Abstract
Purpose
Aromatase catalyzes the conversion of androgens to estrogens; its high expression in breast cancers may be responsible for the local high levels of estrogen and may promote tumor growth and progression; however, the clinical importance of aromatase remains unclear and needs to be further researched.
Methods
By immunochemistry, we detected aromatase, MMP2 and MMP9 immunoreactivity in 244 axillary lymph node negative breast cancers.
Results
Aromatase immunoreactivity was positively associated with co-expression of MMP2 and MMP9 (MMP2/9) in the estrogen receptor and/or progestin receptor- (ER/PR) positive patients (P < 0.05), but not in the ER and PR negative patients (P > 0.05); aromatase status positively associated with tumor size in the postmenopausal patients (P < 0.05) but not in the premenopausal patients (P > 0.05). The proportional hazards assumption was violated for aromatase status (global test, P < 0.05), and aromatase was an unfavorable prognostic factor for disease-free survival (DFS) (P = 0.04) in multivariate analysis of time-dependent non-proportional Cox regression. In the ER/PR-positive patients, positive aromatase staining was significantly associated with decreased overall survival (OS) (P = 0.04), but there was no such association in the ER and PR negative patients (P > 0.05).
Conclusions
Our study suggested that local estrogen production by aromatase plays important roles in the growth and invasiveness of breast cancer; tumor aromatase status may be indicative of breast cancer prognosis in some patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well accepted that estradiol plays an important role in the genesis and progression of breast cancer (Pasqualini and Chetrite 2005). Aromatase, known as estrogen synthetase, involves in estradiol bioformation and mediates the conversion of estrogens from androgens (Brodie et al. 1999). Human breast cancer tissues contain aromatase for its mRNA, and protein as well as enzyme activity (Esteban et al. 1992; Evans et al. 1993; Lu et al. 1996) were detected in the breast cancer specimens. Observations on transgenic mice with over-expressed aromatase (Kirma et al. 2001; Mandava et al. 2001; Tekmal et al. 1996) indicate that locally high concentrations of estrogen produced by aromatase may play an important role in breast carcinogenesis; furthermore, a study of aromatase gene-transfected breast cancer cells (Dowsett et al. 1996) showed that tumor growth is supported by this intracrine source of estrogen in the absence of endocrine estrogen stimulation.
There was evidence of elevated serum estrogen levels associated with a high risk of breast cancer (Hankinson et al. 1998; Key et al. 2003; Missmer et al. 2004); however, it is interesting that local concentrations of estradiol in specimens of breast carcinomas from postmenopausal patients were found to be several-fold higher than those in the plasma (Pasqualini et al. 1996; van Landeghem et al. 1985). More than 50% of all breast cancers are sensitive to hormone treatment, indicating an important role of estrogens in the progression of the disease. Whether high expression of aromatase indicates unfavorable prognosis, and whether it is associated with classic prognostic factors in node negative breast cancers remain to be resolved.
Matrix-metalloproteinases (MMPs) play important roles in the aggressiveness of malignant cells, especially in the process of metastasis to distant sites (Duffy et al. 2000). MMP-2 and MMP-9 can degrade type-IV collagen effectively, and the latter is the main component of the basement membrane (Duffy et al. 2000). Talvensaari-Mattila observed that MMP-2 has been associated strongly with a shortened survival, independent of major prognostic factors in breast cancers (Talvensaari-Mattila et al. 1998); MMP9 was found to be related to lymph node metastasis in breast cancers (Iwata et al. 1996) and vascular invasion in esophageal cancers (Koyama et al. 2000). The over-expression of MMP2 and MMP9 in breast cancers and their clinical significance were also observed in other studies (Hirvonen et al. 2003; Pacheco et al. 2001). Wolczynski observed that both MMP-2 and MMP-9 biosynthesis could be stimulated by estradiol in ER-positive MCF-7 cells (Wolczynski et al. 2001). All these may indicate that estrogen could enhance the invasive ability of breast cancers through the ER pathway; however, whether aromatase is related to MMPs in breast cancers needs further research.
In this study, we examined the expression of aromatase, MMP-2 and MMP-9 using immunohistochemistry in 244 primary axillary node negative breast cancers, correlated aromatase with MMPs and clinicopathological factors and evaluated its prognostic values.
Materials and methods
Patient characteristics
A total of 244 patients, who underwent surgery of modified radical mastectomy or radical mastectomy from 1990 to 1998 in the Cancer Hospital of Fudan University, were recruited in this study; all patients were pathologically diagnosed with primary breast cancer with negative axillary lymph node metastasis. The histological grade was determined according to the modified Scarff–Bloom–Richardson criteria (Elston and Ellis 2002). All specimens were fixed with 10% formalin and embedded in paraffin wax.
The patients did not receive irradiation, chemotherapy or hormonal therapy before surgery. The patients were aged 30–90 years, with a median age of 52.5 years and a mean age of 53.9 years; the median follow-up time was 59.0 months and the mean follow-up time was 56.9 months, which ranged from 11–120 months; the distribution of follow-up times for patients still alive at the time of follow- up ranged from 20 to 120 months, with a median of 58.4 months. About 45.5% (111/244) patients received adjuvant chemotherapy (CMF: cyclophosphamide, 600 mg/m2 i.v. bolus, dl, 8; methotrexate 40 mg/m2, i.v. bolus, d1, 8; fluorouracil 600 mg/m2, i.v. infusion, d1, 8; every 4 weeks × 6 cycles), 47.1% (115/244) patients received adjuvant endocrine therapy (tamoxifen 10 mg/day, po, bid × 3–5 years) (Table 1). Among tamoxifen treated patients, 72.2% (83/115) were ER- and/or PR-positive, and among those without tamoxifen, 65.1% (84/129) were ER- and/or PR-positive.
Antibodies and immunohistochemistry
Monoclonal mouse antibody against aromatase (MCA2077) was ordered from Serotec Company, Kidlington, UK; it was produced by the immunogen of synthetic peptide corresponding to amino acids 376–390 of human aromatase and works in immunohistology on paraffin embedded slice (Turner et al. 2002). Polyclonal rabbit anti-human-MMP-2 antibody (sc-10736) and polyclonal rabbit anti-human-MMP-9 (sc-10737) antibody were ordered from Santa Cruz Biotechnology, Santa Cruz, CA.
Immunohistochemical analysis was performed employing the avidin–biotin–immuno-peroxidase technique using ABC Staining Systems from Santa Cruz Biotechnology, Santa Cruz, CA, as previously described (Hsu et al. 1981). Antigen retrieval for aromatase was performed by boiling the slides in a cooker for 10 min in citric acid buffer [2 mM citric acid and 9 mM trisodium citrate dehydrate (pH 6.0)] after deparaffinization. Antigen retrieval for MMPs was performed by treating the sections with 0.4% pepsin (Sigma, St Louis, MO) for 20 min at room temperature. The dilutions of primary antibodies used in this study were as follows: aromatase, 1:100 and MMP-2 and MMP-9, 1:150. The antigen–antibody complex was visualized with 3,3′-diaminobenzidine (DAB) solution [1 mmol/l 3,3′-DAB, 50 mmol/l Tris–HCl buffer (pH 7.6) and 0.006% H2O2] and the sections were counterstained with hematoxylin. Human placenta (Sasano et al. 2001) was used as positive control for detecting aromatase, and previously known positive samples of MMP-2 and MMP-9 were used as positive controls, respectively. As negative controls, normal mouse or rabbit IgG was used instead of the primary antibodies. No specific immunoreactivity was detected in these sections.
Scoring of immunoreactivity
Previously described scoring methods (Shenton et al. 1998; Talvensaari-Mattila et al. 1998) for aromatase and MMPs were used with modifications. After reviewing the entire slides of immunostained sections, the sections were evaluated microscopically by two independent investigators, who were blinded to the patients’ outcome, using objectives with ×10 and ×40 magnifications.
The results of the immunohistochemistry were evaluated by estimating the percentage of cancer cells with positive cytoplasmic immunoreactivity. Cancers were classified as aromatase positive if at least 10% of the cells were stained, whereas those with no reactivity or staining in less than 10% of the cells were regarded as aromatase negative. For evaluation of the expression of MMPs, the results were divided into two groups as follows: (−) no immunoreactivity (+) ≥1% positive cytoplasmic stained cells.
Steroid-receptor assays
Before 1994, the standard dextran-coated charcoal assay was used as described by Kute et al. (1992), the Scatchard-plot analysis was done with eight points and the protein content in the reaction was 1 mg/ml. Receptor levels of 10 fmol/mg or more of the protein were considered positive. Immunohistochemistry was used since 1994 and ≥10% cells with nucleus staining were considered positive for both ER and PR. The rabbit polyclonal antibodies for ER (sc-542) and PR (sc-538) from Santa Cruz Biotechnology were used as primary anybodies.
Statistical methods
Distributions of aromatase status and other categorical variables were compared using standard χ 2 tests. The disease-free interval was calculated from the date of primary surgery of the breast. First recurrence or metastasis was scored as an event, and patients without recurrence or metastasis were censored at the time of the last follow-up. OS was calculated from the date of primary surgery with death from breast cancer being scored as an event. Patients who were alive at the last follow-up were censored at the last follow-up date. In the univariate survival analysis, postoperative DFS and OS were analyzed by the Kaplan–Meier method, and the differences between subgroups were compared by means of a log rank test. The multivariate analysis was tested with the Cox proportional hazards model and the time-dependent Cox non-proportional hazards model. The proportional hazards assumption was tested by a global test (Hilsenbeck et al. 1998). All statistical tests were two-sided at the 5% level of significance and were performed using SPSS (SPSS Company, Chicago, IL) and Stata software (Stata Corporation, College Station, TX). Relative Risks (RRs) are presented with their 95% confidence intervals (CIs).
Results
Expression of aromatase, MMP-2 and MMP-9
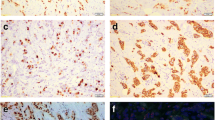
Immunoreactivity for aromatase was detected mainly in the cytoplasm of cancer cells and in the partially surrounding stromal cells. Positive stain of aromatase was 54.9 % (134/244) (Fig. 1a). Both MMP-2 and MMP-9 were localized in the cytoplasm of carcinoma cells, and a few in the stromal cells (Fig. 1b, c). Positive rate of MMP-2 was 58.6% (143/244), and positive rate of MMP-9 was 62.7% (153/244); co-expression rate of both MMP2 and MMP9 was 48.4% (118/244) (Table 1).
Immunohistochemistry for aromatase and MMP2 and MMP9 in breast cancer. Aromatase protein was detected in the cytoplasm of invasive breast cancer cells (a). MMP2 was detected in the cytoplasm of invasive breast cancer cells (b). MMP9 was detected in the cytoplasm of carcinoma cells (c). Original magnification ×200
Correlation between aromatase and MMPs
Aromatase immunoreactivity was positively associated with both MMP-2 and MMP-9, respectively (P < 0.01), and also positively associated with the co-expression of MMP2/9 (P < 0.01) (Table 1). It was interesting that a positive correlation between aromatase and co-expression of MMP2/9 was observed in the ER- and/or PR-positive patients (P < 0.01), but not in the ER- and PR-negative patients (P > 0.05) (Table 2).
Correlation between aromatase and clinicopathological parameters
A significantly positive correlation was observed between aromatase immunoreactivity and tumor size (P = 0.02) (Table 1). In the subgroup analysis, there was a significant relationship between tumor size and aromatase immunoreactivity in postmenopausal patients (P = 0.01), but not in premenopausal patients (P = 0.464) (Table 3). There were no statistically significant correlation found between aromatase immunoreactivity and histological type, grade, age, ER status, PR status and their combination (Table 1).
Survival analysis in the 244 patients and in different ER/PR subgroups
In the univariate survival analysis, the aromatase-positive group showed a lower 10-year DFS rate (71.1%) than the aromatase-negative group (95.3%), but without statistical significance (P = 0.058). There was no statistically significant association between positive-aromatase immunoreactivity and decreased OS (10-year OS rate, 87.5%, for aromatase-positive group compared with 98.2% for aromatase-negative group, P = 0.148); however, the prognostic factors for DFS included tumor size, tamoxifen therapy and grade (P < 0.05) (Table 4).
In Cox proportional hazards regression analysis, tumor size, grade and adjuvant tamoxifen therapy were independent prognostic factors for DFS (P < 0.05), and aromatase status was not included in the model (P > 0.05) (Table 4). To further evaluate whether the proportional hazards assumption is valid across aromatase categories, the test for the lack of proportionality was performed and (Hilsenbeck et al. 1998), however, it was statistically significant (global test, P = 0.03). This suggested that the hazard ratio of aromatase was not constant and the time-dependent non-proportional Cox regression was suitable for analyzing this data other than the Cox proportional hazards regression. In the time-dependent Cox analysis, aromatase status (P = 0.04, RR = 1.529, 95%CI: 1.017–2.299) as well as tumor size, grade, and tamoxifen therapy (P < 0.05) were included in the model, when aromatase was taken as a time-dependent covariate (Table 4).
The univariate survival analysis showed that aromatase positivity significantly associated with decreased OS (P = 0.04) in the ER- and/or PR-positive patients (10-year OS 100% for the aromatase-negative group compared with 85.1% for the aromatase-positive group ) (Fig. 2a). However, in the multivariate survival analysis, we did not find this association significant (P > 0.05). In the ER- and PR-negative cases, there were not any significant OS differences between aromatase-negative and aromatase-positive patients in either univariate (Fig. 2b) or multivariate analysis (P > 0.05) (data not shown).
Discussion
At present, there are three main kinds of methods to evaluate the aromatase status in cancer specimens. Biochemical measurement is classic and regarded as the gold standard method for quantitative assessment of aromatase activity (Shenton et al. 1998; Silva et al. 1989). However, there are unsolved disadvantages that make it difficult to apply for routine clinical measurements. For example, the aromatase assay is lengthy and requires a relatively large volume of tissue, and the tissues should be frozen in liquid nitrogen immediately after resection to avoid degradation until the beginning of measurement (Brodie et al. 1998; Lu et al. 1996; Sasano et al. 2001). The second method used in recent research is reverse transcriptase polymerase chain reaction (RT–PCR) to detect the expression of aromatase mRNA in the tissues. Although it does not require a large volume of sample and costs less time than the biochemical assay, the specimen still should be stored in nitrogen. Immunohistochemistry may overcome the disadvantages and could be widely used in clinic and so is highly desirable (Sasano et al. 2001; Shenton et al. 1998). Although both monoclonal and polyclonal antibodies were used in several research studies, immunoreactivity with a monoclonal antibody, but not with a polyclonal antibody, was strongly correlated to biochemical aromatase activity in breast cancer specimen (Shenton et al. 1998), which was supported by observations of in situ hybridization and aromatase activity measurement in cryosections (Brodie et al. 1998; Lu et al. 1996). So, to detect the expression of aromatase in this study, we used a monoclonal antibody.
Consistent with Shenton and Brodie’s reports (Brodie et al. 1998; Brodie et al. 1997; Shenton et al. 1998), we found that aromatase was expressed in the cytoplasm of carcinoma cells and in the surrounding stromal cells, with the cancer cells comprising a major portion of cells reactive to the anti-aromatase antibody. The aromatase-positive rate was 52% in Silva’s study by biochemistry aromatase activity assay (Silva et al. 1989), 52.6–64.29% in immunohistochemistry study using anti-aromatase monoclonal antibody (Brodie et al. 2001; Lu et al. 1996; Shenton et al. 1998) and similarly in our study, the positive rate was 54.9%.
Previous studies indicated no consistent association between ER, PR status and aromatase status (Eppenberger et al. 2001; Silva et al. 1989) In our study, there was no relationship between ER, PR and aromatase status (P > 0.05), furthermore, we did not find any significant relationship between the combination of the ER and PR status and the aromatase status (P > 0.05). This observation was also supported by cell research, because both ER-positive (e.g., MCF-7) and ER-negative (e.g., SK-BR-3, MDA-MB-231) breast cancer cells express aromatase protein and can produce E2 by themselves (Kinoshita and Chen 2003; Zhou et al. 1996).
In breast cancer, MMP2 and MMP9 are both produced in the early stage of metastasis and enable the cancer cells to metastasize (Duffy et al. 2000; Mitropoulou et al. 2003; Talvensaari-Mattila et al. 1998). We found significant positive association of aromatase with the MMP2/9 co-expression status (P < 0.05), and the association was of statistical significance in the ER/PR-positive patients (P < 0.01), but not in the ER- and PR-negative patients (P > 0.05). This phenomenon indicates that the regulation of MMP2 and MMP9 expression may occur in the same way, up regulated by local estrogen from autocrine mediated by ER. Previous studies (Abbas Abidi et al. 1997; Mitropoulou et al. 2003) supported this hypothesis; they found that aromatase inhibitor letrozole could suppress both MMPs expressions in MCF-7 cells, and that estrogen could stimulate their expression.
Evidence for the functional significance of aromatase was indicated by a correlation between aromatase activity and expression of proliferating cell nuclear antigen (PCNA) in the tumor (Brodie et al. 1997; Lu et al. 1996). The in situ production of estrogens may play an important role in the proliferation and growth of breast cancer and consistent with it, we found that there was a positive relationship between aromatase and the tumor size (P < 0.05). In the subgroup analysis, we found only in postmenopausal women a positive relationship between aromatase and tumor size, which was statistically significant (P < 0.05). This may suggest that in postmenopausal women, in situ estrogens mainly come through the aromatase pathway in an autocrine manner; as we know that in premenopausal women ovarian estrogens predominate in the circulation, but after menopause the relative proportion of estrogen synthesized in the extragonadal sites increases as ovarian function declines, and local synthesis of estrogens may contribute to breast cancer growth in postmenopausal women than in premenopausal women (Ellis et al. 2004). Bolufer’s study (Bolufer et al. 1992) was consistent with ours. They reported that there was a strong direct association between aromatase activity and tumor size in postmenopausal patients (P = 0.001).
As we know, aromatase is regarded as an important enzyme in the intra-tumor estrogen biosynthesis and can produce a local high level of estrogen sufficient to stimulate the proliferation of cancer cells (Lu et al. 1996). Results from both animal experiments (Brodie and Mouridsen 2003) and clinical trials (Buzdar 2003) showed that aromatase inhibitors are powerful inhibitors of estrogen synthesis and can significantly suppress the growth and recurrence of ER-positive breast cancers. So, the prognostic significance of aromatase was expected. However, only several studies on the prognostic significance of aromatase in breast cancer could be found in medical literature. Silva et al’s study showed that there was marginal inverse correlation between aromatase activity measured by biochemical assay and relapse-free survival (P < 0.1), and they further found in univariate analysis (Silva et al. 1989), a significant correlation between aromatase activity and survival of patients after relapse (P < 0.05). By RT-PCR, Eppenberger (Eppenberger et al. 2001) found in univariate survival analysis, women with their breast cancers expressing higher aromatase mRNA significantly correlated with increased risk of relapse and death (P < 0.05). However, also by RT-PCR, Miyoshi did not find the prognostic significance of the tumor aromatase mRNA level in the breast cancer specimen (P > 0.05) (Miyoshi et al. 2003). These differences may be due to the method’s disadvantage, as Lu’s study indicated that biochemical assay of aromatase activity may underestimate the true aromatase activity in the breast cancer microenvironment (Lu et al. 1996). The disadvantage of both biochemical assay and RT-PCR is that they just treat the tissue as a whole mass and provide no location information, while in the different types of cancers, there are different ratios of carcinoma cells to stoma cells and the result may be greatly influenced (Sasano et al. 2001; Shenton et al. 1998). Immunohistochemistry may overcome these disadvantages (Sasano et al. 2001). In our study, however, we did not find the significant association between aromatase immunoreactivity and DFS in univariate analysis (P > 0.05).
Hilsenbeck believed that substantial violation of the proportional hazards assumption may be a more common phenomenon than is currently appreciated in medical literature (Hilsenbeck et al. 1998). So, the verification of the proportional hazards assumption could better be performed before using the Cox proportional hazards regression analysis. The time-dependent Cox regression is suitable for analyzing the data if any violation of the proportional hazards assumption was found (Hilsenbeck et al. 1998). Our results indicated that hazard ratios of aromatase changed across time and was a prognostic factor independent of other clinical parameters (P < 0.05). As previous reports suggested, using Cox proportional regression, ignoring the violation of it, would lead us to underestimate the prognostic factor’s important effects when the follow-up time was long, or overestimate the strength of their prognostic relationship when time was short (Hilsenbeck et al. 1998). So, based on this study, we would underestimate the aromatase prognostic significance if we use the Cox proportional hazard regression in multivariate analysis.
Estrogen can enhance the abilities of proliferation and invasiveness of the ER-positive breast cancer cells (Brodie and Mouridsen 2003; Mitropoulou et al. 2003), so the clinical significance of aromatase in different ER status patients may be different. We observed that in the ER/PR-positive subgroup, the OS of patients with positive aromatase immunoreactivity was worse than that of patients without aromatase expression (P < 0.05); on the contrary in the ER and PR negative patients, there was not any association between aromatase expression and decreased OS (P > 0.05). These indicate that the ER/PR-positive breast cancers would be stimulated to progression and metastasis by local estrogens produced by cancer aromatase; however, this finding should be considered cautiously because of the limited number of events, and further studies are needed to evaluate this issue.
In summary, our data raise the possibility that in breast cancers, aromatase may enhance the invasive ability by up-regulating both MMP2 and MMP9 through the ER pathway and stimulate cancer growth especially in postmenopausal patients. The relapse hazards of aromatase-positive patients are changing across time and the prognostic significance will be lost in patients with long time follow-up, while tumor aromatase status may be indicative of breast cancer prognosis in some patients.
References
Abbas Abidi SM, Howard EW, Dmytryk JJ, Pento JT (1997) Differential influence of antiestrogens on the in vitro release of gelatinases (type IV collagenases) by invasive and non-invasive breast cancer cells. Clin Exp Metastasis 15:432–439
Bolufer P, Ricart E, Lluch A, Vazquez C, Rodriguez A, Ruiz A, Llopis F, Garcia-Conde J, Romero R (1992) Aromatase activity and estradiol in human breast cancer: its relationship to estradiol and epidermal growth factor receptors and to tumor-node-metastasis staging. J Clin Oncol 10:438–446
Brodie A, Long B, Lu Q (1998) Aromatase expression in the human breast. Breast Cancer Res Treat 49(Suppl 1):S85–S91, discussion S109–S119
Brodie A, Lu Q, Long B (1999) Aromatase and its inhibitors. J Steroid Biochem Mol Biol 69:205–210
Brodie A, Lu Q, Nakamura J (1997) Aromatase in the normal breast and breast cancer. J Steroid Biochem Mol Biol 61:281–286
Brodie AH, Mouridsen HT (2003) Applicability of the intratumor aromatase preclinical model to predict clinical trial results with endocrine therapy. Am J Clin Oncol 26:S17–S26
Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, DeJong PC, Blankenstein MA, Nortier JW, Slee PH, van de Ven J, van Gorp JM, Elbers JR, Schipper ME, Blijham GH, Thijssen JH (2001) Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol 79:41–47
Buzdar AU (2003) Advances in endocrine treatments for postmenopausal women with metastatic and early breast cancer. Oncologist 8:335–341
Dowsett M, Detre S, Rowlands M, Grimshaw R (1996) Oestrogen formation in breast: clinical and biological importance. J Endocrinol 150:S59–S63
Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N (2000) Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2:252–257
Ellis MJ, Hayes DF, Lippman ME (2004) Treatment of metastastatic breast cancer In: Harris Jr, Lippmen Me, Morrow M, Osborne Ck (eds) Disease of the breast. Lippincott Williams & Wilkins, Philadephia, pp 1101–1159
Elston CW, Ellis IO (2002) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991; 19:403–410. Histopathology 41:151
Eppenberger U, Levano S, Schoumacher F, Muller H, Eppenberger-Castori S (2001) Molecular epidemiology of aromatase expression in 1182 primary breast cancers. In: Miller W, Santen R (eds) Aromatase inhibition and breast cancer. Marcel Dekker, New York, pp 199–212
Esteban JM, Warsi Z, Haniu M, Hall P, Shively JE, Chen S (1992) Detection of intratumoral aromatase in breast carcinomas. An immunohistochemical study with clinicopathologic correlation. Am J Pathol 140:337–343
Evans TR, Rowlands MG, Silva MC, Law M, Coombes RC (1993) Prognostic significance of aromatase and estrone sulfatase enzymes in human breast cancer. J Steroid Biochem Mol Biol 44:583–587
Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE (1998) Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 90:1292–1299
Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM (1998) Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat 52:227–237
Hirvonen R, Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T (2003) Matrix metalloproteinase-2 (MMP-2) in T(1-2) no breast carcinoma. Breast Cancer Res Treat 77:85–91
Hsu SM, Raine L, Fanger H (1981) Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Iwata H, Kobayashi S, Iwase H, Masaoka A, Fujimoto N, Okada Y (1996) Production of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human breast carcinomas. Jpn J Cancer Res 87:602–611
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C (2003) Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95:1218–1226
Kinoshita Y, Chen S (2003) Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res 63:3546–3555
Kirma N, Gill K, Mandava U, Tekmal RR (2001) Overexpression of aromatase leads to hyperplasia and changes in the expression of genes involved in apoptosis, cell cycle, growth, and tumor suppressor functions in the mammary glands of transgenic mice. Cancer Res 61:1910–1918
Koyama H, Iwata H, Kuwabara Y, Iwase H, Kobayashi S, Fujii Y (2000) Gelatinolytic activity of matrix metalloproteinase-2 and–9 in oesophageal carcinoma; a study using in situ zymography. Eur J Cancer 36:2164–2170
Kute TE, Shao ZM, Sugg NK, Long RT, Russell GB, Case LD (1992) Cathepsin D as a prognostic indicator for node-negative breast cancer patients using both immunoassays and enzymatic assays. Cancer Res 52:5198–5203
Lu Q, Nakmura J, Savinov A, Yue W, Weisz J, Dabbs DJ, Wolz G, Brodie A (1996) Expression of aromatase protein and messenger ribonucleic acid in tumor epithelial cells and evidence of functional significance of locally produced estrogen in human breast cancers. Endocrinology 137:3061–3068
Mandava U, Kirma N, Tekmal RR (2001) Aromatase overexpression transgenic mice model: cell type specific expression and use of letrozole to abrogate mammary hyperplasia without affecting normal physiology. J Steroid Biochem Mol Biol 79:27–34
Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE (2004) Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst 96:1856–1865
Mitropoulou TN, Tzanakakis GN, Kletsas D, Kalofonos HP, Karamanos NK (2003) Letrozole as a potent inhibitor of cell proliferation and expression of metalloproteinases (MMP-2 and MMP-9) by human epithelial breast cancer cells. Int J Cancer 104:155–160
Miyoshi Y, Ando A, Hasegawa S, Ishitobi M, Taguchi T, Tamaki Y, Noguchi S (2003) High expression of steroid sulfatase mRNA predicts poor prognosis in patients with estrogen receptor-positive breast cancer. Clin Cancer Res 9:2288–2293
Pacheco MM, Nishimoto IN, Mourao Neto M, Mantovani EB, Brentani MM (2001) Prognostic significance of the combined expression of matrix metalloproteinase-9, urokinase type plasminogen activator and its receptor in breast cancer as measured by Northern blot analysis. Int J Biol Markers 16:62–68
Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C (1996) Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre–and postmenopausal breast cancer patients. J Clin Endocrinol Metab 81:1460–1464
Pasqualini JR, Chetrite GS (2005) Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol 93:221–236
Sasano H, Suzuki T, Moriya T (2001) Immunohistochemistry of aromatase: a recent new development. In: Miller W, Santen R (eds) Aromatase inhibition and breast cancer. Marcel Dekker, New York, pp 191–198
Shenton KC, Dowsett M, Lu Q, Brodie A, Sasano H, Sacks NP, Rowlands MG (1998) Comparison of biochemical aromatase activity with aromatase immunohistochemistry in human breast carcinomas. Breast Cancer Res Treat 49(Suppl 1):S101–S107, discussion S109–S119
Silva MC, Rowlands MG, Dowsett M, Gusterson B, McKinna JA, Fryatt I, Coombes RC (1989) Intratumoral aromatase as a prognostic factor in human breast carcinoma. Cancer Res 49:2588–2591
Talvensaari-Mattila A, Paakko P, Hoyhtya M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T (1998) Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer 83:1153–1162
Tekmal RR, Ramachandra N, Gubba S, Durgam VR, Mantione J, Toda K, Shizuta Y, Dillehay DL (1996) Overexpression of int-5/aromatase in mammary glands of transgenic mice results in the induction of hyperplasia and nuclear abnormalities. Cancer Res 56:3180–3185
Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, Groome NP, Sharpe RM, Fraser HM, Saunders PT (2002) Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol 172:21–30
van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH (1985) Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res 45:2900–2906
Wolczynski S, Surazynski A, Swiatecka J, Palka J (2001) Estrogenic and antiestrogenic effects of raloxifene on collagen metabolism in breast cancer MCF-7 cells. Gynecol Endocrinol 15:225–233
Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, Chen S (1996) Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J Steroid Biochem Mol Biol 59:163–171
Acknowledgments
Funded by Outstanding Young Investigator Award of National Natural Science Foundation of China (30025015), Natural Science Foundation of Shanghai, China (32R14021) and Science Research Foundation for Youths of Fudan University, Shanghai, China (EXF000312).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, J., Li, H., Cao, D. et al. Clinical significance of aromatase protein expression in axillary node negative breast cancer. J Cancer Res Clin Oncol 133, 401–409 (2007). https://doi.org/10.1007/s00432-006-0186-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-006-0186-5