Abstract
Introduction
In advanced ovarian cancers (OCs), p53 mutations are frequently observed. The objective of this study was to explore the value of the p53 mutational status, using four different techniques, in advanced OC patients as a predictive marker for responsiveness to platinum-based chemotherapy.
Methods
One hundred and four, mostly serous papillary OC specimens were analyzed, of which all received a platinum containing chemotherapy after optimal cyto-reductive surgery. To verify the p53 mutational status, immunohistochemical staining with monoclonal antibodies, functional yeast assay (FASAY), single-strand conformation polymorphism analysis (SSCP) and genomic sequencing was performed in parallel.
Results
Out of ten OC patients [2 low malignant potential (LMP)/8 G1] only two had a mutant p53, whereas eight showed a wild-type p53. 40 out of 63 (G2/3) patients with G2/3 OC showed mutant p53 and 23 patients showed a wild-type pattern. p53 status was significantly different between these two groups (LMP/G1 vs. G2/3) (P = 0.015). A progressive disease after chemotherapy completion was noted in 35.6% of the patients (26 out of 73); in 69.2%, a mutated p53 and in 30.8%, a wild-type p53 was found. Nine (12.3%) patients showed a complete response at the end of the first-line chemotherapy. Out of these nine patients five had a mutated and four a wild-type p53. A partial response was observed in nine (12.3%) patients of whom four had a mutated p53. With respect to response to first-line chemotherapy (six cycles of platinum containing regimen), the p53 status was not predictive; no statistical significance regarding the p53 mutational status was observed when the two extreme groups PD versus PR/CR were compared (P > 0.05).
Conclusion
In this study, the p53 mutational status was not predictive for responsiveness to platinum-based chemotherapy; but p53 was significantly more frequently mutated in poorly differentiated OCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is the second most common gynecological cancer but is still the one with the worst prognosis. Around 8,000 and 22,000 patients per year are newly diagnosed with OC in Germany and US (Jemal and Siegel et al. 2008), respectively.
Up to date there is no established OC screening program available. Standard therapy is cyto-reductive surgery followed by an adjuvant chemotherapy combination of paclitaxel and carboplatin (Neijt and Engelholm et al. 2000; du Bois and Luck et al. 2003; Ozols and Bundy et al. 2003). The vast majority of patients do respond to this regimen but some of these patients do relapse within 6 months; these cases are considered to be platinum resistant. In such situations, the prognosis is very limited. In cases where the cancer does recur later, a platinum-based chemotherapy is often re-induced but response rates are lower than after adjuvant therapy (Bolis and Scarfone et al. 2001; Pfisterer and Plante et al. 2006).
Established prognostic factors in OCs are FIGO stage and post-surgery residual tumor burden (Heintz and Van Oosterom et al. 1988). Other factors of less prognostic value are grading, histopathological subtype—clear cell differentiation being the worst (Chan and Teoh et al. 2008; Kurman and Shih 2008)—ascites, and afore mentioned the time period to relapse. So far none of the established markers is predicting response to chemotherapy.
Looking for additional markers which could predict prognosis and response to chemotherapy would be extremely important to subgroup patients and improve patient’s outcome and to avoid inefficient treatments.
OC does arise from the cubic endothelial layer covering the ovary but the genesis is still not fully understood. Different genetic alterations for tumors of low malignant potential (LMP) together with low-grade invasive OCs have been described when compared to high-grade OCs (Singer and Kurman et al. 2002; Singer and Shih et al. 2003). For the latter group, mutations in the p53 gene were found in up to 60%. In comparison, only 8% of the LMP tumors show mutated p53 (Singer and Stohr et al. 2005; Schuijer and Berns 2003). In a recent publication, we showed that LMP tumors and well-differentiated serous OCs cluster together sharing a similar molecular profile. In contrast, moderate and poorly differentiated serous OC did also exhibit a similar profile that was distinctly different from the profile of the LMP and well-differentiated tumors (Kurman and Shih 2008). Kurman et al. introduced type I and II OC. They based this differentiation on their findings that in low-grade OC and precursor lesions, type I, KRAS and BRAF are frequently mutated. On the contrary, in type II OCs consisting of high-grade lesions, p53 is almost exclusively mutated and combined with genomic instability in these tumors. These interrogations let people believe that there are two distinct groups of OCs with different routes of origin.
P53 is still considered to be one of the most important guardians for DNA replication and a major player in cell cycling (Prives and Hall 1999). P53 gets activated in response to DNA damage, induced, e.g., by platinum intercalation and causes a cell cycle arrest at G1/G2 checkpoints until the damage has been repaired or apoptosis is induced (Vogelstein et al. 2000). P53 is involved in regulating different cell cycling genes such as p21/waf 1, a potent inhibitor of cyclin-dependent kinases (Harper et al. 1993) and GADD45 sensoring environmental and physiological stress (Smith et al. 1994). It is also involved in regulating pro- and anti-apoptotic factors such as caspase activation via mitochondrial cytochrome and Smac/Diabolo release again controlled by the Bcl 2-family (Gadducci et al. 2002).
In OC, the occurrence of p53 gene alterations is dependent on different factors. Shelling et al. (1995) describes an increase with higher stage (58% III/IV vs. 37% I/II). Also, the histological subtype seems to have influence on the p53 status. Most mutations and over-expressions are depicted in serous OCs with about 58%. Endometrioid, mucinous and clear cell differentiated tumors show less frequent p53 alterations, 28, 16 and 10%, respectively (Skilling et al. 1996). In a very recent publication, Salani et al. (2008) showed that in purified tissue samples of serous OC, the mutation rate of p53 is about 80%. These findings again fit into the proposal of two types of OCs and two pathways of tumorigensis.
The half-life of the p53 protein is short so that it is barely detectable by routine methods in normal tissue. Immunohistochemical (IHC) staining using monoclonal antibodies detect over-expression of p53, which is normally considered mutated, this is however not exact, because p53 could be either over-expressed because of increased translation followed by transcription or a mutation that leads to stabilization or resistance toward degradation. Although many studies have been carried out using this IHC method, there is still a debate about their validity because different studies showed differing results. Next to IHC staining (Bali et al. 2004; Bartel et al. 2008), many different methods—such as sequencing (Galic et al. 2007), chemical mismatch cleavage (Curiel et al. 1990), denaturing gradient gel electrophoresis (DGGE) (Holmila and Husgafvel-Pursiainen 2006), functional yeast assay (FASAY) and single-strand conformation polymorphism assay (SSCP) (Bartel et al. 2008)—have been used to elucidate the importance of p53 mutations with regard to prognosis and chemotherapy response. None of these studies could proof the association of p53 mutation and response to chemotherapy, but because of uncertainty of the p53 testing, it still cannot be ruled out.
Despite all the efforts, there are still conflicting results regarding the association of p53 over-expression/mutation and its correlation with overall survival and responsiveness to chemotherapy in OC patients (Psyrri et al. 2007; Bartel et al. 2008).
We have recently shown that it is unlikely that one single method can recognize all possible mutations. From our previous study comparing four different methods (SSCP, FASAY, IHC, and DNA sequencing), we draw the conclusion that at least two of these methods should be combined to detect the p53 mutational status to reach a high level of confidence (Meinhold-Heerlein et al. 2001). In order to contribute to this complex subject, we analyzed 104 patients with mainly serous ovarian carcinomas using these four different techniques to determine the p53 status (Meinhold-Heerlein et al. 2001) and did correlate these results with histological subtypes, grading, stage, age, response to chemotherapy and survival.
Materials and methods
Materials and methods for mutational analyses have been published previously (Meinhold-Heerlein et al. 2001), in brief. One hundred and four women with OC presenting at the Department of Gynecology of the University of Freiburg between 1987 and 1994 were included in this study. One portion of the tumor was immediately snap frozen in liquid nitrogen and stored at −80°C until further processing. Another portion was fixed in 8% formalin and paraffin embedded.
IHC analysis
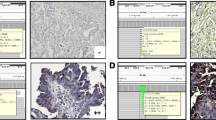
Five micrometer sections of paraffin-embedded tissue were attached to slides pretreated with aminoalkylsilane as described in the protocol of Rentrop et al. (1986). The tissue was dewaxed with xylene and rehydrated according to standard procedures. Endogenous peroxidase was blocked with 3% hydrogen peroxidase in PBS for 10 min. Slides were then treated with Target unmasking Fluid (TUF, Dianova SP 0025, Hamburg, Germany) for 20 min at 60°C to enhance antigen accessibility. After blocking non-specific binding of the antibody with 1.5% horse serum in PBS for 30 min, the sections were incubated with DO-1 anti-p53 monoclonal primary mouse antibody (Dianova/Oncogene Science OP43, Hamburg, Germany) for 12 h at 4°C (1:100 dilution). Secondary biotinylated rabbit anti-mouse antibody (1:200 dilution) was detected with Vectastain ABC kit (Serva, Heidelberg, Germany) and diaminobenzidine (DAB chromogen kit, DAKO; Germany) with metal enhancement (0.5%; w/v; CuSO4). Counterstaining was performed with 1% (w/v) hemalaun. Sections were dehydrated with increasing concentrations of ethanol and mounted with Entellan (Merck, Darmstadt, Germany). Primary antibody was omitted in duplicate test section as negative control. Cases were considered mutated by immunohistochemistry if >10% of the nuclei stained positively with the monoclonal antibody DO-1 (Fig. 1).
SSCP analysis
Genomic DNA was isolated from a homogenized aliquot of the tumor tissue as described previously (Miller et al. 1988). PCR amplification of exons 5–8 of the p53 gene was performed using Taq polymerase (Pharmacia, Freiburg, Germany) and two suitable oligonucleotide primers (Mazars et al. 1991), published previously (Meinhold-Heerlein et al. 2001). Codons 126–163 and 147–186 of exon 5 were analyzed separately and they are called as 5 and 5′. SSCP analysis was performed with a 12% polyacrylamide gel containing 10% (v/v) glycerol. Electrophoresis was carried out at 30 W for 16 h 4°C. Placental tissue served as a negative control. Silver staining procedure of the gel was performed as published elsewhere (Budowle et al. 1991). In cases where a band shift was detected, a second independent radioactive PCR–SSCP was performed. Elution of the abnormal band was followed by an asymmetric PCR of the single-strand transcript (Gyllensten and Erlich 1988). Purified PCR-DNA was sequenced.
Functional assay for the separation of alleles in yeast (FASAY)
Functional assay for the separation of alleles in yeast was performed as described by Waridel et al. (1997). Tissue samples for RT-PCR were homogenized. After binding to 75 μl oligo-dt-coated magnetic beads, the mRNA was purified using a Dynabeads mRNA direct kit and MPC-E magnet (Dynal, Hamburg, Germany). RNA was eluted in 20 μl elution solution containing 50 U human placental RNase inhibitor (Boehringer, Mannheim, Germany) and stored at −20°C. p53 cDNA was synthesized using superscript II reverse transcriptase (Gibco Eggenstein, Germany) and the primer RT-1 (5′-CGG GAG GTA GAC-3′; Genset, Paris, France). PCR was performed with Pfu DNA polymerase (Boehringer Mannheim) and the primers as described by Flaman et al. (1995). The amplification product was checked by gel electrophoresis. For gap repair, the yeast expression vector pRDI-22 (Waridel et al. 1997) was digested with HindIII and StuI. The linearized plasmid was dephosphorylated with calf intestinal alkaline phosphatase (Boehringer Mannheim, Germany) and extracted with ethanol. The gap lies between codons 67 and 347 (Ishioka et al. 1993). The reporter strain yIG397 was grown and transformed as described (Flaman et al. 1995). If more than 19% of the colonies were red, the FASAY classified positive (Fig. 2; Flaman et al. 1995; Inga et al. 1997; Kashiwazaki et al. 1997; Lomax et al. 1997; Waridel et al. 1997). RT-PCR products were sequenced on a Licor automated sequencer using the primers described by Waridel et al. (1997).
DNA sequencing
Shifted bands in SSCP analysis were eluted and the mutation was confirmed by dideoxy sequencing (Sanger et al. 1977) using a T7 sequencing kit (Pharmacia) according to the manufacturer’s instructions. The oligonucleotide primers have been described previously (Mazars et al. 1991). Genomic DNA of placental tissue served as a negative control.
Clinical data
Patients did sign confirmed consent allowing the collection of clinical data, this procedure is in accordance with the ethical standard.
For all available samples, at least two different methods were performed to determine the mutational status. In cases were at least one of the performed tests showed a positive signal, the case was classified as mutated. If tests showed consistently a wild-type signal, the case was considered to have a functional p53.
Interpretations of the results and statistical analysis
Statistical analyses were run with the SPSS program Version XVI. To find correlation between the p53 status, clinical and histopatholgical parameters the Chi-square test and the Fisher exact test were performed. Statistical significance was assumed with a P value of <0.05.
Results
We analyzed 104 patients with OC who were admitted to the Department of Gynecology at the University Hospital Freiburg, Germany between 1987 and 1994. Tumor samples were partly snap frozen and partly paraffin embedded for further analysis. To determine the status of the p53 gene, four different techniques were employed; IHC analysis, SSCP and functional assay for the separation of alleles in yeast (FASAY), genomic sequencing was performed in those cases which were highly suspicious to contain p53 mutations. We also collected clinico-pathological data: survival data, FIGO stage, age at diagnosis and response to platinum containing chemotherapy and documented histo-pathological characteristics such as grading and histological subtypes. These data were available in 73 cases. In our analyses, we classified 42 cases (57.5%) out of 73 as mutated when at least 1 method showed a positive signal (IHC, SSCP, or FASAY). In those cases which were highly suspicious to contain p53 mutations, genomic sequencing was performed additionally. Mutation loci and amino acid exchange are extensively described in our previous study (Meinhold-Heerlein et al. 2001). In 31 cases (42.5%), no method gave a positive signal; these cases were classified as wild type (Tables 1, 2, 3).
Response to platinum-based first-line chemotherapy and p53
Twenty-six (35.6%) patients out of 73 had a progressive disease under platinum-based first-line chemotherapy. 18 cases were classified as p53 mutant and eight cases had a wild-type p53 status. 20 (27.4%) patients showed no evidence of disease; based on the report, these patients had no evaluable tumor left at the end surgery. Nine (12.3%) patients showed a complete response at the end of the first-line chemotherapy. Out of these nine patients five had a mutated and four had a wild-type p53. A partial response was observed in nine (12.3%) patients of whom four had a mutated p53. In the group of patients with no change (n = 9), four cases had a mutated p53. With respect to response to first-line chemotherapy (six cycles of platinum containing regimen), the p53 status was not predictive; no statistical difference between wild type and mutant p53 was observed.
Survival and stage
We had data from 71 patients. The 2-year survival differed significantly between FIGO stages I/II (early stage) and FIGO III/IV (late stage). None of the early stage OC patients (n = 7) died within 2 years, but 31 of 64 late stage OC patients died within 2 years (P < 0.05).
Survival and p53
Analyzing the 2-year survival between patients with wild type and mutant p53; data for 71 patients were available. No statistical significant difference could be observed (P > 0.05). The median survival for patients carrying a mutation within the p53 gene was 29 months (confidence interval 18.4–39.6) versus 27 months (confidence interval 14.9–39.1) for patients having a wild-type p53 (Fig. 3).
Stage and p53
Seven patients out of 73 (9.6%) were diagnosed with a stage I or II disease. 90.4% (66 out of 73) of the patients had a stage III or IV advanced disease. Three patients with an early stage disease had mutant p53 and four had wild-type p53. In the group of patients with advanced diseases, 39 patients showed a mutated p53, whereas 27 patients were classified to have a wild-type p53 gene. When comparing the p53 status, there was no statistical significant difference between early and late stage OCs (P > 0.05).
Grading and p53
Ten patients had a LMP disease (n = 2) or a well-differentiated invasive OC (n = 8), two had a mutant p53, whereas the other eight showed a wild-type p53. 40 out of 63 patients carrying a moderate or poorly differentiated invasive OC had a mutant p53; 23 patients showed a wild-type pattern. Comparing these two groups, LMP/G1 versus G2/3, the p53 status did show a significant correlation with the tumor cell differentiation (P = 0.015), showing that with de-differentiation, the p53 mutation rate increases.
Histological subtypes of OC and p53
In our study cohort, we had 52 (71.2%) cases of serous papillary differentiation, 11 cases (15.1%) endometrioid, 7 (9.6%) cases mucinous, and 3 (4.1%) cases clear cell differentiated OCs, respectively.
When grouped into serous and non-serous OCs, no statistically significant difference regarding the p53 status was observed (P > 0.05).
Age and p53
Looking at the age at diagnosis, we could not find a significant difference in the mutational status of the p53 gene in correlation to age (Fig. 4).
Patients with mutated p53 had a middle age of 61.3 years (±10.8); patients with a wild-type p53 status were in mean 56.6 years old (±14.2). This difference was not statistically different (P = 0.111).
Discussion
Ovarian cancer is still the gynecological malignancy with the highest mortality rate, mainly due to its detection in advanced stage, where the tumor has spread beyond the pelvis. OC does cause only unspecific clinical symptoms. Up to date there is no early detection marker available for clinical routine procedures. Established treatment options for advanced OC is the sequential approach of radical surgery followed by a combination of intravenous chemotherapy with carboplatin and paclitaxel for six cycles.
Also, there are only very few prognostic markers, which are of clinical nature, most importantly the residual tumor burden after surgery. Grading and histological subtype are markers of limited prediction value. The biological profile of OC is still not considered being of predictive value in daily routine. There is still a lack of markers that could predict disease response toward chemotherapy and risk of recurrence; this is in some part due to inconsistency in publications with regard to key proteins.
P53 is considered to be one of the key regulators for correct DNA replication and is mutated in about half of all human malignancies (Hollstein et al. 1994; Levine 1997). Being involved in many signaling pathways, p53 has been intensively studied in various cancer types, i.e., in breast cancer where up to 25% of the study population had a mutated p53 (Offersen et al. 2008). Irrespective of the nodal status, mutations of the p53 was one of the strongest predictors of poor prognosis.
One genetic disease is directly linked to disrupted p53 function, in HPNCC families (Lynch syndrome) colon polyps occur at a high frequency which in turn do convert into pre-neoplastic lesions and eventually into colon cancer.
In OC, p53 has been investigated in various studies in which a mutation rate between 23 and 80% has been found (Hollstein et al. 1991, 1994; Levine et al. 1991; Levine 1997; Galic et al. 2007; Kupryjanczyk et al. 2008; Salani et al. 2008). P53 mutations in OC have been related to poor prognosis (Wen et al. 1999; Ueno et al. 2006) as well as to better survival (Ueno et al. 2006) but still no large scale prospective studies have been conducted to determine whether the p53 status is of definitive predictive value for survival.
However, some studies did analyze the question whether the p53 status could predict the response to platinum-based chemotherapy. The response is predictive of the overall outcome of OC patients, given that patients who do have a progressive disease under chemotherapy or those whose tumor recurred with in 6–12 months after the last chemotherapy have a unfavorable prognosis. Many attempts have been undertaken to find out who would more likely benefit from chemotherapy (those patients with wild type or those with mutated p53). Especially in OC, the published results are contrary; some groups showed that OCs with mutated p53 do respond better than the wild-type ones (Lavarino et al. 2000; Ueno et al. 2006); other groups did show the opposite (Nakayama et al. 2003; Gadducci et al. 2006; Bartel et al. 2008) or did find that p53 is non-predictive (Gadducci et al. 2000). Considering histological subtypes, p53 was found to be predictive for subgroups, i.e., non-serous subtypes as shown by Ueno et al. (2006).
In most studies, the p53 status was almost exclusively determined by IHC staining using monoclonal antibodies. Theoretically the accumulation of otherwise short lived p53 protein could point indirectly toward a mutation within the p53 gene. But the accumulation could also be potentially caused indirectly by protein stabilizing cofactors and/or by disrupted p53 degradation leading ultimately to inadequate protein signaling.
Lavarino et al. (2000) descript a correlation between the p53 status and the response rate, showing that tumors carrying mutated p53 are significantly more responsive. But looking at the different methods employed, they showed that compared with SSCP, the protein accumulation check by IHC was much less predictive, not even statistically significant.
In this study, we employed mainly three different techniques, SSCP, FASAY and IHC staining, to determine the p53 status (Meinhold-Heerlein et al. 2001). In some exclusive cases which were highly suspected to contain a mutated p53 gene, we performed DNA sequencing. From our previous study, comparing four different methods (SSCP, FASAY, IHC, and DNA sequencing), we hypothesized that at least two methods should be performed to detect the p53 mutational status to reach a high level of confidence. As published by Meinhold-Heerlein et al. (2001), the detection rate was quite different between these techniques (SSCP 50%, IHC 62%, and FASAY 77%). In the present study, p53 was considered mutated when one out of the three techniques detected a mutation (31.5% SSCP, 39.7 IHC, and 49.3% FASAY); the overall detection rate was 57.5% and thus lower than previously published (Meinhold-Heerlein 2001). This may be due to the smaller collection of cases. Nevertheless, the FASAY again proved to be the most sensitive test. Therefore, we are confident about the accuracy of our data concerning the mutational status. Of note, the distribution of the FIGO stages is somewhat imbalanced; seven cases of FIGO stage I and II and 66 cases of FIGO stage III and IV were included, respectively. This imbalance might account for the fact that in this study the p53 status is not link to the FIGO stage.
Two recently published studies: one using IHC only (Psyrri et al. 2007) and the other one using IHC and SSCA (Bartel et al. 2008) yet again demonstrated that there is still no consensus about the predictive value of p53 in OC. Both studies included over 100 patients, respectively, but despite this larger numbers, most of the earlier published data had rather small numbers, the findings were contrary. Psyrri et al. (2007) shows that high nuclear and cytoplasmic p53 expression is associated with better outcome, reflected in improved overall survival and disease-free survival. Bartel et al. (2008) show in their investigation that 49% of patients with a wild-type p53 do over-express it. Patients over-expressing wild-type p53 were less responsive to chemotherapy and did have an unfavorable prognosis. In our analysis, we also could not demonstrate a correlation between p53 mutational status and survival irrespective of residual tumor burden. One may also bear in mind that the choice of the second line therapy has a big impact on OC patients’ survival, which has not been linked to the p53 status yet.
P53 itself being involved in maintaining accurate DNA replication gets activated by DNA damage, i.e., caused by DNA intercalating substrates such as platin derivates. Disrupted p53 function could lead to inefficacy of DNA intercalating substrates because correct DNA replication is no longer surveyed. No signal to arrest the cell cycle or to even induce apoptosis gets transduced because of disrupted p53 signaling. Therefore, we reasoned that information about the p53 status could contribute to predict the response to platinum containing chemotherapy. Hence, p53 in our cohort is not predictive of response to platinum-based therapy. In discordance with Bartel’s study, we did not find a correlation between the p53 status and response to chemotherapy. This might be due to the fact that a combination therapy is overcoming the p53 insufficiency. The introduction of paclitaxel as a standard chemotherapeutic agent might have overcome the effects of an ineffective p53 signaling. Taxanes do interact with the spindula and is p53 independent.
There is still a debate about how OC develops and how it does progress. There are several pieces of evidence that OC does not follow a stepwise model via hyperplasia to a LMP tumor to a fully developed OC, as it is established for colon cancer by Vogelstein. Recent results suggest that there are two separate ways of OC development. In LMP tumors and well-differentiated OCs, RAS mutations are frequently observed, whereas only a few such mutations have been found in G2/3 OCs (Shih and Kurman 2004; Dehari et al. 2007). Our group (Meinhold-Heerlein et al. 2007) recently demonstrated by gene expression profiling that LMP/G1 differentiated OCs clustered together and G2/3 OCs shared similarities in gene expression profiles. On the contrary, p53 has been shown to be frequently mutated in moderately and poorly differentiated OC (Salani et al. 2008). With loss of cell differentiation, we did find a significant correlation with mutated p53, showing the highest rate of p53 mutation in poorly differentiated cells. Tumor cells with inadequate differentiation do acquire higher rates of DNA alterations to progress through the cell cycle, i.e., p53 as one major guardian of correct DNA replication needs to be disarmed. This notion again supports the hypothesis introduced by Kurman and Shih that there are two types I and II of OC with profound molecular differences.
OC has different histological subtypes; most commonly is a serous papillary differentiation. Each subtype can respond differently to chemotherapy and even survival rates are somewhat dependent on the histological subtype; tumors with clear cell differentiation seem to have a less favorable prognosis. Chan et al. (2008) recently published data where patients with clear cell OC have a significantly shorter disease-free survival that those with serous papillary differentiated OC (Chan et al. 2008). Looking at the mutational status among the different histological subtypes of our cohort, no statistical significant differences could be observed. Also, when comparing all non-serous versus serous differentiation, no difference with regard to the p53 status was observed.
Observing cancer as a disease of aging, one could expect that with increasing age the probability of gathering genetic alterations increases. Feng et al. (2007) show that a decreased p53 activity over life time occurs in mice. With respect to the cohorts age at diagnosis we could not show a significant correlation between age and the p53 status.
Conclusion
Linking p53 alterations to chemotherapy response or outcome prediction in patients suffering form OC is still a challenge. P53 has been studied extensively but results and conclusions are still conflicting. Part of this problem is that most studies have used monoclonal antibodies to describe p53 alterations. Also, in many studies the sample number is small. Another issue is that the patients’ cohorts do contain different histological subtypes which do differ with respect to patients’ outcome and response to chemotherapy. In some studies, patients did receive different types of chemotherapy which makes interpretation of responsiveness challenging. We analyzed the p53 in 73 patients with mostly serous OC status by four different techniques. Whenever one out of four showed an alteration, p53 was considered to be mutated. This gives us a high confidence in our results concerning the p53 mutation because there is not a single test that could claim to detect all p53 mutations. All patients did receive platinum containing adjuvant therapy for six cycles after having received optimal surgical debulking. In our study, we can show that with loss of cell differentiation we did find a significant correlation with mutated p53, showing the highest rate of p53 mutation in poorly differentiated cells. Tumor cells with inadequate differentiation do acquire higher rates of DNA alterations to progress through the cell cycle, i.e., p53. But we were not able to provide a link between the p53 status in OC and response either to platinum-based chemotherapy or to patients’ 2-year survival.
OC still has a limited overall survival rate and so far no molecular marker is widely accepted to predict the response to chemotherapy or even the prognosis. Clinico-pathological markers such as stage, histological subtype, grading, and postoperative tumor burden, in particular, are still leading the clinician in predicting response and outcome.
References
Bali A, O’Brien PM et al (2004) Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res 10(15):5168–5177
Bartel F, Jung J et al (2008) Both germ line and somatic genetics of the p53 pathway affect ovarian cancer incidence and survival. Clin Cancer Res 14(1):89–96
Bolis G, Scarfone G et al (2001) Carboplatin alone vs carboplatin plus epidoxorubicin as second-line therapy for cisplatin- or carboplatin-sensitive ovarian cancer. Gynecol Oncol 81(1):3–9
Budowle B, Chakraborty R et al (1991) Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet 48(1):137–144
Chan JK, Teoh D et al (2008) Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 109(3):370–376
Curiel DT, Buchhagen DL et al (1990) A chemical mismatch cleavage method useful for the detection of point mutations in the p53 gene in lung cancer. Am J Respir Cell Mol Biol 3(5):405–411
Dehari R, Kurman RJ et al (2007) The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol 31(7):1007–1012
du Bois A, Luck HJ et al (2003) A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95(17):1320–1329
Feng Z, Hu W et al (2007) Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci USA 104(42):16633–16638
Flaman JM, Frebourg T et al (1995) A simple p53 functional assay for screening cell lines, blood, and tumors. Proc Natl Acad Sci USA 92(9):3963–3967
Gadducci A, Cianci C et al (2000) p53 status is neither a predictive nor a prognostic variable in patients with advanced ovarian cancer treated with a paclitaxel-based regimen. Anticancer Res 20(6C):4793–4799
Gadducci A, Cosio S et al (2002) Molecular mechanisms of apoptosis and chemosensitivity to platinum and paclitaxel in ovarian cancer: biological data and clinical implications. Eur J Gynaecol Oncol 23(5):390–396
Gadducci A, Di Cristofano C et al (2006) P53 gene status in patients with advanced serous epithelial ovarian cancer in relation to response to paclitaxel-plus platinum-based chemotherapy and long-term clinical outcome. Anticancer Res 26(1B):687–693
Galic V, Willner J et al (2007) Common polymorphisms in TP53 and MDM2 and the relationship to TP53 mutations and clinical outcomes in women with ovarian and peritoneal carcinomas. Genes Chromosom Cancer 46(3):239–247
Gyllensten UB, Erlich HA (1988) Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci USA 85(20):7652–7656
Harper JW, Adami GR et al (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75(4):805–816
Heintz AP, Van Oosterom AT et al (1988) The treatment of advanced ovarian carcinoma (I): clinical variables associated with prognosis. Gynecol Oncol 30(3):347–358
Hollstein M, Sidransky D et al (1991) p53 mutations in human cancers. Science 253(5015):49–53
Hollstein M, Rice K et al (1994) Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res 22(17):3551–3555
Holmila R, Husgafvel-Pursiainen K (2006) Analysis of TP53 gene mutations in human lung cancer: comparison of capillary electrophoresis single strand conformation polymorphism assay with denaturing gradient gel electrophoresis and direct sequencing. Cancer Detect Prev 30(1):1–6
Inga A, Iannone R et al (1997) Determining mutational fingerprints at the human p53 locus with a yeast functional assay: a new tool for molecular epidemiology. Oncogene 14(11):1307–1313
Ishioka C, Frebourg T et al (1993) Screening patients for heterozygous p53 mutations using a functional assay in yeast. Nat Genet 5(2):124–129
Jemal A, Siegel R et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58(2):71–96
Kashiwazaki H, Tonoki H et al (1997) High frequency of p53 mutations in human oral epithelial dysplasia and primary squamous cell carcinoma detected by yeast functional assay. Oncogene 15(22):2667–2674
Kupryjanczyk J, Kraszewska E et al (2008) TP53 status and taxane-platinum versus platinum-based therapy in ovarian cancer patients: a non-randomized retrospective study. BMC Cancer 8:27
Kurman RJ, Shih IeM (2008) Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 27(2):151–160
Lavarino C, Pilotti S et al (2000) p53 gene status and response to platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma. J Clin Oncol 18(23):3936–3945
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88(3):323–331
Levine AJ, Momand J et al (1991) The p53 tumour suppressor gene. Nature 351(6326):453–456
Lomax ME, Barnes DM et al (1997) Two functional assays employed to detect an unusual mutation in the oligomerisation domain of p53 in a Li-Fraumeni like family. Oncogene 14(15):1869–1874
Mazars R, Pujol P et al (1991) p53 mutations in ovarian cancer: a late event? Oncogene 6(9):1685–1690
Meinhold-Heerlein I, Ninci E et al (2001) Evaluation of methods to detect p53 mutations in ovarian cancer. Oncology 60(2):176–188
Meinhold-Heerlein I, Bauerschlag D et al (2005) Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene 24(6):1053–1065
Meinhold-Heerlein I, Bauerschlag D et al (2007) An integrated clinical-genomics approach identifies a candidate multi-analyte blood test for serous ovarian carcinoma. Clin Cancer Res 13(2 Pt 1):458–466
Miller SA, Dykes DD et al (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3):1215
Nakayama K, Takebayashi Y et al (2003) Prognostic value of overexpression of p53 in human ovarian carcinoma patients receiving cisplatin. Cancer Lett 192(2):227–235
Neijt JP, Engelholm SA et al (2000) Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol 18(17):3084–3092
Offersen BV, Alsner J et al (2008) A comparison among HER2, TP53, PAI-1, angiogenesis, and proliferation activity as prognostic variables in tumours from 408 patients diagnosed with early breast cancer. Acta Oncol 47(4):618–632
Ozols RF, Bundy BN et al (2003) Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21(17):3194–3200
Pfisterer J, Plante M et al (2006) Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 24(29):4699–4707
Prives C, Hall PA (1999) The p53 pathway. J Pathol 187(1):112–126
Psyrri A, Kountourakis P et al (2007) Analysis of p53 protein expression levels on ovarian cancer tissue microarray using automated quantitative analysis elucidates prognostic patient subsets. Ann Oncol 18(4):709–715
Rentrop M, Knapp B et al (1986) Aminoalkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J 18(5):271–276
Salani R, Kurman RJ et al (2008) Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer 18(3):487–491
Sanger F, Nicklen S et al (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74(12):5463–5467
Schuijer M, Berns EM (2003) TP53 and ovarian cancer. Hum Mutat 21(3):285–291
Shelling AN, Cooke IE et al (1995) The genetic analysis of ovarian cancer. Br J Cancer 72(3):521–527
Shih IeM, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164(5):1511–1518
Singer G, Kurman RJ et al (2002) Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol 160(4):1223–1228
Singer G, Shih IeM et al (2003) Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor). Int J Gynecol Pathol 22(1):37–41
Singer G, Stohr R et al (2005) Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol 29(2):218–224
Skilling JS, Sood A et al (1996) An abundance of p53 null mutations in ovarian carcinoma. Oncogene 13(1):117–123
Smith ML, Chen IT et al (1994) Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 266(5189):1376–1380
Ueno Y, Enomoto T et al (2006) Prognostic significance of p53 mutation in suboptimally resected advanced ovarian carcinoma treated with the combination chemotherapy of paclitaxel and carboplatin. Cancer Lett 241(2):289–300
Vogelstein B, Lane D et al (2000) Surfing the p53 network. Nature 408(6810):307–310
Waridel F, Estreicher A et al (1997) Field cancerisation and polyclonal p53 mutation in the upper aero-digestive tract. Oncogene 14(2):163–169
Wen WH, Reles A et al (1999) p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol 18(1):29–41
Conflict of interest statement
None of the authors has to state a conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bauerschlag, D.O., Schem, C., Weigel, M.T. et al. The role of p53 as a surrogate marker for chemotherapeutical responsiveness in ovarian cancer. J Cancer Res Clin Oncol 136, 79–88 (2010). https://doi.org/10.1007/s00432-009-0639-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0639-8