Abstract
Purpose
Galectin-3 has been implicated in advanced stage of cancer disease. In the current study we examined the possibility of urinary galectin-3 levels to stage cancer disease and to follow up therapy.
Experimental design
Urine was collected from all types of cancer patients at different stages including patients undergoing radio/chemotherapy. Galectin-3 level was determined by anti-galectin-3 based ELISA and agglutination assays. Immunoblotting and purification on lactosyl affinity column further confirmed the presence of galectin-3.
Results
Cancer samples exhibited stage dependent expression of galectin-3 approx. ranging from 1.0 to 3.3, 4.4 to 5.4, 5.4 to 24.7, 13.1 to 31.9, 13.9 to 32.9 ng/mg C (creatinine) for stage I–V, respectively, at P ~ <0.05 level. Galectin-3 levels were decreased by approx. threefolds after 5th day of therapy.
Conclusions
Sample collection being simple and non-invasive, urinary galectin-3 may be used as a potential diagnostic tool for monitoring or follow up of the stage of cancer disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Galectin-3 is a member of a growing family of multifunctional galactoside-binding proteins (Herrmann et al. 1993). Members of this gene family share affinity for β-galactoside-containing glycoconjugates (Barondes et al. 1994). Galectin-3 is approx. 16–36 kDa protein composed of three distinct structural domains: (a) a short NH2 terminus that controls its cellular targeting, (b) a repetitive collagen-like sequence which serves as a substrate for matrix metalloproteinases, and (c) the COOH-terminal domain, a globular structure encompassing the carbohydrate-binding site (Oda et al. 1991; Raz et al. 1989). Galectin-3 is found in the cytoplasm, but depending on cell types and proliferative states, it can also be detected on the cell surface (Davidson et al. 2002), within the nucleus (Van den Brule and Castronovo 2000) and in the extracellular compartment (Takenaka et al. 2002). In some human tumors, a direct relationship was shown between galectin-3 levels and the stage of tumor progression (Nangia-Makker et al. 2000; Bresalier et al. 1997). We have recently reported the expression of higher levels of galectin-3 in metastatic cells as apposed to only insignificant levels in normal cells (Sathisha et al. 2007). Immuno-histological studies conducted in our laboratory demonstrated that only metastatic tissues showed higher levels of galectin-3; while benign tissues express only marginally (unpublished observation). The distribution of galectin was associated with stage III–V with cellular changes such as dysplasia, cancer cells’ nest formation, breakage of basement membrane, and infiltration of cells into a non-native tissue origin etc., characteristic of stage III–V. Studies conducted by Lotz et al. 1993 also reveal that galectin-3 expression is related to neoplastic transformation and progression towards metastasis in colon carcinoma (Schoeppner et al. 1995). In gastric carcinoma, it was found that tissue levels of galectin-3 were higher in certain primary tumors and their metastasis than in the adjacent normal mucosa (Miyazaki et al. 2002). Down-regulation of galectin-3 expression by colon carcinoma cells resulted in a significant decrease in liver colonization ability, whereas up-regulation of galectin-3 increased metastatic potential (Schoeppner et al. 1995). In ovarian carcinoma, however, no correlation was observed (Woynarowska et al. 1994). The introduction of r-galectin-3 into null-expressing non-tumorigenic BT-549 cells resulted in the acquisition of anchorage-independent growth properties and tumorigenicity, suggesting a relationship between galectin-3 expression and malignancy of human carcinoma cell lines (Song et al. 2002). These findings imply an involvement of galectin-3 in malignant progression of carcinomas. The biological functions of galectin-3 remain elusive. Galectin-3 has been found to be associated with the inhibition of apoptosis, proliferation, and the progression of cancer (Nakahara et al. 2005), as well as a mediator of inflammation (Robertson et al. 1990).
There has been a growing interest in proteome of human urine that can be used for diagnosis and clinical monitoring. Recently quantification of formaldehyde in urine was shown to be a promising tool in the investigation of cancer, particularly bladder cancer (Siemiatycki et al. 1994). Prostate cancer foundations detected 95% of cancers and reduced unnecessary biopsies by 20% by testing for prostrate specific antigen in urine samples. Dr. Claims detected cancer based on DNA methylation of selected genes in urine (Cottrell 2004). Urinary proteome has infinite potential for diagnosis and disease monitoring because of its non-invasive, simple, and stress-free sampling from patients; while tumor and tissue biopsies are not obtained as easily. Some peptides originated from oncogene family proteins, have been documented as potential biomarker candidates related to certain disease states (Hong and Kwon 2005). To date phosphoglucoisomerase enzyme (Baumann et al. 1990), 8 hydroxy guanosine, a DNA degraded product (Kanabrocki et al. 2006), and other carcino embryonic antigen levels in the urine have been shown to correlate with the cancer progression. However, they lack either specificity or quantitation.
In the current study therefore we analyzed the urine of patients with cancers for galectin-3 that may be of value for monitoring the disease status time to time and to monitor the efficacy of the therapy. Since one can anticipate lots of changes in the metabolism of cancer due to a fight between normal cell and a cancer cell and also due to logistic suppression of normal cells by cancer cells, the above study was undertaken to determine galectin-3 in urine and its reliability to consider it as a common metastatic marker. Serum being difficult to obtain from cancer patients, a detailed study in urine samples would throw some light on the potential use of non-invasive tool for easy, reliable and inexpensive determination of galectin-3.
Materials and methods
Collection of urine from cancer patients and normal controls
Urine samples were collected from patients who were being monitored and tested for cancer in the oncology day—hospital—Bharath Hospital and Institute of Oncology at Mysore, India. A total of 259 patients with histologically proven diagnosis of cancer and some being treated with chemo/radiotherapy were included in this study (Table 1); 61 cervical cancer; 52 oesophageal carcinoma; 44 breast carcinoma; 16 ovary; 12 neck node and 63 miscellaneous including tongue; larynx; non Hodgkin’s lymphoma; lymphnode; liver; vaginal; pharyngeal; squamous cell carcinoma; peripheral lymphoma; buccal mucosal; postcricoid; leukemia; colon scalp; pleural; nasal; vertebrae; penis; osteogenic; adenocarcinoma1 and multiple myeloma with different stages of cancers. In cancer samples—both male and female patients with age group—range 10–80 years were included. Approximately about 13, 30, and 56% of cancer patients were belonging to the age group ranges—10–25, 25–45, and >45–80%, respectively.
Approximately 60% of the patients were with epithelial cancer having clinical evidence of metastases. Urine samples were also collected from patients undergoing chemotherapy as indicated above, 1–5 days of post-chemotherapy. All urine samples were centrifuged at 5,000g for 10 min at 4°C followed by storage of the supernatant at −20°C adding traces of toluene to avoid microbial contamination until further use. Samples were further filtered through Amicon ultra centrifugal device (Millipore) with 5,000 Da cut off to remove interfering substances and the concentrated (tenfold), filtered samples were screened for galectin-3. Samples were also obtained from 12 urine donors (normal) with a median age of 30 years (range 25–45, one of 12 patients (8%) was 80 years-old) to obtain normal values. Attention was paid in the control groups to select volunteers from group, who neither attacked by the diseases nor taking drugs. Total protein content was determined by Folin-phenol method (Lowry et al. 1951), UV spectra between 400 and 200 nm was recorded in a UV spectrophotometer (Smith et al. 1976). Urea and creatinine levels were estimated using a Monozyme (Monozyme India Ltd., Secundarabad, India) kit method.
Measurement of galectin-3 by ELISA
Urine galectin-3 concentrations were assayed with a previously standardized ELISA procedure (Rajeshwari et al. 1998). The concentrated urinary proteins were immobilized onto the Immuno ELISA plates (96 well micro titer plates, Nunc—immunoplate maxisorp) by binding buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and incubated at 4°C overnight. Next day ELISA blocking buffer (20 mM phosphate buffered saline, PBS, pH 7.4 with 5% skimmed milk powder) was added to mask the non specific binding sites of the antibody, incubated for 1 h at 37°C at room temperature, followed by the addition of anti-human galectin-3 monoclonal antibody (Becton Dickinson Co., USA) and rabbit anti-mouse IgG-alkaline phosphatase conjugate (GENEI, Bangalore, India) at 1:1,000 and 1:10,000 (v/v) dilution, respectively. After incubation for 1 h at room temperature, 100 μl of paranitrophenyl phosphate-PNPP (1 mg ml−1) was added as substrate. Plates were washed four times after each step using wash buffer (PBS containing 0.5% Tween-20). Absorbance was read at 410 nm in a microplate ELISA reader (Molecular Devices, Spectramax 340, Germany). A calibration curve was generated using 20–100 ng ml−1 of galectin-3 standard (Sigma chemical co., USA, 99% pure). Values were equalized for creatinine since there were no significant differences found between healthy controls and those of cancer patients, except for some samples under extreme conditions. ELISA was also performed for urine samples from patients before and after chemotherapy.
Hemagglutination assay
Microplate agglutination assay was performed for the evaluation of galectin content as per the protocol of (Nowak et al. 1976). The results were compared with ELISA. This attempt was made particularly for its easiness to employ in even common laboratories in developing countries. Briefly, human erythrocytes were prepared from 10 mL of fresh blood (collected in Alsever’s medium), washed four times with five volumes of 0.15 M NaCl. A 4% erythrocyte suspension in 0.02 M PBS, pH 7.4 containing 1 mg mL−1 trypsin was incubated for 1 h at 37°C. The trypsin treated cells were washed with five volumes of 0.15 M NaCl and fixed in five volumes of 0.02 M PBS pH 7.4 containing 1% glutaraldehyde for 1 h at room temperature. Glutaraldehyde fixation was terminated by the addition of five volumes of 0.1 M glycine in PBS, pH 7.4 at 4°C and the fixed erythrocytes were employed for the hemagglutination assay. To the RBC thus isolated, urine samples from the various cancer patients were added at concentration of 200 μg equivalent of protein. The sample containing higher galectin level agglutinates more compared to less galectin concentration. The lower agglutination level was indicated as 1+ and the highest as 5+ units.
Electrophoresis and Western blotting
Randomly selected concentrated urine samples were resolved by 12% SDS-PAGE. After, electrophoresis, the proteins were transferred from gel to PVDF membrane (Nangia-Makker et al. 2002), membrane was blocked with 1% BSA with TBS (0.5 M Tris base, 9% NaCl, pH 7.6), followed by washing with wash buffer TBS with 0.05% Tween 20. Then added primary antibody—anti-human galectin-3 at 1:500 (v/v) dilutions and incubated for 1 h. Membrane was washed with wash buffer (3×), prior to the addition of secondary antibody—goat anti-mouse IgG alkaline phosphatase conjugate at 1:1,000 dilutions (v/v) followed by BCIP—(5-bromo-4-chloro-3-indolyl phosphate) substrate. Upon reaction of substrate with the bound antibody showed the presence of galectin-3 bands (~36 kDa) in the urinary proteins.
Purification of galectin-3 from urine samples
One of the concentrated urine samples containing higher levels of galectin-3 in ELISA was allowed to run on lactosyl–sepharose 4B column (1 × 10 mm) and the bound proteins were eluted with 0.2 ml of 0.1 M lactose in [20 mM phosphate buffered saline (PBS), pH 7.4] (Iurisci et al. 2000). Aliquots of the eluent were resolved by 12% SDS-PAGE and immunoblot was performed as described above. The membrane was observed for galectin-3 band in urine samples from various cancer patients.
Statistical analyses
Because galectin-3 values were not normally distributed, the 5th and 9th percentile values were chosen for data description. Differences between patient groups were tested with the non-parametric Mann–Whitney U test. The correlation between the agglutination activity, ELISA and stage of cancer were performed. The normal and the cancer patients’ galectin-3 levels were compared by one-way ANOVA at P value of 0.05 level of significance using Smith statistical package. Linearity of standard was established using origin 6.1 of SPSS-10 statistical programme. Regression analysis was performed for each types of cancers between the stage I–V with galectin-3 levels to understand the significance of galectin-3 during advancement of the disease.
Results
Galectin-3 measurement by ELISA in normal and cancer subjects
The galectin-3 used to prepare the standard curve of galectin-3 ELISA was >99% pure as per sigma standard-galectin-3 and judged by SDS-PAGE. The dose-response curve obtained with standard galectin was linear in the range of 20–100 ng with the absorbance of 0.1–0.5 with approx. fivefold increase in the signal over the blank for the final concentration, indicating the sensitivity of the assay (Fig. 1). When plates were probed with preimmune mouse serum, no signal above background level was observed suggesting the specific reactivity by the antibody.
Calibration curve of standard galectin-3. Standard galectin-3 obtained from Sigma chemical co., USA was used as standard at the range of 0.02–0.1 μg/100 μL and detected employing ELISA using monoclonal anti-galectin-3 antibody as described under materials and methods. >5-fold difference between the noise to signal ratio was observed. Values are the mean value taken as average of triplicates with three individual measurements, hence expressing as mean data with standard deviation. Data validates the standard curve which showed a linearity with a correlation coefficient—r of ~1.0, when analyzed using origin 6.1 version of SPSS-10 programme
Urine galectin-3 concentrations of healthy individuals and cancer patients are depicted in Table 1. The levels of galectin-3 in urine of 12 healthy controls varied between 1.0 and 1.2 ng/mg C (median 1.13 ng/mg C, 95th percentile 1.21 ng/mg C; Table 1). The 95th percentile of galectin-3 levels (1.20 ng/mg C) was arbitrarily taken as the upper limit of normal. There was no correlation between urinary galectin-3 concentration and age groups studied between 25 and 45. one sample out of 12 (8%) was from age approx. 80 years with the normal health condition and showed the value (1.09 ng/mg C) within the normal range indicating that galectin-3 has no significance in normal health condition irrespective of the age groups. Among all types of cancer patients, several cases showed an increase in galectin-3 levels in the urine and this increase was significantly higher (P < 0.003 to < 0.0001) in metastatic patients than with those of healthy individuals. In 109 patients with localized tumors (stage I and II), the median galectin-3 concentration in urine was 2.4 ng/mg C (ranging from 1.05 to 3.36 ng/mg C) and 4.9 ng/mg C (ranging from 4.4 to 5.4 ng/mg C), respectively. A total of 54 out of 63 (86%) of stage I and all the 46 (100%) of stage II patients showed galectin-3 levels above upper limit of normal controls. Approximately 2.7- and 4.2-fold increase in galectin-3 levels were observed in upper limit samples of stage I and stage II patients, respectively. In stage III 95th percentile value showed approx. 10–25 ng/mg C with 10- to 20-fold higher than normal and approx. twofold to fourfold higher than stage II patients. One breast cancer stage III patient out of 9 (10%) and 6 of 57 (10%) of stage III patients showed galectin-3 levels similar to upper limit value of stage II (4.5 ng/mg C). However, studies on tissue pathology sections revealed that they were actually of stage III. This reduction in galectin-3 levels may be associated with the degradation of galectin-3 that is not detectable by the antibody. However, 36 of 57 (63%) and 15 out of 57 (27%) stage III patients showed six- to tenfold and 12- to 24-fold higher galectin-3 levels than healthy controls. All oesophagus-14 of 14 (100%) showed 15- to 20-fold higher value with P ~ 0.034. In stage III, migration of cancer cells is within the tissue but in secondary, tertiary locations. In stage IV patients six out of ten (~60%) of cervical, 8 out of 14 (~57%) of other types of cancer patients showed a median galectin-3 levels as approx. 13 ng/mg C which is approx. 13-fold higher than normal controls. Remaining 4 of 10 (40%) of cervical cancer patients and 6 of 14 (43%) of other types of cancer patients showed a median levels of approx. 25 ng/mg C, which is approx. 25-fold higher than healthy controls at P ~ <0.001. 100% (12 of 12) of breast, oesophagus (13 of 14) and 90% of other types of stage V cancer patients revealed a median galectin level of approx. 27 ng/mg C, while only 1—ascending colon cancer patient, out of 15 (6%) of stage V cancer patients showed value matching with stage II (5.5 ng/mg C). Indeed this individual was in the last stage of his life, almost bed ridden and semiconscious. Renal failure was also reported. Hence the observed lower galectin-3 value could be due to higher levels of creatinine in the urine (~3 mg/mL) or due to leaky excretion of several proteins that reduce the specific levels of galectin-3. Overall 76% of cancer patients with metastatic stage III and IV showed sixfold higher galectin-3 levels than stage I and stage II indicating that majority of the tissues at stage III express galectin-3. Although similar fold increase was found in stage III patients, P value is more significant (P < 0.001) in stage IV than that of stage III patients P ~ 0.034). As high as approx. 34-fold increase in galectin-3 levels was observed in stage V cancer patients. Thus a significant difference in urine galectin-3 was seen between carcinoma patients with non metastatic and those with metastatic condition (P < 0.032).
Notably, maximum urine concentrations of galectin-3 occurred in patients with metastatic oesophageal carcinoma (median 32 ng/mg C, range 20–37 ng/mg C; Table 1). Also, galectin-3 urine levels did not differ among the various oesophageal cancer patients of similar neoplastic conditions.
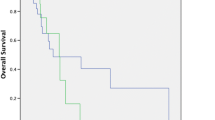
Further, in five each of cervical, oesophagus, and breast carcinoma patients, urinary galectin-3 levels were measured before and 5 days after chemotherapy (Fig. 2). In four of each cases (80%), urine galectin-3 level decreased drastically from 57 to 18.5 ng/mg C within 3–5 days after surgery. The fifth patient might possibly require more number of days to lower the level of galectin-3, indicating the minor variation in responses of patients to treatments. In one localized breast tumor patient there was only a normal galectin-3 levels before and after surgery, while in advanced breast cancer patient, approx. threefold decline in galectin-3 level was observed after surgery. Data thus indicate that galectin-3 level corroborates with advanced cancer pathogenicity. Overall regression analysis (Fig. 3) between stage I and stage V with galectin-3 levels indicated R value of 0.93–1.0 with P < 0.01 for all types of cancer patients except for breast cancer samples which showed a P value of approx. 0.5.
Urine galectin-3 concentrations in carcinomas before and after treatment with chemotherapy. Urine galectin-3 concentrations were assayed by ELISA in random cancer patients in oesophagus, cervical and non-Hodgkin’s lymphoma cancer patients before and after 2–5 days of chemotherapy. Decrease in galectin-3 levels noted were plotted. Reduction in galectin-3 levels after therapy suggests that galectin-3 can be a potent urinary marker for staging, diagnosing and follow up of the disease. All the data are mean ± SD of three replicates
Correlation between the stage of the disease and galectin-3 levels. Regression analysis was performed individually on each type of cancers between galectin-3 levels as determined by ELISA with the stage of the disease and regression coefficient-R and the level of significance was obtained employing SPSS programme of Origin 6.1 statistical programme. Gradational increase in galectin-3 levels was observed with the advancement of the stage of the disease irrespective of type of cancer. R value calculated separately for different types of samples in the similar programme is as follows: cervical—R −0.93, P ~ 0.019; oesophagus—R −0.957, P ~ 0.042; breast—R −0.925, P ~ 0.247; ovary—R −0.934, P ~ 0.012 and other types of cancers—R −0.952, P ~ 0.01. All the data are mean ± SD of three replicates
Hemagglutination assay
The presence of galectin-3 in urine samples was also confirmed by agglutination and inhibition of agglutination assay by galactose. The agglutination level was very high in advanced stage cancer patients, while only traces of agglutination was observed in patients with first stage, non-metastatic cancer patients (Table 2). Increase in agglutination intensity reveals increased galectin-3 and is indicated in grades as 1 to 5+. A good correlation was found between agglutination activity and ELISA units, with a correlation coefficient—r approx. 1.0 varying from 0.8 with a range of P < 0.00005 in cervical and oesophagus cancers; while r value of 1.0 with P < 0.08 in ovary and <0.03 and 0.003 for breast samples, respectively. In different types of cancer samples, r value ~0.6 with P < 0.0005 was observed.
Western blotting
In order to confirm the presence of galectin-3 in the urine sample, random samples were examined for protein profile (Fig. 4) and western blot (Fig. 5) analysis. There were some proteins excreted in various cancer patients. Results were substantiated by western blot analysis. Similar approx. 36-kDa band was observed in all tested samples irrespective of the type of cancers, slight shifts were observed; however, it is not clear whether, there are some minor changes in the protein sequences. Attempt will be made to isolate and purify them to determine their sequence for further understanding.
Protein profile in urine samples of cancer and normal subjects. 12% SDS-PAGE was performed loading cancer patient’s urine samples. Lane 1 C1 cervical-stage IV, Lane 2 C2 oesophagus-stage V, Lane 3 C3 stomach-stage V, Lane 4 C4 cervical-stage 111, Lane 5 C5 Breast-stage V and those of normal urine sample in Lane 6 (N). After electrophoresis, gels were stained using silver nitrate staining reagent for proteins. Lane 7 (M) shows the profile of molecular weight markers. Negligible proteins were found in normal urine samples; while differential protein profiles were observed in the urine of advanced cancer patients
Western blot analysis of galectin-3 in urine samples of cancer patients. 12% SDS-PAGE was performed loading cancer patient’s urine samples. Lane 1 C1 cervical-stage IV, Lane 2 C2 oesophagus-stage V, Lane 3 C3 stomach-stage V, Lane 4 C4 cervical-stage 111, Lane 5 C5 breast-stage V and those of normal urine sample in Lane 6 (N). After electrophoresis, proteins were transferred to a nitrocellulose membrane. Blots were probed with anti-human galectin-3 antibody followed by alkaline phosphatase conjugated goat anti-mouse IgG. BCIP (5-bromo-4-chloro-3-indolyl phosphate) was used to detect alkaline phosphatase in western blot analysis. Bands were visualized by enzyme substrate reaction as described under materials and methods and the molecular size was determined comparing to molecular markers which were stained by silver nitrate staining. Gel documentation was performed to understand differences in profile, in cancer samples
These data hence, suggest that galectin-3 can be an universal metastatic marker which has advantages over other markers and assays in serum since sample collection is by non-invasive method and also negatives, as encountered in other immunological detection methods due to the narrow specificity of immunological reagents for only certain cell/tissue specific markers are minimized. Results were further substantiated by its specific binding to galectin-specific lactosyl sepharose affinity column followed by elution with lactose.
Discussion
In this study, we have determined urine galectin-3 concentrations in patients having various types of cancers and compared with the data of that in healthy individuals. Urine galectin-3 levels were significantly higher in sub-populations of patients having each type of tumor. In approx. 90% of cancer patients irrespective of the type of cancer, the incidence of supernormal levels of galectin-3 was elevated in relation to tumor progression. Galectin-3 levels were significantly higher in the urine samples of patients with metastatic condition than in patients’ with localized tumors. This tendency of increase in urine galectin-3 levels, and its association with the occurrence of metastasis was also observed by other investigators where galectin-3 levels were measured in the serum of cancer patients (Iurisci et al. 2000).
Breast cancer samples showed moderately significant value (P = 0.0038) in their first stage. But as the tumor progresses, the galectin-3 level was increased accordingly. Enhancement of the metastatic potential of human breast carcinoma-BT549 cells by galectin-3 provide additional evidence to our observation.
The indication by the studies of Califice et al. 2004 that dissociated cytoplasmic galectin-3 expression increases in later phases of tumor progression reveal more and more reduction in cytoplasm due to increased nuclear activity which is predominantly found in tumor progression. Increasing resistance often found during such tumor progression state and this could be due to the reactive nitrogen and oxygen species such as NO and ONOO, most likely through the bcl-2—like anti-apoptotic function of galectin-3 (Yang et al. 1996).
The biological role of galectin-3 remains elusive; galectin-3 is found at elevated levels in a variety of neoplastic cells, and several experimental observations suggest that it is involved in tumor metastasis in vivo (Shimonishi et al. 2001; Raz et al. 1989). In majority of the cases galectin-3 of cancer cells appear to initiate the cascade of metastatic events by interacting with extracellular matrix molecules of normal cells, subsequently activating basement membrane hydrolyzing enzymes—matrix metalloproteinases that in turn may be responsible for the dissemination of tumor cells into the circulation. The role of galectin-3 in enhancing proliferation and inhibition of apoptosis is also evident and may add to its cause for aggressive metastatic events.
The results of the present study could imply that the metastatic spread of malignant tumors involve, among other factors, higher levels of expression of galectin-3 in the circulation in the early stages itself leading to its excretion into the urine, which can be used as an efficient urinary marker. As proposed by Takenaka et al. (2004) the changes in the level of expression of galectin-3 may favor metastasis by either one or all of the above-mentioned modalities such as (a) enhancing the adhesive interactions between tumor cells and the extracellular matrix, (b) promoting tumor cell embolization through increased cell–cell adhesion, and (c) conferring a selective survival advantage to metastatic cells.
No significant changes in galectin-3 levels in urine of cancer patients till day 2–3 after therapy may indicate the removal of galectin-3 levels in the urine by collapsed cancer cells. Lowered levels after day 3–5 may indicate the successful elimination of galectin-3 expressing cancer cells in the body. The levels of galectin-3 in urine of healthy controls showed almost nil or trace amounts; there was a significant difference between the localized (stage I) tumor and the metastatic (stage III and V) cancer patients at P value 0.005 level (Tables 1, 2); hence galectin-3 can be a potential biomarker to monitor metastatic stage of cancer.
In conclusion, the data generated indicate the involvement of galectin-3 in tumor invasion and spread of varieties of cancers suggesting the universal role of galectin-3 in metastasis. Indeed this may explain, how tumor cells can get into metastasis with the similar pathogenicity irrespective of the tissue affected by cancer. Increased galectin-3 levels in the urine of cancer patients reflect biological aspects of tumor cell behavior and its implications in its association with a metastazing phenotype. The current data together with our previous observations (Sathisha et al. 2007) on the antimetastatic ability of galectin-3 inhibitors from the selected glycoconjugates not only imply the critical role of galectin-3 in tumor invasion and metastasis; but provides a non-invasive sample collection that may favor cancer victims, in terms of monitoring of the disease; if need be a targeting molecule for prediction and prevention of tumor metastasis.
References
Barondes S, Catronava V, Cooper D, Cummings R, Drickamer K, Feizi T et al (1994) Galectins: a family of animal β-galactoside binding lectins. Cell 76:597–598. doi:10.1016/0092-8674(94)90498-7
Baumann M, Kappl A, Lang T, Brand K, Siegfried W, Paterok E (1990) The diagnostic validity of the serum tumor marker phosphohexose isomerase (PHI) in patients with gastrointestinal, kidney and breast cancer. Cancer Invest 8:51–56. doi:10.3109/07357909009012053
Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A (1997) Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer Res 80:776–787
Califice S, Castronovo V, Bracke M, Van den Brule F (2004) Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene 23:7527–7536. doi:10.1038/sj.onc.1207997
Cottrell SE (2004) Molecular diagnostic applications of DNA methylation technology. Clin Biochem 37:595–604. doi:10.1016/j.clinbiochem.2004.05.010
Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL (2002) Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology 12:329–337. doi:10.1093/glycob/12.5.329
Herrmann J, Truck CW, Atchison RE, Huflej ME, Poulter L, Gitt MA et al (1993) Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline, glycine, tyrosine rich sequence with bacterial and tissue collagenase. J Biol Chem 208:26704–26711
Hong SS, Kwon SW (2005) Profiling of urinary proteins by nano-high performance liquid chromatography/tandem mass spectrometry. J Liquid Chromatogr Relat Technol 28:805–822. doi:10.1081/JLC-200051465
Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res 6:1389–1393
Kanabrocki EL, Ryan MD, Murray D, Jacobs RW, Wang J, Hurder A et al (2006) Circadian variation in multiple sclerosis of oxidative stress marker of DNA damage. A potential cancer marker? Clin Ter 157:117–122
Lotz MM, Andrews CW, Korzelins CA, Lee EC, Steele GD, Jrclarke A et al (1993) Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci USA 90:3466–3470. doi:10.1073/pnas.90.8.3466
Lowry OH, Rosebrough NJ, Farr AL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Miyazaki J, Hokari R, Rato S, Tsuzuki Y, Kawaguchi A, Nagao S et al (2002) Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep 9:1307–1312
Nakahara S, Oka N, Raz A (2005) On the role of galectin-3 in cancer apoptosis. Apoptosis 10:267–275. doi:10.1007/s10495-005-0801-y
Nangia-Makker P, Akahani S, Bresalier R, Raz A (2000) The role of galectin-3 in tumor metastasis. In: Lectins and pathology. Taylor and Francis Incorporation, London
Nangia-Makker P, Hogan V, Honjo Y, Yuichiro H, Baccarini S, Tait L et al (2002) Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst 94:1854–1862
Nowak TP, Haywood PL, Barondes SH (1976) Developmentally regulated lectin in embryonic chick muscle and a myogenic cell line. Biochem Biophys Res Commun 68:650–657. doi:10.1016/0006-291X(76)91195-5
Oda Y, Leffler H, Sakakura Y, Kasai KI, Barondes SH (1991) Human breast carcinoma cDNA encoding a galactoside binding lectin homologous to mouse Mac-2 antigen. Gene 99:279–283. doi:10.1016/0378-1119(91)90139-3
Rajeshwari N, Shylaja MD, Krishnappa M, Shetty HS, Mortensen CN, Mathur SB (1998) Development of ELISA for the detection of Ralstonia solanacearum in tomato: its application in seed health testing. World J Microbiol Biotechnol 14:697–704. doi:10.1023/A:1008892400077
Raz A, Pazerini G, Carmi P (1989) Identification of the metastasis associated galactoside binding lectin as a chimeric gene product with homology to an IgE binding protein. Cancer Res 49:3489–3493
Robertson MW, Albrand K, Keller D, Liu FT (1990) Human IgE binding sequence and differential recognition of IgE glycoforms. Biochemistry 29:8093–9000. doi:10.1021/bi00487a015
Sathisha UV, Smitha J, Harish Nayaka MA, Dharmesh SM (2007) Inhibition of Galectin-3 mediated cellular interactions by pectic polysaccharides from dietary sources. Glycoconj J 24:497–507. doi:10.1007/s10719-007-9042-3
Schoeppner HL, Raz A, Ho SB, Bresalier RS (1995) Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer 75:2818–2826. doi:10.1002/1097-0142(19950615)75:12<2818::AID-CNCR2820751206>3.0.CO;2-#
Shimonishi T, Miyazaki K, Kono N, Sabit H, Tuneyama K, Harada K et al (2001) Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol 32:302–310. doi:10.1053/hupa.2001.22767
Siemiatycki J, Dewar R, Nadon L, Gérin M (1994) Occupational risk factors for bladder cancer: results from a case-control study in Montreal, QC, Canada. Am J Epidemiol 140:1061–1080
Smith RB, Smith RV, Yakatan GJ (1976) Spectrofluorometric determination of hydroflumethiazide in plasma and urine. J Pharm Sci 65:1208–1211. doi:10.1002/jps.2600650819
Song YK, Billiar TR, Lee YJ (2002) Am J Pathol 160:1069–1075
Takenaka Y, Fukumori T, Raz A (2002) Galectin-3 and metastasis: Special issue on Galectin. Glycoconj J 19:543–549. doi:10.1023/B:GLYC.0000014084.01324.15
Takenaka Y, Fukumori T, Raz A (2004) Galectin-3 and metastasis. Glycoconj J 19:543–549. doi:10.1023/B:GLYC.0000014084.01324.15
Van den Brule FA, Castronovo V (2000) Laminin binding lectins during invasion and metastasis. In: Lectins and pathology. Taylor and Francis Inc., London
Woynarowska B, Skrincosky DM, Haag A, Sharma M, Matta K, Bernacki RJ (1994) Inhibition of lectin-mediated ovarian tumor cell adhesion by sugar analogs. J Biol Chem 269:22797–22803
Yang RY, Hsu DK, Liu FT (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA 93:6737–6742. doi:10.1073/pnas.93.13.6737
Acknowledgments
Authors thank Dr. V. Prakash, Director, CFTRI, for his keen interest in the work and encouragement. Authors are also thankful to Dr. S. G. Bhat, former Head, Dr. P. V. Salimath, current Head, Department of Biochemistry and Nutrition. SMD thank Department of Biotechnology, New Delhi, India and Department of Science and Technology, New Delhi, India for financial assistance. SMD and SUV acknowledges the financial assistance from Indian Council of Medical Research as Senior Research Fellowship to SUV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balasubramanian, K., Vasudevamurthy, R., Venkateshaiah, S.U. et al. Galectin-3 in urine of cancer patients: stage and tissue specificity. J Cancer Res Clin Oncol 135, 355–363 (2009). https://doi.org/10.1007/s00432-008-0481-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0481-4