ygyno.2004.01.022
Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I (2002) Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett 184:105–116. doi:10.1016/S0304-3835(02)00190-8
Acknowledgment
We thank Professor E. Sivridis for eagerly providing the material for this study and the facilities of the Department of Pathology, Democritus University of Thrace.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lambropoulou, M., Papadopoulos, N., Tripsianis, G. et al. Co-expression of survivin, c-erbB2, and cyclooxygenase-2 (COX-2): prognostic value and survival of endometrial cancer patients. J Cancer Res Clin Oncol 136, 427–435 (2010). https://doi.org/10.1007/s00432-009-0673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0673-6