Abstract
Purpose
Chemokines play multiple roles in the development and progression of many different tumors. Our cDNA array data suggested that chemokine CXCL5 was upregulated in gastric cancer. Here, we analyzed CXCL5 protein expression in gastric cancer and investigated the clinical implications of CXCL5 upregulation.
Methods
Immunostaining for CXCL5 was performed on gastric tissue microarrays of tissue specimens obtained by gastrectomy. The intensity of immunostaining in tumor tissue was considered strong when tumor tissue staining was more intense than in normal tissue; the intensity was null when staining was weaker in the tumor than in normal tissue; and the intensity was weak when staining was similar in both tissues. Serum CXCL5 levels and microvascular density in tumor tissue were measured by ELISA and monoclonal antibody to Factor VIII.
Results
Strong CXCL5 expression correlated with tumor stage. CXCL5 expression did not correlate with T stage. However, N stage positively correlated with CXCL5 expression. Serum CXCL5 levels in late stage (IIIB, IV) gastric cancer patients were higher than in patients with benign conditions. Microvascular density was higher in tumors with strong CXCL5 expression, but the correlation with CXCL5 was not linear. Multiple logistic regression analyses showed that, compared to no or weak expression, strong expression of CXCL5 was a significant risk factor for high N stage (N2, N3).
Conclusions
CXCL5 overexpression was associated with late stage gastric cancer and high N stage. These results suggest a role for CXCL5 in the progression of gastric cancer, specifically in lymph node metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second most common cause of cancer-related deaths worldwide (Parkin et al. 2001). Surgery is the only potential curative treatment. Early diagnosis and treatment contribute to decreased mortality in Asian countries (Nagata et al. 1983; Abe et al. 1984). However, there are still numerous patients with advanced gastric cancer whose survival is not improved by surgery (Hundahl et al. 2000; Wanebo et al. 1993; Cenitagoya et al. 1998). Lymph node metastasis is a major prognostic factor for advanced gastric cancer (Kattan et al. 2003, Hyung et al. 2002). Adjuvant chemotherapy is used to negate the effects of lymph node metastasis, but the survival benefit is only marginal. Thus, understanding the mechanism of metastasis is critical to develop new treatments that can improve the survival of patients with advanced gastric cancer.
Many events are necessary for tumor cell metastasis. Cells must degrade the basement membrane, migrate through surrounding tissues, intravasate into lymphatic or blood vessels, exit from these vessels, survive, and proliferate (Fidler 2002). Chemokines are believed to be involved in these complex processes (Balkwill 2004). The chemokine family is comprised of CXC and CC chemokines. Chemokines are small, secreted proteins that act as immune modulators and chemoattractants (Zlotnik and Yoshie 2000; Bacon et al. 2002). In tumor cells, the stimulation of chemokine receptors results in the activation of molecular pathways involved in actin reorganization and motility (Muller et al. 2001).
Recently, we found that the CXC-chemokine CXCL5 was upregulated in gastric cancer using cDNA array technology. Compared to normal gastric mucosa, early and advanced gastric cancers expressed 3.7 and 7.2 times more CXCL5, respectively (unpublished data). CXCL5 is an important angiogenic factor in human non-small cell lung cancer (Arenberg et al. 1998). It is also a strong neutrophil chemoattractant (Qiu et al. 2003). Recently Miyazaki et al. showed that CXCL5 was highly expressed in metastatic cells of head and neck squamous cell carcinomas, as compared to cells of the primary tumor. Using RNA interference, they were also able to show that CXCL5 played a role in cancer cell migration and invasion (Miyazaki et al. 2006). Their results are supported by other studies showing the involvement of CXCR2, a CXCL5 receptor, in the regulation of cell motility (Schraufstatter et al. 2001). In addition, antibodies to CXCL5 reduce the metastatic potential of lung cancer (Arenberg et al. 1998).
Even though there are studies on CXCL5 in several cancer types, there are no reports on the role of CXCL5 in gastric cancer. In this study, we analyzed the level of CXCL5 protein expression in gastric cancer and discuss the clinical implications of our findings.
Methods
Tissue samples were randomly selected and obtained from consenting individuals who underwent gastrectomy for gastric cancer between Jan 1999 and Dec 2001 at Severance Hospital, Yonsei University College of Medicine. We used samples from 161 patients. All patients underwent gastrectomy for curative purposes. A pathologist reviewed samples, and pathological staging was conducted independently of this study. Tumor stage was determined based on the AJCC staging system. After pathological evaluation, tissue cores for tumor and adjacent normal tissue from each specimen were arrayed into a new paraffin block to create a tissue microarray (TMA). All patients gave informed consent, and the Ethical Committee for the Clinical Research of the Institutional Review Board of Yonsei Medical Center, Korea, approved the study protocol. Patient demographic characteristics and tumor status are summarized in Table 1.
Immunostaining was performed on the TMA using standard procedures. The CXCL5-specific antibody (R&D Systems, Inc., Minneapolis, MN, USA) was used at a dilution of 1:100. TMA slides were deparaffinized in xylene and rehydrated in graded alcohol. Endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxide at room temperature for 20 min. Microwave antigen retrieval was performed in citrate buffer (0.01 M, pH 6.0) for 10 min. Then sections were blocked with 10% normal donkey serum for 1 h to reduce non-specific background staining. Blocked sections were incubated in primary antibody at 4°C. The subsequent reaction was performed using the LSAB+ kit (DakoCytomation, Carpinteria, CA, USA) according to the manufacturer’s instructions. Finally, the immunoreaction was developed using 3-amino-9-ethylcarbazole and counterstained with hematoxylin.
Dark brown granules in the cytoplasm of tumor cells or in that of normal gastric epithelial cells indicated positive immunoreactivity. The intensity of immunostaining in tumor tissue was scored using normal tissue as an internal control. Tumor tissue was considered to have strong expression if it showed stronger intensity than normal tissue. If the intensity was weaker than in normal tissue, we considered the sample to have no expression. If the staining intensity was similar to normal tissue, we considered the sample to have weak expression. The samples were evaluated by two pathologists who were blinded to patients’ clinical information.
To measure serum CXCL5 levels by ELISA, serum samples were collected from nine patients with gastric cancer and from ten patients with benign conditions such as gastritis and hyperplsatic polyp. Samples were stored at -80°C until they were assayed. Serum CXCL5 levels were measured with a Quantikine Human ENA78 immunoassay kit (R&D systems, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Gastric tissues from these patients were not included in the immunohistochemical analysis.
To measure microvascular density (MVD) in tumor tissue, immunostaining using monoclonal antibody to Factor VIII (DakoCytomation, Denmark) was performed as described above. Stained vessels were counted under high-power microscopic fields (X200). The average number of vessels counted in the best-visualized area was recorded for each case (Wulfing et al. 2004).
Medical records were reviewed for clinical information. Tumor information, such as cancer cell differentiation, T stage, N stage, overall stage, and MVD, were correlated to tumor immunoreactivity using the Spearman correlation. Serum CXCL5 levels in the patients with benign tumors or gastric cancer were compared using the non-parametric Mann–Whitney U test. Multivariate logistic regression analysis was performed to determine factors associated with N stage.
Results
Association between CXCL5 overexpression and late tumor stage
Normal gastric epithelium and foveloar gland expressed CXCL5 in a heterogeneous way. Normal gastric epithelium as well as intestinal metaplsia expressed CXCL5, and its level in intestinal metaplsia was not higher than normal gastric epithelium. In all cases, immunoreactivity was observed in the cytoplasm of tumor cells. Demographic characteristics and tumor status were analyzed according to CXCL5 expression levels (Table 1). In this study, we grouped the tumor stages as follows: IA and IB as I, II as II, IIIA as III, and IIIB and IV as IV. As tumor expression of CXCL5 increased, so did the overall tumor stage (P < 0.05). Thirteen (72.2%) of 18 tumors with strong CXCL5 expression were stage IV, but only 2 (4.2%) of 48 tumors and 27 (28.4%) of 95 tumors with no and weak CXCL5 expression, respectively, were stage IV. We observed no correlation between cancer cell differentiation and CXCL5 expression. Also, CXCL5 expression did not correlate with T stage. However, N stage positively correlated with CXCL5 expression, as 2 (4.2%) of 48 tumors with no expression and 12 (12.6%) of 95 tumors with weak expression were N3 stage, compared to 11 (61.1%) of 18 tumors with strong expression (P < 0.05).
Higher CXCL5 serum levels in late stage gastric cancer than benign conditions
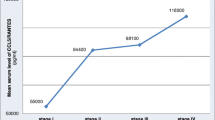
Ten non-tumor cases were included as a control group. Two cases had hyperplastic gastric polyps. The other eight cases simply had gastritis. The gastric cancer group included two cases with stage I cancer, three cases with stage III cancer, and four cases with stage IV cancer. Stage IV tumors were considered late stage tumors (n = 4) and stages I to III were considered early stage tumors (n = 5). By ELISA, the median CXCL5 level in the control group was 588.92 pg/ml (range 420.46–983.43 pg/ml). In early stage cancer, it was 906.53 pg/ml (range 536.52–1,015.58 pg/ml). The median CXCL5 level in last stage cancer was 1,473.91 pg/ml (range 757.74–3,200.32 pg/ml), which was significantly higher than in the control group (P < 0.05, Figs. 1, 2).
No correlation between CXCL5 expression and MVD
MVD was calculated as the number of vessels per high-power microscopic field. According to statistical analysis, MVD correlated with overall tumor stage (P < 0.05). MVD for stages I, II, III, and IV were 12.2 ± 9.8, 12.2 ± 6.9, 15.0 ± 8.9, and 15.4 ± 10.3, respectively. MVD positively correlated with overall stage (P < 0.05). The average MVD was 14.5 ± 9.1 for tumors with no CXCL5 expression, 12.4 ± 9.4 for tumors with weak CXCL5 expression, and 16.8 ± 10.1 for tumors with strong expression. MVD tended to be higher in tumors with strong CXCL5 expression, but the correlation with CXCL5 expression levels was not linear (P > 0.05).
Factors associated with high N stage
N2 and N3 stage tumors were considered high N stage tumors. Based on univariate analysis, cancer cell differentiation, T stage, MVD, and CXCL5 levels were significantly associated with high N stage. Multivariate logistic regression analysis with cancer cell differentiation, T stage, MVD, and CXCL5 levels showed that CXCL5 was the only factor significantly associated with high N stage (Table 2). Poorly differentiated cancer cell was associated with high N stage, but it was not statistically significant. Strong CXCL5 expression had 14.7 and 4.7 times higher risk for high N stage compared to no and weak CXCL5 expression, respectively (P < 0.05 and P < 0.05, respectively). Multivariate analysis showed 71.7% sensitivity and 81.7% specificity for predicting high N stage.
Discussion
Even though early diagnosis and treatment improve the survival of patients with gastric cancer, there are still a significant number of patients who die of advanced disease. To further improve survival, treatments based on a better understanding of cancer progression are needed (Miyazaki et al. 2006). Our study revealed a strong association between overexpression of CXCL5 and late tumor stage. Patients with late stage tumors also had CXCL5 serum levels that were higher than in controls. CXCL5 expression was not unique to tumor tissues, as early stage tumor CXCL5 expression was similar to that in normal tissues. We believe that CXCL5 is involved in late stage tumor progression through one of many possible mechanisms, as chemokines play multiple roles in the development and progression of many tumors (Balkwill 2004).
CXCL5 expression was significantly associated with high N stage, proven with multivariate analysis. Tumors with strong CXCL5 expression had higher risks for high N stage compared to no and weak CXCL5 expression. Even though CXCL5 expression correlated with N stage, it did not correlate with T stage. These results suggest that CXCL5 may be involved in cancer migration and invasion, and association between strong CXCL5 expression and late stage gastric cancer may contribute to its association with high N stage. There is only one report relating CXCL5 expression to cancer cell migration and invasion (Miyazaki et al. 2006). However, there is much indirect evidence supporting this relationship. The receptor of CXCL5, CXCR2, can regulate cell motility, and the activation of other chemokine receptors induces actin polymerization as well as cell migration and invasion (Schraufstatter et al. 2001; Wang et al. 2002). Various intracellular pathways are also involved in actin reorganization, and motility can be activated by chemokine receptors (Schraufstatter et al. 2001; Wang et al. 2002; Venkatakrishnan et al. 2000; Bonacchi et al. 2001; Chandrasekar et al. 2003, 2004; Ridley 2001). For CXCL5 and its related pathways to become potential targets for cancer treatment, it is important to determine the mechanistic roles of CXCL5 and whether CXCL5 plays a role in cancer progression.
CXCL5 is a strong neutrophil attractant. In non-cancerous conditions, such as ischemic/reperfusion injury and chronic obstructive pulmonary disease, neutrophils recruited by CXCL5 cause tissue damage (Walz et al. 1991; Colletti et al. 1995; Lentsch et al. 1998). There are several reports that Helicobacter infection is associated with increased chemokine production, but CXCL5 seems to be not related to Helicobacter infection (Shimoyama et al. 1998; Suzuki et al. 1998; Sieveking et al. 2004). One report suggests that increasing amounts of tumor infiltrating neutrophils in advanced gastric cancer are associated with reduced mortality (Caruso et al. 2002). However, neutrophils can either eliminate tumor cell populations or contribute to their invasive potential (Di Carlo et al. 2001; Welch et al. 1989). Based on our results, neutrophil recruitment by CXCL5 may help gastric cancer cells to metastasize to lymph nodes. Neutrophils may enable tumor cells to migrate through the extracellular matrix, helping them to enter the vasculature (De Larco et al. 2004). Even though neutrophil infiltration could not be analyzed and the role of neutrophil in tumors was controversial, this could be another hypothesis supporting our results.
Studies on non-small cell lung cancer suggest that high expression of CXCL5 in vivo and in vitro is associated with poor prognosis. These studies point out that high CXCL5 is associated with angiogenesis and that high levels of ELR+CXC chemokines, including CXCL5, are associated with enhanced tumor growth. CXCL5 expression correlates with tumor vascularity and tumor growth, and high levels of CXC-chemokines are associated with risk of recurrence after tumor resection (Arenberg et al. 1996, 1998; White et al. 2003). In our study, CXCL5 expression and microvessel count were evaluated on serial TMA sections to observe a correlation between CXCL5 expression and MVD within a certain area of the tumor (Wulfing et al. 2004). Interestingly, tumor samples with strong CXCL5 expression had a high MVD, but the correlation between CXCL5 expression and MVD was not linear. CXCL5 may not have as important a role in the angiogenesis of gastric cancer as in lung cancer. Tumors may induce angiogenesis by preferentially secreting one type of angiogenic factor over another. Furthermore, there may be more than one pathway regulating angiogenesis (White et al. 2003). However, we cannot rule out the possibility that CXCL5 helped tumor cells to survive by supplying blood vessels to late stage gastric cancers.
In conclusion, overexpression of CXCL5 was associated with advanced tumor stage, specifically high N stage. Through multiple mechanisms, CXCL5 may be involved in the migration and invasion of cancer cells and in late stage tumor progression. Further studies are needed to confirm and understand this interesting observation and to determine whether CXCL5 may serve as a therapeutic target for cancer treatment.
References
Abe S, Ogawa Y, Nagasue N, Sasaki Y, Akamizu H, Hirose S, Yukaya H, Suehiro S (1984) Early gastric cancer: results in a general hospital in Japan. World J Surg 8:308–314
Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM (1996) Inhibition of interleukin-8 reduces tumorigensis of human non-small cell lung cancer. J Clin Invest 97:2792–2802
Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM (1998) Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 102:465–472
Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, Maysushima K, Murphy P, Nomiyama H, Oppenheim J, Rot A, Schall T, Tsang M, Thorpe R, Van Damme J, Wadhwa M, Yoshie O, Zlotnik A, Zoon K; IUIS/WHO Subcommittee on Chemokine Nomenclature (2002) Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res 22:1067–1068
Balkwill F (2004) Cancer and the chemokine network. Nat Rev Cancer 4:540–550
Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M, Gentilini P, Marra F (2001) Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem 276:9945–9954
Caruso RA, Bellocco R, Pagano M, Bertoloi G, Riogoli L, Inferrera C (2002) Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Modern Pathol 15:831–837
Cenitagoya GF, Bergh CK, Klinger-Roitman J (1998) A prospective study of gastric cancer. Real 5-year survival rates and mortality rates in a country with high incidence. Dig Surg 15:317–322
Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, Dulin NO, Singh IS (2003) Chemokine-Cytokine Cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-nB pathway. J Biol Chem 278:4675–4686
Chandrasekar B, Bysani S, Mummidi S (2004) CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, InB kinase, and nuclear factor-nB and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem 279:3188–3196
Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM (1995) Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest 95:134–141
De Larco JE, Wuertz BR, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10:4895–4900
Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P (2001) The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 97:339–345
Fidler IJ (2002) Critical determinants of metastasis. Semin Cancer Biol 12:89–96
Hundahl AS, Phillips JL, Menck HR (2000) The National Cancer Data Base report on poor survival of US gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on cancer staging, proximal disease, and the ‘‘different disease’’ hypothesis. Cancer 88:921–932
Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, Lee JH, Choi SH, Min JS (2002) Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg 26:323–329
Kattan MW, Karpeh MS, Mazumdar M, Brennan MF (2003) Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol 21:3647–3650
Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ (1998) Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-1 and Kupffer cells. Hepatology 27:507–512
Miyazaki H, Patel V, Wang H, Edmunds RK, Gutkind JS, Yeudall WA (2006) Down-regulation of CXCL5 inhibits squamous carcinogenesis. Cancer Res 66:4279–4284
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Nagata T, Ikeda M, Nakayama F (1983) Changing state of gastric cancer in Japan: histologic perspectives of the past 76 years. Am J Surg 145:226–233
Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The global picture. Eur J Cancer 37:S4–S66
Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK (2003) Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Resp Crit Care 168:968–975
Ridley AJ (2001) Rho GTPases and cell migration. J Cell Sci 114:2713–2722
Schraufstatter IU, Chung J, Burger M (2001) IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol 280:L1094–L1103
Shimoyama T, Everett SM, Dixon MF, Axon AT, Crabtree JE (1998) Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol 1998 51:765–770
Sieveking D, Mitchell HM, Day AS (2004) Gastric epithelial cell CXC chemokine secretion following Helicobacter pylori infection in vitro. J Gastroenterol Hepatol 19:982–987
Suzuki H, Mori M, Sakaguchi AA, Suzuki M, Miura S, Ishii H (1998) Enhanced levels of C-X-C chemokine, human GROalpha, in Helicobacter pylori-associated gastric disease. J Gastroenterol Hepatol 13:516–520
Venkatakrishnan G, Salgia R, Groopman JE (2000) Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem 275:6868–6875
Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM (1991) Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin-8. J Exp Med 174:1355–1362
Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, Osteen R (1993) Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 218:583–592
Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A (2002) PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry 41:7100–7107
Welch DR, Scissel DJ, Howrey RP, Aeed PA (1989) Tumor-elicited polymorphonuclear cells, contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. P Natl Acad Sci USA 86:5859–5863
White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA (2003) Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res 9:853–860
Wulfing P, Kersting C, Tio J, Fischer RJ, Wulfing C, Poremba C, Diallo R, Bocker W, Kiesel L (2004) Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res 10:2393–2400
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12:121–127
Acknowledgment
This study was supported in part by grant FG03-32-01 of the 21C Frontier Functional Human Genome Project from the Ministry of Science and Technology of Korea and by a grant from the Brain Korea 21 Project for Medical Sciences of Yonsei University College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.Y., Park, K.H., Bang, S. et al. CXCL5 overexpression is associated with late stage gastric cancer. J Cancer Res Clin Oncol 133, 835–840 (2007). https://doi.org/10.1007/s00432-007-0225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-007-0225-x