Abstract
Purpose
Metabolic dependence on glucose utilisation has been described for different tumours characterised by activation of Akt, upregulation of GLUT1, M2PK and TKTL1. To date, however, little is known about glucose metabolism in breast cancer tissue.
Methods
We analysed 55 breast cancer specimens, 26 adjacent ductal carcinomas in situ (DCIS) and 23 adjacent normal breast tissues for expression of glycolytic markers by immunohistochemistry.
Results
We found expression of pAkt in 49%, GLUT1 in 25%, M2PK in 68% and TKTL1 in 31% of the tumours investigated. Expression of pAkt and Her2neu are positively correlated with borderline significance (P = 0.055). Expression of pAkt, GLUT1 and TKTL1 were higher in breast cancer and DCIS than in normal tissue. Surprisingly, M2PK expression was highest in normal breast tissue.
Conclusions
We found a glycolytic phenotype in a high percentage of breast cancer samples. Inhibition of glycolysis might evolve as a future option for breast cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer amongst women in Western Europe and North America and the leading cause of cancer deaths. According to the Cancer Statistics 2005 of the American Cancer Society, the chance of developing invasive breast cancer during a woman’s lifetime is approximately 1 in 7 (about 14%). The prognostic factors currently accepted for clinical use are age, nodal status, tumour size, histological grade and steroid receptor status (Singletary and Connolly 2006). Overexpression of Her2neu (ErbB2, epidermal growth factor receptor) has also been shown to predict poor clinical outcome (Slamon et al. 1987), and thus immunohistochemical analysis of Her2neu expression has become a clinical routine. Breast cancer is a very well-studied tumour entity, but, up to now, little is known about glucose metabolism in breast cancer. Herein, we investigate whether specific markers are expressed in breast cancer which indicates a metabolic situation characterised by dependence on aerobic glycolysis and whether such characteristics might be indicative for more aggressive tumour growth.
Already in 1924, Otto Warburg showed that cancer cells display high rates of aerobic glycolysis (Warburg 1924), but it is only recently, that an interest in Warburg’s theory has reawakened. Whereas Otto Warburg proposed that the cause for cancer was linked to defects in oxidative phosphorylation, more recent models suggest several molecular changes as responsible factors for aerobic glycolysis in carcinoma cells.
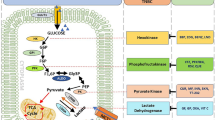
Glucose uptake seems to be critical in triggering cellular transformation. Cells which are able to take up glucose continuously and independently of environmental signals do not have to utilise glucose efficiently by oxidative phosphorylation. However, they are capable of increasing the glycolytic rate, in order to gain energy and metabolites, in a less efficient but faster manner. This phenomenon serves as a possible explanation why 18FDG-PET (fluorodeoxyglucose positron emission tomography) detects malignant tumours by using a radiolabeled glucose analogue. Glucose uptake in cancer cells is increased by activation of the oncogene Akt (protein kinase B) (Sun et al. 2001). Activation of Akt increases transcription and plasma membrane localisation of glucose transporter GLUT1 (glucose transporter 1), the glucose transporter expressed in most cell types (Barnes et al. 2005; Rathmell et al. 2003). Akt activation also leads to translocation of hexokinase I and II to the mitochondrial outer membrane, thereby increasing enzymatic phosphorylation of glucose (Buzzai et al. 2005). Enzymatic phosphorylation of glucose yields glucose-6-phosphate, which can either be metabolised via fructose-6-phosphate to phosphoenolpyruvate or enter the pentose phosphate cycle. Normal cells can maintain the required cellular energy level via the utilisation of fatty acids. Since Akt can block fatty acid oxidation, tumour cells with constitutively active Akt are highly dependent on glucose as energy source. Accordingly, they have been shown to die upon glucose withdrawal (Sun et al. 2001; Cross et al. 1995). Kinase activity of Akt is often increased in breast and ovarian cancers and appears to be associated with poor prognosis (Barnes et al. 2005).
The tetrameric form of the glycolytic M2PK (M2 pyruvate kinase isoenzyme) catalyses the phosphoryl group transfer from phosphoenolpyruvate to ADP, yielding pyruvate and ATP. In contrast the dimeric form of M2PK leads to an accumulation of glycolytic metabolites upstream of phosphoenolpyruvate, thereby providing substrates of synthetic processes such as amino acid production or nucleic acid synthesis via the pentosephosphate cycle. In tumour cells, the dimeric form is dominant (Mazurek et al. 2005). Tumour cells also have the ability to synthesise fatty acids (Lee et al. 1998), which are necessary for the assembly of new cell membranes. This process requires CoA and NADPH. The oxidative pentose phosphate cycle is an alternative NADPH producing pathway. Transketolase enzyme reactions enable oxygen-independent glucose degradation and play a crucial role in nucleic ribose synthesis utilising glucose carbons in tumour cells. Activation of transketolase by thiamine stimulates tumour growth (Comin-Anduix et al. 2001), whereas transketolase inhibitors such as oxithiamine inhibit tumour cell proliferation (Langbein et al. 2006). So far, three human transketolase genes have been described. Upon them, TKTL1 (transketolase like enzyme 1) has been shown to be overexpressed in different tumours, e.g. in colon and urothelial cancer (Coy et al. 2005), epithelial ovarian cancer (Krockenberger et al. 2007), as well as in granulosa cell tumours of the ovary (Schmidt et al. 2008). Overexpression of TKTL1 may result in glucose degradation via the alternative pentose phosphate pathway, yielding acetyl-CoA as well as energy and fatty acids for cell membrane assembly.
Constitutive activation of Akt, upregulation of GLUT1, dimerisation of M2PK and overexpression of TKTL1 characterise a phenotype of carcinoma cells that is highly dependent on glucose utilisation for the production of energy and synthetic processes. Herein, we evaluate breast cancer tissue, adjacent ductal carcinoma in situ and normal breast tissue for expression of pAkt, GLUT1, M2PK and TKTL1. The findings are compared with established breast cancer specific properties to reveal correlations or prognostic significance of glycolytic markers in breast cancer.
Materials and methods
Formalin-fixed and paraffin-embedded samples of 55 breast cancer specimens, 26 DCIS and 23 normal breast tissues were cut at 2 μm, placed on slides (Superfrost, Langenbrinck, Emmendingen, Germany), and deparaffinised with xylene. Rinsing of the slides in decreasing concentrations of ethanol was followed by antigen unmasking in 10 mM sodium citrate buffer (pH = 6.0) in a microwave oven at 600 W for 5 min. After rinsing sections in distilled H2O, endogenous peroxidase was inhibited by incubation for 10 min in 3% H2O2 in methanol. Slides were washed with PBS and incubated with 1% goat serum in PBS for 15 min. Subsequently, slides were incubated with different antibodies. The antibodies and dilutions used were: pAkt (Abcam ab28821, rabbit polyclonal, 1:100), M2PK (DF4, ScheBo Biotech AG, 1:250) and GLUT1 (rabbit-anti human polyclonal, 1:100, US Biologicals, Biomol, Hamburg, Germany). For detection of TKTL1, we used a mouse monoclonal antibody (clone JFC12T10, Linaris, Wertheim, Germany) previously described by Langbein et al. (2006), diluted in antibody diluent (DAKO, Hamburg, Germany) at 1:200–1:400. The antibody we used to stain for M2PK recognises only the dimeric form of M2PK, which has been described as the characteristic form present in tumour tissue (Schneider et al. 2003). The proliferation marker Ki-67 nuclear protein was stained using a mouse monoclonal antibody (clone MIB1, DAKO, dilution 1:100).
After 45–60 min of incubation at room temperature with the primary antibody in a humidified chamber, slides were washed with PBS and incubated with an appropriate biotinylated secondary antibody, washed and incubated with streptavidin-peroxidase according to the manufacturer’s protocol (LSAB-kit, DAKO, Hamburg, Germany). Staining was developed by adding 3,3′-diaminobenzidine (DAB, DAKO) with subsequent counterstaining using haematoxylin. Sections were dehydrated in graded ethanol and embedded in Vitro Clud (Langenbrinck).
Statistical analysis and clinical correlation
Expression levels of pAKT, GLUT1, M2PK and TKTL1 were evaluated by two independent observers experienced in histopathology and immunohistochemistry. For semiquantitative evaluation of staining distribution, a scoring scale from 1 to 4 was defined: score 1 indicating less than 10%, score 2 indicating 10–49%, score 3 indicating 50–79% and score 4 indicating staining of 80% or more of tumour cells. Staining intensities were scored as well. Score 0 indicates no staining, whereas score 1 indicates weak, score 2 moderate and score 3 strong staining. By multiplying the scores for percentage of positive tumour cells with the score for staining intensity, we obtained an Immunoreactive Score (IRS) as established for hormone receptor determination in breast cancer (Remmele and Stegner 1987). For statistical analysis IRS ≤ 4 was rated negative and IRS > 4 was rated positive for marker expression. Adjacent areas of normal breast and ductal carcinoma in situ were evaluated for marker expression as well. For clinical correlation file records of the patients were investigated, and Her2neu status was extracted from the clinical records. Her2neu overexpression was determined by the Department of Pathology by immunohistochemical staining with HercepTestTM (DAKO) and evaluated as recommended (Hatanaka et al. 2001). The statistical analysis was performed by a professional statistician applying SPSS for Windows, version 15.0 (SPSS Inc., USA). The study was performed according to the regulations of the local ethical committee.
Results
We evaluated 104 breast specimens of 55 patients via immunohistochemistry. We stained 55 breast cancer tissues and 26 available adjacent ductal carcinomas in situ as well as 23 normal breast tissues from the same patients for expression of pAkt, GLUT1, M2PK and TKTL1. No significant differences between the IRS scores determined by each of the two observers were evident. The mean age of the patients was 53 ranging from 27 to 96 (53 ± 13 years). The clinico-pathological and immunohistochemical characteristics are summarised in Table 1. Applying Spearmans Rho test, we confirmed that the expression of the markers of interest were not age dependent (result not shown). The Kolmogorov Smirnov Test confirmed that our patient sample does not follow Gaussian distribution and is stochastically independent (results not shown).
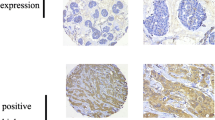
In Fig. 1 examples for expression patterns of pAkt (Fig. 1a, e, i), GLUT1 (Fig. 1b, f, j), M2PK (Fig. 1c, g, k) and TKTL1 (Fig. 1d, h, l) in breast cancer tissue (Fig. 1a–d), in DCIS (Fig. 1e–h), and in normal breast tissue (Fig. 1i–l) are shown. GLUT1 showed a characteristic membranous staining, whereas pAkt, M2PK and TKTL1 stained cytoplasmatically. M2PK and TKTL1 staining of DCIS showed intense staining of the myoepithelial cell layer in some but not all sections (Fig. 1g, h).
Immunohistochemical staining for pAkt, GLUT1, M2PK and TKTL1 in breast cancer, DCIS and normal breast tissue. Representative staining patterns for pAkt (a, e, i), GLUT1 (b, f, j), M2PK (c, g, k) and TKTL1 (d, h, l) in breast cancer (a–d), DCIS (e–h) and normal breast tissue (i–l) are demonstrated. IRS scores were higher in breast cancer and DCIS for pAkt (a, e), GLUT1 (b, f) and TKTL1 (d, h) than in normal breast tissue (i, j, l). In contrast, IRS scores for M2PK were higher in normal breast tissue (k) than in breast cancer (c) and DCIS (g)
Expression (IRS > 4) of pAkt was found in 49%, GLUT1 in 25%, M2PK in 68% and TKTL1 in 31% of the breast cancer samples we investigated (Table 1). We compared the IRS scores for pAkt with histopathological prognostic factors and found that patients with ErbB (Her2neu) overexpressing tumours frequently expressed high levels of pAkt (Fig. 2; Fishers exact test, P = 0.055). Only staining results for Her2neu evaluated as +++ were rated positive. Three breast cancer samples were assessed to be Her2neu++. They were rated negative for Her2neu expression. Looking at these borderline samples in detail, one of them was negative for Akt expression and two of them were positive. We also found a correlation between expression of GLUT1 and the proliferation marker Ki-67 (Mann–Whitney U test, P = 0.02; Fig. 3). Comparing the immunohistochemical staining results for pAkt, M2PK, TKTL1 and GLUT1 with clinico-pathological data we found no further correlation. The statistical tests we performed and the significance levels are listed in Table 2.
In addition, we compared the immunohistochemical staining results for pAkt, M2PK, TKTL1 and GLUT1 in breast cancer, adjacent DCIS and normal breast tissue. We found that pAkt expression was higher in carcinoma and DCIS than in normal breast tissue, yet the difference was not significant (Fig. 4; Kruskal–Wallis test, P = 0.081).
The mean expression of GLUT1 was about twice as high in carcinoma and DCIS than in normal breast tissue. However, this was not statistically significant (Fig. 5; Kruskal–Wallis test, P = 0.36), probably due to the relatively small sample size.
M2PK expression was significantly higher in normal breast tissue than in carcinoma (Mann–Whitney U test, P = 0.013) and higher—though not quite significantly higher—than in DCIS (Fig. 6; Mann–Whitney U test, P = 0.056).
TKTL1 expression was higher in carcinoma than in normal breast tissue (Mann–Whitney U test, P = 0.58) and expression was significantly higher in DCIS than in normal breast tissue (Fig. 7; Mann–Whitney U test, P = 0.026).
Discussion
Several molecular markers such as tyrosine kinase receptors are known to play a role in breast cancer progression and serve as targets for new therapeutic approaches. Up to now, little attention has been drawn towards glycolytic markers as indicators for altered glucose metabolism characteristic for tumour cells. We investigated the expression of a panel of glycolytic markers, pAkt, GLUT1, M2PK and TKTL1 in breast cancer tissue, adjacent DCIS and normal breast tissue. M2PK and TKTL1 seemed to show intense staining in the myoepithelial cell layer. The contribution of myoepithelial cells to breast cancer development has only recently been recognised. Myoepithelial cells have been discussed to be paracrine inhibitors of tumour progression (Sternlicht et al. 1997). Strong expression of M2PK and TKTL1 in the myoepithelial layer of DCIS might indicate that these cells are highly metabolic and therefore strongly dependent on glucose utilisation. Whether there is a different staining pattern of luminal and myoepithelial cells has to be analysed in future experiments.
We found high expression of pAkt in tumours overexpressing ErbB2. This is consistent with previous reports describing pAkt expression as indicative for poor outcome (Sun et al. 2001). Expression of GLUT1 correlated with expression of the proliferation marker Ki-67, indicating that fast growing tumours are dependent on high amounts of glucose as energy source. In breast cancer and DCIS, pAkt expression was found to be higher than in normal breast tissue. Similarly, expression of GLUT1 and TKTL1 was higher in carcinoma and DCIS than in normal tissue. Even though the number of tissue samples analysed was rather small, some of our observations proved to be statistically significant, other results show a clear tendency. The finding of higher expression levels of pAkt, GLUT1 and TKTL1 in tumour cells and DCIS as compared to normal breast tissue supports our hypothesis that glycolysis is characteristic for tumour cells and that the switch from aerobic fermentation to tumour glycolysis is an early step in transformation of tumour cells. To our surprise, M2PK shows a contrasting distribution pattern. Expression of M2PK is high in normal breast tissue and lower in DCIS and tumour cells. The antibody we used for M2PK staining is specific for the dimeric form of M2PK leading to an accumulation of glycolytic metabolites upstream of phosphoenolpyruvate and thereby providing substrates for synthetic processes. The dimeric form of M2PK is known to be dominant in tumour cells (Mazurek et al. 2005). We found higher expression of M2PK in adjacent normal breast tissue than in DCIS or carcinoma cells itself. A possible explanation could be that the predominance of the dimeric form represents a very early step in transformation and is therefore mainly found in benign tissue surrounding DCIS and carcinoma cells. An alternative explanation might be that primary breast cancer is mostly associated with relatively slow tumour growth as compared to colon carcinoma, ovarian carcinoma or granulosa cell tumours of the ovary, which were shown to overexpress M2PK (Schmidt et al. 2008). Therefore, energy supply might be a more decisive factor than the assembly of new cells. This might explain the presence of glycolytic markers even though the supply with metabolites for synthetic processes does not need to be triggered. The distribution patterns for dimeric M2PK found by us in breast cancer and surrounding tissue suggests that the role of M2PK in breast cancer might have to be re-evaluated.
Herein, we describe a metabolic phenotype in breast cancer characterised by activation of Akt, up-regulation of GLUT1, TKTL1, and, surprisingly, down-regulation of M2PK. Consideration of these glycolytic markers in breast cancer diagnosis might open up new prognostic and therapeutic approaches. Several inhibitors of glycolysis are currently under clinical investigation (reviewed in Pelicano et al. 2006), e.g. glucose analogues such as 2-deoxyglucose, a glucose analogue inhibiting phosphorylation of glucose by hexokinase or lonidamine suppressing glycolysis in cancer cells by inhibition of hexokinase bound to mitochondria. Alone or in combination with chemotherapeutics, these and other inhibitors of glycolysis might evolve to be effective in breast cancer therapy.
References
Barnes K, McIntosh E, Whetton AD et al (2005) Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene 24:3257–3267. doi:10.1038/sj.onc.1208461
Buzzai M, Bauer DE, Jones RG et al (2005) The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene 24:4165–4173. doi:10.1038/sj.onc.1208622
Comin-Anduix B, Boren J, Martinez S et al (2001) The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur J Biochem 268:4177–4182. doi:10.1046/j.1432-1327.2001.02329.x
Coy JF, Dressler D, Wilde J et al (2005) Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin Lab (Zaragoza) 51:257–273
Cross DA, Alessi DR, Cohen P et al (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. doi:10.1038/378785a0
Hatanaka Y, Hashizume K, Kamihara Y et al (2001) Quantitative immunohistochemical evaluation of HER2/neu expression with HercepTestTM in breast carcinoma by image analysis. Pathol Int 51:33–36. doi:10.1046/j.1440-1827.2001.01162.x
Krockenberger M, Honig A, Rieger L et al (2007) Transketolase-like 1 (TKTL1) expression correlates with subtypes of ovarian cancer and the presence of distant metastases. Int J Gynecol Cancer 17:101–106. doi:10.1111/j.1525-1438.2007.00799.x
Langbein S, Zerilli M, Zur Hausen A et al (2006) Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 94:578–585. doi:10.1038/sj.bjc.6602962
Lee WN, Boros LG, Puigjaner J et al (1998) Mass isotopomer study of the nonoxidative pathways of the pentose cycle with [1, 2–13C2]glucose. Am J Physiol 274:843–851
Mazurek S, Boschek CB, Hugo F et al (2005) Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 15:300–308. doi:10.1016/j.semcancer.2005.04.009
Pelicano H, Martin DS, Xu RH et al (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646. doi:10.1038/sj.onc.1209597
Rathmell JC, Fox CJ, Plas DR et al (2003) Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23:7315–7328. doi:10.1128/MCB.23.20.7315-7328.2003
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8:138–140
Schmidt M, Kammerer U, Segerer S et al (2008) Glucose metabolism and angiogenesis in granulosa cell tumors of the ovary: activation of Akt, expression of M2PK, TKTL1 and VEGF. Eur J Obstet Gynecol Reprod Biol 139:72–78. doi:10.1016/j.ejogrb.2008.02.009
Schneider J, Neu K, Velcovsky HG et al (2003) Tumor M2-pyruvate kinase in the follow-up of inoperable lung cancer patients: a pilot study. Cancer Lett 193:91–98. doi:10.1016/S0304-3835(02)00720-6
Singletary SE, Connolly JL (2006) Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 56:37–47. doi:10.3322/canjclin.56.1.37
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182. doi:10.1126/science.3798106
Sternlicht MD, Kedeshian P, Shao ZM et al (1997) The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 3:1949–1958
Sun M, Wang G, Paciga JE et al (2001) AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol 159:431–437
Warburg O (1924) Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften 50:1131–1137. doi:10.1007/BF01504608
Acknowledgments
We thank André Schmidt and Kurt Weigand for their valuable discussions, Marianne Florey for critical review of the manuscript, as well as Tanja Kottmann for statistical analysis. This study was supported by the Medical Faculty of the University of Wuerzburg and “Hilfe im Kampf gegen Krebs e.V.”
Conflict of Interest Statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, M., Voelker, HU., Kapp, M. et al. Glycolytic phenotype in breast cancer: activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J Cancer Res Clin Oncol 136, 219–225 (2010). https://doi.org/10.1007/s00432-009-0652-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0652-y