Abstract
Background
Colorectal cancer (CRC) is the third most common cancer in the USA. While various lifestyle factors have been shown to alter the risk for colorectal cancer, recommendations for the early detection of CRC are based only on age and family history.
Methods
This case-only study examined the age at diagnosis of colorectal cancer in subjects exposed to tobacco smoke. Subjects included all patients who attended RPCI between 1957 and 1997, diagnosed with colorectal cancer, and completed an epidemiologic questionnaire. Adjusted linear regression models were calculated for the various smoking exposures.
Results
Of the 3,540 cases of colorectal cancer, current smokers demonstrated the youngest age of CRC onset (never: 64.2 vs. current: 57.4, P < 0.001) compared to never smokers, followed by recent former smokers. Among never smokers, individuals with past second-hand smoke exposure were diagnosed at a significantly younger age compared to the unexposed.
Conclusion
This study found that individuals with heavy, long-term tobacco smoke exposure were significantly younger at the time of CRC diagnosis compared to lifelong never smokers. The implication of this finding is that screening for colorectal cancer, which is recommended to begin at age 50 years for persons at average risk should be initiated 5–10 years earlier for persons with a significant lifetime history of exposure to tobacco smoke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most commonly incident cancer among both men and women. It is estimated that there will be almost 145,300 new cases of colorectal cancer in the USA in 2006 (ACS 2006). Genetic syndromes such as familial adenoma polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC) account for approximately 10% of total colorectal cancer cases, while greater than 75% of cases may arise from environmental/lifestyle risk factors and sporadic mutations (Fuchs et al. 1994; Strate and Syngal 2005). Those environmental and lifestyle risk factors may include, but are not limited to, a diet low in vegetables, a diet high in red meat, lack of physical activity, high body mass index (BMI), tobacco exposure, lack of NSAID use and alcohol use.
Although smoking is a significant risk factor for a number of cancers, studies published between 1950 and 1980 failed to find an association between smoking and colorectal cancer (Doll et al. 1980; Doll and Peto 1976; Hammond 1966; Hammond and Horn 1958; Kahn 1966; Weir and Dunn Jr 1970). Subsequent studies then linked smoking to colorectal adenomas, a well-established colorectal cancer precursor lesion (Almendingen et al. 2000; Boutron et al. 1995; Demers et al. 1988; Erhardt et al. 2002; Giovannucci et al. 1994a, 1994b; Hoff et al. 1987; Ji et al. 2006; Lee et al. 1993; Longnecker et al. 1996; Martinez et al. 1995; Monnet et al. 1991; Nagata et al. 1999; Potter et al. 1999; Terry and Neugut 1998), and a 2001 review paper found that 15 of 16 studies published after 1970 for men or after 1990 for women reported an association between smoking and colorectal cancer (Giovannucci 2001). Assuming an approximate 30-year induction period (Giovannucci and Martinez 1996) and a sharp increase in male smoking rates in the 1930s, an increase in the cases of colorectal cancer should have been observed. Ecologic data support this suggestion: colorectal cancer rates increased from the mid-1970s, peaked in 1985 and have since declined by 20% (Jemal et al. 2004; Tomeo et al. 1999). Thirty years previously, in 1955, male smoking rates began to decline (CDC 2005).
Recent studies have linked second-hand smoke (SHS) exposure to colorectal cancer; increases in risk were observed among never smokers who were exposed to high amounts of SHS (Lilla et al. 2006; Nishino et al. 2001; Slattery et al. 2003). Despite recent evidence of an association between smoking and colorectal cancer, as well as knowledge about other etiologic factors, only age and family history factor are taken into consideration for recommendations on screening for CRC (Smith et al. 2003; Winawer et al. 2003). The Surgeon General had not considered smoking in relation to colorectal cancer until 2001 and subsequently concluded in 2004 that, “the evidence is suggestive but not sufficient to infer a causal relationship between smoking and colorectal adenomatous polyps and colorectal cancer.”(US Office of the Assistant Secretary for Health 2004; US Public Health Service.2001)
Only a few studies have examined the issue of smoking, colorectal cancer, and age at diagnosis (Buc et al. 2006; Michalek and Cummings 1987; Zisman et al. 2006). All three of the studies reported a significantly younger age at colorectal cancer diagnosis for those exposed to tobacco smoke compared to those not exposed. For smokers, our group was the first to report an earlier age at diagnosis of cancer at a variety of cancer sites, including colorectal cancer (Michalek and Cummings 1987). These studies have hypothesized that cigarette smoking reduces the body’s resistance to malignancies, which could hasten tumor growth and result in a younger age at diagnosis (Michalek and Cummings 1987; Zisman et al. 2006).
Although only a few studies have examined this issue, there is biological plausibility for a younger age at diagnosis among smokers. It has been hypothesized that carcinogens from cigarette smoke can reach the bowel via circulation following transoral uptake (Yamasaki and Ames 1977), by direct inhalation of cigarette smoke (Kune et al. 1992), or through direct exposure to tobacco carcinogens that were brought up from the lungs by the ciliated epithelial cells, swallowed and passed through the intestines. Various cellular mechanisms have been proposed for the carcinogenic action of these substances on the colonic epithelium.
The goal of the present study was to examine the age at colorectal cancer diagnosis based on smoking exposure. This was investigated by examining the age at diagnosis and smoking status of all patients, diagnosed with colorectal cancer, who were treated at Roswell Park Cancer Institute between 1957 and 1997.
Methods
Data sources
This study used data gathered from over 40 years of patient admission to Roswell Park Cancer Institute (Buffalo, New York). Patients who were diagnosed between 1957 and 1997 with a primary, histologically confirmed incident case of colorectal cancer were eligible for inclusion into this case-only study (n = 3,540). Eligible patients completed an epidemiologic questionnaire, which involved three different versions over the years. The first questionnaire, administered during Period #1 (1957–1965), inquired about lifestyle, tobacco, alcohol and dietary patterns. The questionnaire used during Period #2 (1965–1975) was a more limited version of the Period #1 questionnaire and focused mainly on tobacco and alcohol use. The Period #3 (1982–1997) questionnaire included items on lifestyle, tobacco, alcohol and dietary patterns, along with family history of colorectal cancer and second-hand smoke (SHS) exposure (Table 1).
Smoking exposure measures
Each patient was classified according to his/her self-reported tobacco exposures. Measures included current smoking status (current, former or never), amount smoked, age at the initiation of smoking, and number of years since quitting smoking. We examined active smoking in combination with second-hand smoke (SHS) exposures in relation to age at colorectal cancer diagnosis using the Period #3 questionnaire data, which was the only one of the three surveys to collect SHS information. SHS exposure was assessed by asking four questions to the participants. The first three items captured current SHS exposure, asking how many hours each day a person was exposed to the smoke of others in their (1) home, (2) at work and (3) in other locations. The final item assessed past SHS exposure by asking if a participant’s parents smoked in the home while he/she was living with the parents (Table 2).
Statistical analysis
One-way ANOVA models were initially run to assess the crude difference in age at diagnosis with the various smoking exposures. Next, multivariate linear regression models were constructed with age at diagnosis as the outcome variable. Each tobacco exposure variable was entered into a separate model. In addition to the smoking variables in each model, gender, year of diagnosis and alcohol use (ever/never) were included as covariates. Other variables such as body mass index (BMI), vegetable consumption, meat consumption, family history and race could not be used in the models as control variables due to the large percentage of questionnaires missing this information. Sensitivity analyses were performed where crude models were restricted to those not missing covariate information. Covariates were then added one at a time, in a stepwise, forward manner to observe the effect of each covariate on the outcome. The analyses examined the stage of diagnosis (local involvement, regional involvement, regional nodes and direct extension, and distant) based on smoking status, and statistical models were also stratified by the stage of diagnosis. All analyses were stratified by gender and anatomic location (colon versus rectum).
The same analyses were completed for SHS exposure, and additional covariates included BMI, race, and vegetable and meat consumption for those analyses. Taking into account the temporal trends in smoking, the analyses were also stratified by 10-year birth intervals, and the difference in age at diagnosis was calculated for each interval. These stratified analyses were run to ensure that the overall results were not due to a birth cohort effect.
Results
Table 3 displays the demographic and lifestyle characteristics of the colorectal cancer patients who were admitted to Roswell Park Cancer Institute between 1957 and 1997, stratified by data series and overall. The results show a significant shift towards cases of colon cancer versus rectal cancer over time. The percentage of patients who reported ever using alcohol increased significantly from Period #1 to Period #3. Smoking habits also changed between 1957 and 1997, with an increase in the percentage of ever smokers and a significant decrease in the percentage of current smokers over this interval. Also, the amount smoked significantly increased over time, while the average age at initiation significantly decreased.
Tobacco smoke exposure and age at diagnosis of colorectal cancer
Table 4 displays the crude and adjusted difference in age at diagnosis based on smoking status, amount smoked and age at smoking initiation. For the smoking status, the largest adjusted difference in age at diagnosis was among current smokers (−6.8 years, P < 0.001), followed by former smokers who quit less than 5 years ago (−4.3 years, P < 0.01); no significant difference was observed for former smokers who quit more than 5 years prior to diagnosis (+1.0, P = 0.21). The results show no significant differences when stratified by gender. The results show that as the amount smoked daily increased, the adjusted age at CRC diagnosis decreased. Individuals who smoked less than a pack/day were diagnosed with colorectal cancer almost 3 years (P < 0.01) before never smokers; those who smoked 1 pack/day were diagnosed 3.6 years (P < 0.01) earlier than never smokers, and those who smoked more than 1 pack/day were diagnosed almost 5 years (P < 0.01) before their never smoking counterparts. There was also a positive trend for decreasing age at diagnosis with the daily amount smoked. Finally, those who were 22 years old or older when they began smoking were 2.7 years (P < 0.01) younger at the time of diagnosis, while those between the ages of 17 and 21 at smoking initiation were 4 years younger at diagnosis (P < 0.01), and those under 17 years old at smoking initiation were 4.8 years (P < 0.01) younger at the age of CRC diagnosis compared to their never smoking counterparts (P < 0.01 for trend).
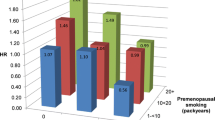
Past SHS exposure had a greater effect on the age on diagnosis of colorectal cancer than current SHS exposure among lifetime never smokers. As shown in Table 5, the age at CRC diagnosis did not vary significantly among individuals reporting current SHS exposure; however, those reporting prior SHS exposure were 8.6 years younger at diagnosis (P < 0.01). Those participants exposed to SHS both currently and in the past were diagnosed with colorectal cancer 11.6 years (P < 0.01) earlier than those who reported no prior SHS exposure. Similar results were seen when the analyses were stratified by gender. When current SHS exposure was examined by the number of hours per day exposed to SHS (none, 0.5–2.5 h, 3 or more hours), never smokers exposed to less than 3 h a day of SHS were diagnosed 4.2 years (P < 0.01) years earlier and never smokers exposed 3 or more hours of SHS a day were diagnosed 3.6 years (P < 0.01) earlier than never smokers not currently exposed to SHS; however, the trend was non-significant. When active and passive smoking exposures were combined, the largest difference in age was observed among current smokers (−9.5 years, P < 0.01), followed by former smokers exposed to SHS (−7.2 years, P < 0.01) and never smokers exposed to SHS (−6.8 years, P < 0.01).
In the data not shown, analyses were stratified by the stage of diagnosis (local, regional, distant) for age at diagnosis by the current smoking status. Clinical stage information was only available for Period #3. Using the stage of diagnosis as a covariate in the linear regression model did not significantly alter the age at diagnosis (beta changed <10%). For Period #3 only, current smokers were diagnosed 4.0 years (P < 0.01) before never smokers, regardless of the stage of diagnosis. When the analyses were stratified, the age at diagnosis was similar for each stage of diagnosis. Compared to never smokers, current smokers with local colorectal cancer were diagnosed 4.8 years (P = 0.11) earlier, while current smokers with regional colorectal cancer were diagnosed 3.6 years (P = 0.03) prior, and current smokers with distant colorectal cancer were diagnosed 3.7 years (P = 0.07) earlier. Although the age at diagnosis for the stratified analyses was similar to the unstratified age at diagnosis, some of the results did not achieve statistical significance. The most likely reason for the lack of statistical significance is that the stratification decreased the number of cases, which reduced statistical power.
Discussion
This study found that persons with tobacco smoke exposures (both active and passive), especially early in life, had a significantly younger age at CRC diagnosis than lifelong never smokers. Active smoking resulted in the youngest age at diagnosis, followed by exposure to passive smoking. The implication of this finding is that screening for colorectal cancer, which is now recommended to begin at age 50 years for average risk individuals should be initiated 5 to 10 years earlier for persons with a significant lifetime history of exposure to tobacco smoke.
The results from this study are consistent with three other published papers that have reported an earlier age of diagnosis for colorectal cancer in those who were current cigarette smokers (Buc et al. 2006; Michalek and Cummings 1987; Zisman et al. 2006). The biological mechanism underlying these observations is unclear. Earlier age of colorectal cancer diagnosis due to smoking may be a consequence of the effects of smoking on the body’s immune resistance to tumors, not just the effect of smoking on the tissue of the large intestine. Animal studies have demonstrated that exposure to tobacco smoke impairs local and systemic immunity (Thomas et al. 1973), depresses primary and secondary immune function (Thomas et al. 1974), and that transplanted tumor cells grow better in mice chronically exposed to tobacco smoke (Thomas et al. 1974). A study on humans found that current smokers have significantly lower residual antibody levels following influenza vaccination than never smokers, supporting this hypothesis of immune impairment from smoking (Finklea et al. 1971).
Natural killer (NK) cells are large granular lymphocytes that are believed to play a role in resistance to neoplasms and viral infections and in the control of metastatic spread of cancer (Tartter et al. 1987). Animal experiments have shown that lower NK cell activity is associated with higher cancer incidence, while studies on NK cell activity on humans have found significantly lower NK activity among smokers compared to non-smokers (Ferson et al. 1979; Ginns et al. 1985; Phillips et al. 1985; Talmadge et al. 1980). Another study found that smoking decreased both the quantity and function of NK cells, and the quantity and function remained impaired after smoking cessation, while other white blood cell functions returned to normal (Tollerud et al. 1989). Other research has linked lower initial NK cell activity to colorectal cancer recurrence and an advanced colorectal cancer stage at diagnosis (Tartter et al. 1987).
Current literature suggests that nicotine may play a significant role in the earlier age at diagnosis for colorectal cancer. After smoking, the levels of nicotine found in the GI tract are higher than that in the plasma (Fukada et al. 2002). While recent research has shown that nicotine may not be carcinogenic by itself, it may induce cell proliferation, increase tumor growth and stimulate angiogenesis (Dasgupta and Chellappan 2006; Heeschen et al. 2001). Nicotine from second-hand smoke has been reported to increase tumor size and promote angiogenesis (Zhu et al. 2003). In two current studies, Wong demonstrated in the first study that nicotine promoted human colon adenocarcinoma cell proliferation and in the other study that nicotine promoted colon tumor growth and angiogenesis (Wong et al. 2007a[k1], 2007b). Other researchers have concluded that nicotine may at least be partially involved in the initiation, promotion and progression of gastrointestinal tumors (Wu and Cho 2004).
The results of this study must be interpreted with caution due to the lack of control for certain confounders. While the analyses examining the effects of active smoking on age at diagnosis controlled for gender, any alcohol use and year of admission, other well-established risk factors for colorectal cancer, such as body mass index (BMI), race, meat consumption, vegetable intake, NSAID use and family history could not be included as covariates because these data were not consistently collected across the study duration. However, where dietary and family history data were available, sensitivity analyses were performed. Table 6 displays the results of those analyses, in which the adjusted difference in age is shown next to the crude difference in age. The addition of these covariates to the model had little effect on age at CRC diagnosis. Also, where data were present, dietary habits, BMI and family history did not significantly differ based on smoking status, suggesting that the lack of complete covariate information did not alter the main conclusions of this analysis.
Smoking patterns over time have been variable, with increased smoking prevalence among male birth cohorts until the 1930s, while increased smoking prevalence among female birth cohorts was observed until the 1950s. It is possible that the association between smoking and age at diagnosis may actually be an artifact of differential smoking behavior among the birth cohorts. To determine whether or not the finding of a younger age at diagnosis among smokers was an artifact of smoking behavior among different birth cohorts, smokers were placed into categories based upon the year of birth (10 year intervals). We found consistent, significant differences, with current smokers having an earlier age at diagnosis within each “birth-cohort”, indicating that the association between smoking and age at diagnosis was not an artifact of differential smoking patterns among birth cohorts.
Zisman et al. raised the possibility of spurious results due to competing causes of mortality (Zisman et al. 2006). There is little doubt that smokers have a shorter life expectancy than never smokers due to a higher death rate from various causes (Bronnum-Hansen and Juel 2001). If competing causes of mortality were the correct explanation, a younger age at diagnosis would be seen across other disease sites. To investigate this, age at diagnosis based on smoking status among other disease sites was examined in this data set. Age at diagnosis did not differ, based on smoking status, for non-malignant diseases such as benign skin, kidney, breast, and uterine disease, along with buccal disease and other GI conditions (gallbladder, pancreatic ailments; P > 0.05). These findings demonstrate that the difference in age at diagnosis is not solely due to the difference in life expectancy between smokers/never smokers and that competing causes of mortality was not a major factor in this analysis.
This study also examined the clinical stage of colorectal cancer at diagnosis based on smoking status. The results showed that the age of diagnosis was similar across the clinical stages (local, regional, distant). The addition of clinical stage as a covariate to the linear regression model and the resulting minimal effect on age at diagnosis ruled out the possibility that smokers were under the increased care of a doctor. Increased doctor visits could have led to increased screening for colorectal cancer, artificially creating an earlier age at diagnosis among smokers. The analysis shows this was not the case in this study. Analyses were also stratified by colorectal cancer stage and found no signs of interaction; a significantly younger age at diagnosis among smokers was consistently observed across all strata.
There are several limitations to the current study. One limitation was the completion rate of the questionnaire. All patients who were treated at Roswell Park Cancer Institute were given the option of completing the questionnaires. The completion rate was the highest for Period #1 (85%), and the rate declined over the two subsequent periods to ∼50% for Period #3. Another limitation was that second-hand smoke (SHS) exposure was only assessed during Period #3 (1982–1997). This greatly limited the number of persons who provided SHS exposure information and reduced the statistical power for some of the passive smoking analyses. Lastly, the limited use of certain covariates (BMI, family history) was another limitation found in this study.
This study has several strengths, including the assessment of tobacco exposures via multiple measures. Previous studies relied upon measure of a more limited scope to assess tobacco exposure, such as current smoking status only. Our results demonstrated a consistent decrease in age at diagnosis across the various exposure measures. Using continuous exposures such as amount smoked and age at initiation, positive trends were observed.
This study is also one of the first studies to find that age at diagnosis may also be affected by second-hand smoke exposure. When both active and passive smoking were placed into the same model, the results showed that active smoking exposure had the largest effect, followed by passive smoking exposure, which is what makes sense from a biological standpoint. Another strength of this study is the large sample size. This large numbers of cases allowed for high statistical power and the ability to stratify the analyses. Cases included in this study were accrued over 40 years, and the large number of colorectal cancer cases included in this study, combined with the more detailed exposure assessment, represents a unique strength.
Conclusions
This study found that smokers with considerable exposure, especially early in life, were at a significantly younger age at CRC diagnosis than lifelong never smokers. The results for active smoking and age at diagnosis are in agreement with the results of other studies that examined age at diagnosis for colorectal cancer and cigarette smoking (Buc et al. 2006; Michalek and Cummings 1987; Zisman et al. 2006). This study is also one of the first to examine the age of patient, with second-hand tobacco smoke exposure, at diagnosis of colorectal cancer. The results demonstrate that never smokers with considerable SHS exposure, especially early in life, were more likely to be diagnosed with CRC at an earlier age than persons without such exposure. The biological mechanism accounting for the observed earlier age at diagnosis among those with heavier exposures to tobacco smoke remains unclear and needs to be explored further. However, the practical implications of our results are that screening for colorectal cancer, which is now recommended to begin at age 50 for average risk individuals might be started 5 to 10 years earlier for those persons with a significant lifetime history of exposure to tobacco smoke.
References
ACS (2006) Cancer facts and figures. ACS Atlanta
Almendingen K, Hofstad B, Trygg K, Hoff G, Hussain A, Vatn MH (2000) Smoking and colorectal adenomas: a case–control study. Eur J Cancer Prev 9:193–203
Boutron MC, Faivre J, Dop MC, Quipourt V, Senesse P (1995) Tobacco, alcohol, and colorectal tumors: a multistep process. Am J Epidemiol 141:1038–1046
Bronnum-Hansen H, Juel K (2001) Abstention from smoking extends life and compresses morbidity: a population-based study of health expectancy among smokers and never smokers in Denmark. Tob Control 10:273–278
Buc E, Kwiatkowski F, Alves A, Panis Y, Mantion G, Slim K (2006) Tobacco smoking: a factor of early onset of colorectal cancer. Dis Colon Rectum 49:1893–1896
CDC(2005) Smoking prevalence among US adults. In: Health. NCfCDPaHPUSOoSa (ed)
Dasgupta P, Chellappan SP (2006) Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle 5:2324–2328
Demers RY, Neale AV, Demers P, Deighton K, Scott RO, Dupuis MH, Herman S (1988) Serum cholesterol and colorectal polyps. J Clin Epidemiol 41:9–13
Doll R, Gray R, Hafner B, Peto R (1980) Mortality in relation to smoking: 22 years’ observations on female British doctors. Br Med J 280:967–971
Doll R, Peto R (1976) Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J 2:1525–1536
Erhardt JG, Kreichgauer HP, Meisner C, Bode JC, Bode C (2002) Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps—a case control study. Eur J Nutr 41:35–43
Ferson M, Edwards A, Lind A, Milton GW, Hersey P (1979) Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int J Cancer 23:603–609
Finklea JF, Hasselblad V, Riggan WB, Nelson WC, Hammer DI, Newill VA (1971) Cigarette smoking and hemagglutination inhibition response to influenza after natural disease and immunization. Am Rev Respir Dis 104:368–376
Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC (1994) A prospective study of family history and the risk of colorectal cancer. N Engl J Med 331:1669–1674
Fukada A, Saito H, Inui K (2002) Transport mechanisms of nicotine across the human intestinal epithelial cell line Caco-2. J Pharmacol Exp Ther 302:532–538
Ginns LC, Ryu JH, Rogol PR, Sprince NL, Oliver LC, Larsson CJ (1985) Natural killer cell activity in cigarette smokers and asbestos workers. Am Rev Respir Dis 131:831–834
Giovannucci E (2001) An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 10:725–731
Giovannucci E, Colditz GA, Stampfer MJ, Hunter D, Rosner BA, Willett WC, Speizer FE (1994a) A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in US women. J Natl Cancer Inst 86:192–199
Giovannucci E, Martinez ME (1996) Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst 88:1717–1730
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, Willett WC (1994b) A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in US men. J Natl Cancer Inst 86:183–191
Hammond EC (1966) Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr 19:127–204
Hammond EC, Horn D (1958) Smoking and death rates; report on forty-four months of follow-up of 187,783 men. II. Death rates by cause. J Am Med Assoc 166:1294–1308
Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP (2001) Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 7:833–839
Hoff G, Vatn MH, Larsen S (1987) Relationship between tobacco smoking and colorectal polyps. Scand J Gastroenterol 22:13–16
Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK (2004) Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 101:3–27
Ji BT, Weissfeld JL, Chow WH, Huang WY, Schoen RE, Hayes RB (2006) Tobacco smoking and colorectal hyperplastic and adenomatous polyps. Cancer Epidemiol Biomarkers Prev 15:897–901
Kahn HA (1966) The Dorn study of smoking and mortality among US veterans: report on eight and one-half years of observation. Natl Cancer Inst Monogr 19:1–125
Kune GA, Kune S, Vitetta L, Watson LF (1992) Smoking and colorectal cancer risk: data from the Melbourne Colorectal Cancer Study and brief review of literature. Int J Cancer 50:369–372
Lee WC, Neugut AI, Garbowski GC, Forde KA, Treat MR, Waye JD, Fenoglio-Preiser C (1993) Cigarettes, alcohol, coffee, and caffeine as risk factors for colorectal adenomatous polyps. Ann Epidemiol 3:239–244
Lilla C, Verla-Tebit E, Risch A, Jager B, Hoffmeister M, Brenner H, Chang-Claude J (2006) Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomarkers Prev 15:99–107
Longnecker MP, Chen MJ, Probst-Hensch NM, Harper JM, Lee ER, Frankl HD, Haile RW (1996) Alcohol and smoking in relation to the prevalence of adenomatous colorectal polyps detected at sigmoidoscopy. Epidemiology 7:275–280
Martinez ME, McPherson RS, Annegers JF, Levin B (1995) Cigarette smoking and alcohol consumption as risk factors for colorectal adenomatous polyps. J Natl Cancer Inst 87:274–279
Michalek AM, Cummings KM (1987) The association between cigarette smoking and age at cancer diagnosis. Hum Biol 59:631–639
Monnet E, Allemand H, Farina H, Carayon P (1991) Cigarette smoking and the risk of colorectal adenoma in men. Scand J Gastroenterol 26:758–762
Nagata C, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S (1999) Cigarette smoking, alcohol use, and colorectal adenoma in Japanese men and women. Dis Colon Rectum 42:337–342
Nishino Y, Tsubono Y, Tsuji I, Komatsu S, Kanemura S, Nakatsuka H, Fukao A, Satoh H, Hisamichi S (2001) Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control 12:797–802
Phillips B, Marshall ME, Brown S, Thompson JS (1985) Effect of smoking on human natural killer cell activity. Cancer 56:2789–2792
Potter JD, Bigler J, Fosdick L, Bostick RM, Kampman E, Chen C, Louis TA, Grambsch P (1999) Colorectal adenomatous and hyperplastic polyps: smoking and N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers Prev 8:69–75
Slattery ML, Edwards S, Curtin K, Schaffer D, Neuhausen S (2003) Associations between smoking, passive smoking, GSTM-1, NAT2, and rectal cancer. Cancer Epidemiol Biomarkers Prev 12:882–889
Smith RA, Cokkinides V, Eyre HJ (2003) American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin 53:27–43
Strate LL, Syngal S (2005) Hereditary colorectal cancer syndromes. Cancer Causes Control 16:201–213
Talmadge JE, Meyers KM, Prieur DJ, Starkey JR (1980) Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst 65:929–935
Tartter PI, Steinberg B, Barron DM, Martinelli G (1987) The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg 122:1264–1268
Terry MB, Neugut AI (1998) Cigarette smoking and the colorectal adenoma–carcinoma sequence: a hypothesis to explain the paradox. Am J Epidemiol 147:903–910
Thomas WR, Holt PG, Keast D (1973) Cellular immunity in mice chronically exposed to fresh cigarette smoke. Arch Environ Health 27:372–375
Thomas WR, Holt PG, Keast D (1974) Recovery of immune system after cigarette smoking. Nature 248:358–359
Thomas WR, Holt PG, Papadimitriou JM, Keast D (1974) The growth of transplanted tumours in mice after chronic inhalation of fresh cigarette smoke. Br J Cancer 30:459–462
Tollerud DJ, Clark JW, Brown LM, Neuland CY, Mann DL, Pankiw-Trost LK, Blattner WA, Hoover RN (1989) Association of cigarette smoking with decreased numbers of circulating natural killer cells. Am Rev Respir Dis 139:194–198
Tomeo CA, Colditz GA, Willett WC, Giovannucci E, Platz E, Rockhill B, Dart H, Hunter DJ (1999) Harvard report on cancer prevention. Volume 3: prevention of colon cancer in the United States. Cancer Causes Control 10:167–180
United States Office of the Assistant Secretary for Health, Surgeon General, National Center for Chronic Disease Prevention and Health Promotion (US), Office on Smoking and Health (2004) The health consequences of smoking: a report of the surgeon general. Department of Health and Human Services, US Public Health Service. For sale by the Superintendent of Doctors US, GPO Atlanta, GA,Washington DC
United States Public Health Service, Office of the Surgeon General, Centers for Disease Control, Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US), US Office on Smoking and Health (2001) Women and smoking: a report of the Surgeon General, US Department of Health and Human Services, Public Health Service Office of the Surgeon General. For sale by the Superintendent of Doctors US GPO, Rockville, MD, Washington DC
Weir JM, Dunn JE Jr (1970) Smoking and mortality: a prospective study. Cancer 25:105–112
Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C (2003) Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 124:544–560
Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (2007a) Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol 221:261–267
Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (2007b) Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci 97:279–287
Wu WK, Cho CH (2004) The pharmacological actions of nicotine on the gastrointestinal tract. J Pharmacol Sci 94:348–358
Yamasaki E, Ames BN (1977) Concentration of mutagens from urine by absorption with the nonpolar resin XAD-2: cigarette smokers have mutagenic urine. Proc Natl Acad Sci U S A 74:3555–3559
Zhu BQ, Heeschen C, Sievers RE, Karliner JS, Parmley WW, Glantz SA, Cooke JP (2003) Second-hand smoke stimulates tumor angiogenesis and growth. Cancer Cell 4:191–196
Zisman AL, Nickolov A, Brand RE, Gorchow A, Roy HK (2006) Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med 166:629–634
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peppone, L.J., Mahoney, M.C., Cummings, K.M. et al. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol 134, 743–751 (2008). https://doi.org/10.1007/s00432-007-0332-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-007-0332-8