Abstracts
Gastric and intestinal phenotypic cell markers are widely expressed in gastric carcinomas, irrespective of their histological type. In the present study, the relations between the phenotypic marker expression of the tumour, histological findings, expression of cell adhesion molecules, and the chromosomal changes in gastric differentiated-type carcinomas were examined. The phenotypic marker expression of the tumour was determined by the combination of the expression of the human gastric mucin (HGM), MUC6, MUC2 and CD10, and was evaluated in comparison with the expression of cell adhesion molecules, such as E-cadherin and β-catenin, and chromosomal changes by comparative genomic hybridization (CGH) in 34 gastric differentiated-type carcinomas. Tumours were classified into the gastric- (G-), gastric and intestinal mixed- (GI-), intestinal- (I-), or unclassified- (UC-) phenotype according to the immunopositivity of staining for HGM, MUC6, MUC2, and CD10. G-phenotype tumours were significantly associated with a higher incidence of differentiated-type tumours mixed with undifferentiated-type component, compared with GI- and I-phenotype tumours (88.9 vs 33.3%, P=0.0498 and 88.9 vs 42.9%, P=0.0397; respectively). HGM-positive tumours were significantly associated with a higher incidence of tumours with abnormal expression of E-cadherin, compared with HGM-negative tumours (66.7 vs 21.1%, P=0.0135). GI-phenotype tumours were significantly associated with a higher incidence of tumours with abnormal expression of E-cadherin, compared with I-phenotype tumours (77.8 vs 21.4%, P=0.0131). HGM-negative tumours were significantly associated with higher frequencies of the gains of 19q13.2 and 19q13.3, compared with HGM-positive tumours (57.9 vs 20.0%, P=0.0382 and 63.2 vs 13.3%, P=0.0051; respectively). MUC6-positive tumours were significantly associated with higher frequencies of the gains of 20q13.2, compared with MUC6-negative tumours (71.4 vs 30.0%, P=0.0349). MUC2-positive tumours were significantly associated with the gain of 19p13.3, compared with MUC2-negative tumours (41.2 vs 5.9%, P=0.0391). I-phenotype tumours were significantly associated with higher frequencies of gains of 5p15.2 and 13q33-34, compared with G-phenotype tumours (66.7 vs 0%, P=0.0481, each) and also associated with higher frequencies of gain of 7p21, compared with GI-phenotype tumours (66.7 vs 0%, P=0.0481). Our present results show that gastric differentiated-type carcinomas have different characteristics according to the phenotypic marker expression of the tumour in terms of histological findings, E-cadherin expression and pattern of chromosomal changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric carcinoma is histologically classified into two types, intestinal and diffuse, differentiated and undifferentiated, based on the gland formation tendency (Lauren 1965; Nakamura et al. 1968). With respect to the histogenesis of these two types of gastric carcinoma, differentiated-type tumours have generally been considered to arise from the gastric mucosa with intestinal metaplasia and undifferentiated-type tumours from the ordinary gastric mucosa without intestinal metaplasia, and the two are considered to follow different genetic pathways during carcinogenesis (Nakamura et al. 1968; Lauren 1965; Saito and Shimada 1986; Tahara 1995). However, immunohistochemical examinations have recently demonstrated that gastric and intestinal phenotypic cell markers are widely expressed in gastric carcinomas, irrespective of their histological type (Tatematsu et al. 1986, 1990, 2003; Egashira et al. 1999; Sasaki et al. 1999; Endoh et al. 1999a, 1999b; Machado et al. 2000; Koseki et al. 2000). Several authors have reported that gastric carcinomas can be classified as having either a gastric- (G-), gastric and intestinal mixed-(GI-) or intestinal- (I-) phenotype, depending on the immunopositivity to staining with human gastric mucin (HGM), MUC6, MUC2 and CD10 (Tajima et al. 2001; Kabashima et al. 2002). Human gastric mucin, MUC6, MUC2 and CD10 are specifically expressed in gastric surface mucous cells, pyloric gland cells, intestinal goblet cells of the mature gastrointestinal tract and brush border of intestinal epithelial cells, respectively. Previous reports showed that G-phenotype tumours accounted for 27.7% of differentiated tumours, often referred to as intestinal-type tumours, according to Lauren (1965), while I-phenotype tumours accounted for 10.1% of undifferentiated tumours (Tajima et al. 2001). The phenotypic marker expression of the tumour is conventionally thought to imitate that of the tissue of origin. It is thus suggested that gastric carcinomas can occur in various types of gastric mucosa. With respect to the clinicopathological significance of the phenotypic marker expression of the tumour, differentiated-type gastric carcinomas of the G-phenotype are more likely to transform into the undifferentiated-type carcinoma and show infiltrative growth to deeper layers of the mucosa or invasion of the surrounding structures through loss of E-cadherin function as compared with those of the I-phenotype (Endoh et al. 1999b; Saito et al. 2001; Tajima et al. 2001). This histological transformation was suggested to occur relatively in the early stage of gastric carcinogenesis. Recently, G-phenotype tumours have been associated with a poorer outcome and greater malignant potential in the incipient phase of invasion and metastasis, compared with tumours of other phenotype (Endoh et al. 1999b; Koseki et al. 2000; Saito et al. 2001; Tajima et al. 2001; Kabashima et al. 2002; Shibata et al. 2003). Phenotypic marker expression in gastric neoplasms has also been suggested to depend on genetic changes (Endoh et al. 2000). However, there are conflicting opinions regarding the relations between the phenotypic marker expression of the tumour and genetic alterations in gastric differentiated-type carcinomas (Shibata et al. 2003) (Fig. 1).
a Human gastric mucin is expressed in the cancer cell cytoplasm (45MI, original magnification ×100). b MUC6 glycoprotein is also expressed in the cancer cell cytoplasm (CLH5, original magnification ×100). c MUC2 glycoprotein is expressed in the cancer cell cytoplasm (Ccp58, original magnification ×100). d CD10 glycoprotein is also expressed on the luminal surfaces of cancerous glands (56C6, original magnification ×100)
Comparative Genomic Hybridization (CGH) is an exceptionally useful method that can simultaneously detect gains or losses in the genetic copy number in tumour DNA with one round of hybridization, and can map those regions on to all the chromosomes (Kallioniemi et al. 1992). With CGH, it is possible to detect chromosomal gains and losses in tumours, including those that were hitherto unknown. Since it was first developed, CGH has been applied to study genetic alterations in many solid tumours (Kokkola et al. 1997; Nessling et al. 1998; Sakakura et al. 1999). However, there are only a few reports about chromosomal changes according to the phenotypic marker expression of tumours using comparative genomic hybridization (Kushima et al. 2003).
In the present study, the relations between the phenotypic tumour cell marker expression, histological findings, expression of cell adhesion molecule, such as E-cadherin and β-catenin, and chromosomal changes in gastric differentiated-type carcinomas were examined (Fig. 2).
Materials and methods
Patients
The materials consisted of clinical specimens obtained from 34 cases of gastric differentiated-type carcinoma who had undergone surgery between January 2000 and March 2004 at the Department of Surgery, Division of General and Gastroenterological Surgery, Showa University, School of Medicine. Of the 34 patients, 26 were men and 8 were women. The mean age of the patients was 71 years (range, 52–88 years). No preoperative radio- and/or chemotherapy had been administered.
Clinicopathological review
Serial 5-mm-thick tissue sections of the entire tumour were prepared from the resected specimens fixed with 10% buffered formalin, embedded in paraffin and then 4°μm consecutive sections were used for histologic examination by haematoxylin and eosin (H&E) staining and immunohistochemical staining. All the tumours were classified according to the criteria established by The Japanese Classification of Gastric Carcinoma (1998). In the present study, the tumours were classified histologically into 16 pure differentiated-type carcinomas and 18 mixed (with undifferentiated-type component)-type carcinomas. The clinical data of the patients are summarized in Table 1.
Immunohistochemical staining for analysis of the tumour differentiation phenotype and adhesion molecules
Sections in which the tumour showed the greatest depth of cancer invasion were subjected to immunohistochemical staining. The following mouse monoclonal antibodies were used: 45M1 (Novocastra Laboratories Ltd, UK), diluted 1:50, to detect HGM; CLH5 (Novocastra Laboratories Ltd), diluted 1:50, to detect MUC6 glycoprotein; Ccp58 (Novocastra Laboratories Ltd), diluted 1:100, to detect MUC2 glycoprotein; and 56C6 (Novocastra Laboratories Ltd), diluted 1:40, to detect CD10 glycoprotein expression. 45M1 and CLH5 were examined as G-phenotype markers, and Ccp58 and 56C6 were examined as I-phenotype markers. 45M1 recognizes the mucin epitope located in the peptide core of HGM, which is synonymous with MUC5AC. This antibody is known to react with surface foveolar cells in the stomach (Bara et al. 1998; Nollet et al. 2002). MUC6 glycoprotein is expressed in mucous cells of the neck zone of the oxyntic mucosa and in antral glands (De Bolos et al. 1995; Reis et al. 1999; Machado et al. 2000). MUC2 glycoprotein, also known as the ‘intestinal-mucin-related protein antigen’, is an intestinal apomucin and also known to be expressed in the supranuclear area of the goblet cells in regions showing intestinal metaplasia in the stomach (Kim and Gum 1995; Sakamoto et al. 1997; Baldus et al. 1998; Utsunomiya et al. 1998; Reis et al. 1999; Machado et al. 2000). CD10 glycoprotein is a 100-kDa cell metalloendopeptidase that inactivates a variety of biologically active peptides and is known to be expressed on the brush border of intestinal epithelial cells as well as in the germinal centres of lymphoid follicles and the microvilli of the kidney (Ronco et al. 1984; Trejdosiewicz et al. 1985). The avidin-biotinyl-peroxidase complex immunohistochemical method was used for all immunohistochemical studies, according to a previously described protocol (Hsu et al. 1981).
With regard to the evaluations of HGM, MUC6, MUC2 and CD10 staining, distinct staining in more than 5% of the tumour cells was recorded as positive immunoreactivity for the relevant marker. These immunohistochemical methods were used to classify the tumours into four different phenotypes: tumours with G-phenotypic cells accounting for more than 5% of their cell population were classified as G-phenotype carcinomas; those with I-phenotypic cells accounting for more than 5% of their cell population were classified as I-phenotype carcinomas; those with both gastric and intestinal phenotypic cells accounting for more than 5% of their cell population were classified as GI-phenotype carcinomas; and those with both gastric and intestinal phenotypic cells accounting for less than 5% of their cell population were regarded as carcinomas of the UC-phenotype (Tajima et al. 2004).
The monoclonal antihuman E-cadherin antibody (HECD-1) (Takara Shuzo, Kyoto, Japan), diluted 1:10,000, monoclonal anti-β-catenin antibody (BD Biosciences, NJ, USA), diluted 1:500, and DAKO envision kits (Dako Cytomation, Copenhagen, Denmark) were also used. The avidin-biotinyl-peroxidase complex immunohistochemical method was used for all immunohistochemical studies, according to a previously described protocol (Hsu et al. 1981). The HECD-1 and anti-β-catenin staining was scored in a semiquantitative fashion from 0 to 3, with 0 denoting absent staining, 1 representing cytoplasmic distribution, 2 representing heterogeneous staining (i.e. when tumours were composed of both normal and abnormally stained areas), and 3 representing the normal membranous pattern of staining. Tumours with more than 10% variation were rated as showing heterogeneous staining. For the purpose of data analysis, all tumours showing loss of the membranous pattern of staining were classified as abnormal, including those with absent, heterogeneous and cytoplasmic patterns of staining (i.e. those with scores 0, 1 and 2) (Jawhari et al. 1997).
The histopathological and immunohistochemical examinations were independently performed by two observers (Morohara and Tajima). The results were then compared, and any discrepancies were resolved by consensus after further histopathological review.
CGH
The method used for the CGH analysis is described below (Nakao et al. 2001).
Laser microdissection and DNA extraction
The tumour samples obtained from the invasive area of the tumour were embedded in OCT and snap-frozen at −80°C. The samples were sectioned into 7 μm-thick sections and fixed in 100% methanol for 3 min, followed by toluidine blue staining. Then, after being air-dried, the sections were laser-microdissected using the PALM. Laser Microdissection System (PALM Microlaser Technologies AG, Germany) for procuring the cancer cells.
The microdissected tumour tissues were homogenized in digestion buffer [100 mM Tris, 15 mM MgCl2, 500 mM KCl, 0.5% tween 20, proteinase K (1 mg/ml; WAKO, Japan) and incubated overnight at 50°C, with shaking. DNA was extracted using the phenol-chloroform-isoamyl alcohol method. After centrifuging the samples at 14,000 rpm for 5 min, the DNA in the aqueous phase was collected and precipitated with 2.5 volumes of 100% ethanol after the addition of 7.5 M ammonium acetate at half the volume. The DNA was pelleted by centrifugation at 14,000 rpm for 30 min. The pellet was then washed in 70% ethanol and centrifuged at 14,000 rpm for 5 min to separate the supernatant. The DNA was then dissolved in TE buffer (10 mM Tris, 1 mM ethylenediamine tetraacetic acid) and weighed.
GenomiPhi
The GenomiPhi DNA Amplification Kit (Amersham Biosciences Corp., New Jersey, USA), which amplifies linear genomic DNA in a novel fashion, was developed for in vitro cloning, library construction, and other molecular biological applications (Dean et al. 2001). The method utilizes bacteriophage Phi29 DNA polymerase to exponentially amplify single- or double-stranded linear DNA templates during isothermal conditions (30°C); no strand displacement reaction thermal cycling is required. DNA is generated in microgram quantities after overnight incubation of nanogram amounts of starting material. DNA replication is extremely accurate because of the proofreading activity of Phi29 DNA polymerase. In brief, 1 μl of a template to be amplified is added to 9 μl of sample buffer and the mixture is heated to 95°C for 3 min to denature the template DNA. The sample is then cooled and mixed with 9 μl of reaction buffer and 1 μl of enzyme mix, before overnight (18 h) incubation at 30°C. After amplification, Phi29 DNA polymerase is heat-inactivated by 10 min incubation at 65°C (Morohara et al. 2005).
Labelled DNA and hybridization
DNA from tumour samples was labelled with Spectrum-Green (Vysis Inc., Chicago, IL, USA), and normal male DNA was labelled with Spectrum-Red (Vysis Inc.) using the nick-translation method. Labelled tumour DNA (400 ng) and normal DNA (200 ng), together with 10 μg of unlabelled Cot-1 DNA (Vysis Inc.) were collected and precipitated with 2.5 volumes of 100% ethanol after the addition of 3 M sodium acetate at 1/10 the volume. The DNA was pelleted by centrifugation at 14,000 rpm for 30 min. The pellet was then washed in 70% ethanol and centrifuged at 14,000 rpm for 5 min to separate the supernatant. The pellet was resuspended with 7 μL of CGH hybridization buffer and 3 μL of purified H2O, denatured at 73°C for 5 min, and then applied to normal lymphocyte metaphase preparations. Prior to hybridization, the metaphase preparations were denatured at 73°C for 5 min in a denaturation solution (70% formamide and 2× standard saline citrate [SSC], pH7), dehydrated in a graded series of ethanol (70, 85 and 100%), and placed on a 37°C slide warmer. The hybridization was conducted at 37°C for 48 h. After hybridization, the slides were washed thrice in 50% formamide/2 × SSC, pH7, once in 2 × SSC at 45°C followed by PN buffer [0.1 M NaH2PO4, 0.1 M Na2HPO4, 0.1% Nonidet P-40], and finally in distilled water at room temperature, each for 5 min. Then, after being air-dried, the slides were counterstained with 4′,6-diamidino-2-phenylindole in an antifade solution (Vysis Inc.), and a coverslip was placed on each hybridization location.
Digital image analysis
Three single-colour images (matching 4′,6-diamidino-2-phenylindole, fluorescein isothiocyanate, and Texas red fluorescence) were collected from each metaphase spread using a cooled charge-coupled device (CCD) camera (KX85, APOGEE, USA). Relative DNA sequence copy number changes were detected by analysing the hybridization intensities of tumour DNA and normal DNA along the length of all the chromosomes in the metaphase spread, as described previously. Each chromosome was analysed using FISH-CGH for Optimas (Optimas Corporation). The absolute fluorescence intensities were normalized so that the average ratio of green to red fluorescence for all chromosome objects in each metaphase was 1:0. The green-to-red ratio was calculated in each image. A ratio <0.8 was evaluated as a loss, whereas a ratio >1.2 was regarded as a gain in the tumour genome. Amplification were defined by a ratio >1.4.
Statistical analysis
The data were analysed with Student’s t test, and the χ 2 test or Fisher’s exact test. Differences with a value of P<0.05 were considered to be significant.
Results
Relations between histological-type and the phenotypic marker expression of the tumour
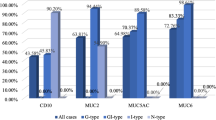
The relations between histological-type and the phenotypic marker expression of the tumour are shown in Table 2. HGM-negative, HGM-positive, MUC6-negative, MUC6-positive, MUC2-negative, MUC2-positive, CD10-negative and CD10-positive tumours composed 57.9, 33.3, 55.0, 35.7, 41.2, 52.9, 42.1 and 53.3% of differentiated-type tumours, respectively; and 42.1, 66.7, 45.0, 64.3, 58.8, 47.1, 57.9 and 46.7% of mixed-type tumours, respectively. There was no significant association between the histological-type tumours and the expressions of HGM, MUC6, MUC2 or CD10. The G-, GI-, I-, and UC-phenotype tumours composed 11.1, 66.7, 57.1 and 50.0% of differentiated-type tumours, respectively; and 88.9, 33.3, 42.9 and 50.0% of mixed-type tumours, respectively. G-phenotype tumours were significantly associated with a higher incidence of mixed-type tumours, compared with the GI- and I-phenotype (P=0.0498 and P=0.0397).
Relations between expressions of E-cadherin and β-catenin and the phenotypic marker expression of the tumour
The relations between expressions of E-cadherin and β-catenin and the phenotypic marker expression of the tumour are shown in Table 3. Abnormal expressions of E-cadherin in HGM-negative, HGM-positive, MUC6-negative, MUC6-positive, MUC2-negative, MUC2-positive, CD10-negative and CD10-positive tumours were observed in 21.1, 66.7, 35.0, 50.0, 29.4, 52.9, 42.1 and 40.0%, respectively. The expression of HGM was significantly associated with a higher incidence of tumours with abnormal expressions of E-cadherin (P=0.0135). Abnormal expression of β-catenin in HGM-negative, HGM-positive, MUC6-negative, MUC6-positive, MUC2-negative, MUC2-positive, CD10-negative and CD10-positive tumours were observed in 47.4, 26.7, 45.0, 28.6, 35.3, 41.2, 26.3 and 53.3%, respectively. There was no significant association between the abnormal expression of β-catenin and expressions of HGM, MUC6, MUC2 or CD10. Abnormal expressions of E-cadherin in G-, GI-, I-, and UC-phenotype tumours were observed in 33.3, 77.8, 21.4 and 50.0%, respectively. GI-phenotype tumours were significantly associated with a higher incidence of tumours with abnormal expression of E-cadherin, compared with I-phenotype tumours (P=0.0131). Abnormal expressions of β-catenin in G-, GI-, I-, and UC-phenotype tumours were observed in 22.2, 44.4, 42.9 and 50.0%, respectively. There was no significant association between the abnormal expression of β-catenin and the phenotypic marker expression pattern of the tumour.
Chromosomal changes in 34 gastric carcinomas
The summary of CGH results in 34 gastric cancers is shown in Table 4. The most frequently gained chromosomal regions in 34 gastric cancers (>40% of all tumours) were 6p (52.9%), 7p (55.9%), 7q (55.9%), 8q (61.8%), 12q (44.1%), 13q (47.1%), 16p (44.1%), 17q (50.0%), 19q (64.7%), 20p (44.1%) and 20q (70.6%); The most frequently lost (>20% of all tumours) chromosomal regions were 4q (23.5%) and 18q (34.3%). With respect to the relations between chromosomal changes and the phenotypic marker expression of the tumour, significant differences were found in gains of 5q (CD10-negative vs CD10-positive, P=0.0282), 18q (CD10-negative vs CD10-positive, P=0.0113) and loss of 18q (MUC6-negative vs MUC6-positive, P=0.0311). With respect to the relations between chromosomal changes and the phenotypic marker expression pattern of the tumour, I-phenotype tumours were significantly associated with the gain of 20p, compared with G-phenotype tumours (P=0.0288).
Relations between minimal overlapping regions of recurrent DNA copy number changes and the phenotypic marker expression of the tumour
The relations between the minimal overlapping regions of recurrent DNA copy number changes and the expression of HGM, MUC6, MUC2 and CD10 are shown in Table 5. HGM-negative tumours were significantly associated with higher frequencies of the gains of 19q13.2 and 19q13.3, compared with HGM-positive tumours (P=0.0382 and P=0.0051). MUC6-positive tumours were significantly associated with a higher frequency of the gain of 20q13.2, compared with MUC6-negative tumours (P=0.0349). MUC2-positive tumours were significantly associated with a higher frequency of the gain of 19p13.3, compared with MUC2-negative tumours (P=0.0391). There was no significant difference between the expression of CD10 and DNA copy number changes. The relations between minimal overlapping regions of recurrent DNA copy number changes and the phenotypic marker expression pattern of the tumour are shown in Table 6. I-phenotype tumours were significantly associated with higher frequencies of gains of 5p15.2 and 13q33-34, compared with G-phenotype tumours (P=0.0481, each) and also associated with higher frequencies of gain of 7p21, compared with GI-phenotype tumours (P=0.0481) (Fig. 3).
DNA copy number alterations in chromosomes 19 and 20 analysed by CGH in 34 gastric carcinomas. The regions of DNA copy number gains and losses are shown as the bars on the right and left sides, respectively. The thick bars indicate amplifications. HGM-negative tumours are significantly associated with higher frequencies of the gains of 19q13.2 and 19q13.3, compared with HGM-positive tumours. MUC6-positive tumours are significantly associated with a higher frequency of the gain of 20q13.2, compared with MUC6-negative tumours. MUC2-positive tumours are significantly associated with a higher frequency of the gain of 19p13.3, compared with MUC2-negative tumours
Discussion
E-cadherin is the major cadherin molecule expressed by epithelial cells (Takeichi 1991). Cadherin forms complexes with cytoplasmic proteins, α-catenin, β-catenin and plakoglobin. Structural abnormalities of E-cadherin and catenin caused by the loss of gene function have been shown to disrupt E-cadherin-mediated intercellular adhesion, causing loose cell-to-cell adhesion in tumour cells (Oda et al. 1994; Oyama et al. 1994). In fact, previous reports showed that the loss or reduced expression of E-cadherin and β-catenin were frequently found in undifferentiated-type gastric carcinomas (Ochiai et al. 1994). However, the abnormal expression of E-cadherin has also been seen in differentiated-type gastric carcinomas. Koseki et al. (2000) detected the abnormal expression of E-cadherin in 26.7% of differentiated-type gastric carcinomas in the early stage. They found abnormal E-cadherin expression more frequently in G-phenotype tumours than in I-phenotype tumours. Furthermore, Endoh et al. (1999b) detected an E-cadherin gene mutation in 21% of early differentiated-type carcinomas of the G-phenotype. It has been suggested that differentiated-type G-phenotype carcinomas are more likely to transform into the undifferentiated-type carcinoma in the relatively early stage of gastric carcinogenesis with progression through loss of E-cadherin function as compared with those of the I-phenotype (Endoh et al. 1999b). In the present study of advanced stage carcinomas, G-phenotype tumours were significantly associated with a higher incidence of mixed-type tumours, as compared with GI- and I-phenotype tumours. HGM-expression was significantly associated with abnormal expression of E-cadherin. GI-phenotype tumours were also significantly associated with a higher incidence of tumours with abnormal expression of E-cadherin, as compared with I-phenotype tumours. Mixed-type tumours have been considered to represent a progressive loss of glandular structure and a histological transformation from differentiated- to undifferentiated-type carcinoma during the progression of the tumour. Therefore, our present results support the previous findings that the phenotypic marker expression of the tumour is associated with histological transformation from differentiated- to undifferentiated-type carcinoma and abnormal expression of E-cadherin in gastric differentiated-type carcinomas.
In the present study, we confirmed previous reports of chromosomal gains of 7q, 8q, 17q, 20p and 20q, and losses of 4q and 18q as frequent events in gastric carcinomas (Kokkola et al. 1997; El-Rifai et al. 1998; Sakakura et al. 1999; van Dekken et al. 1999; Nessling et al. 1998; Vidgren et al. 1999; Knuutila et al. 2000; Noguchi et al. 2001; Wu et al. 2002; Peng et al. 2003). Koizumi et al. (1997) reported that gains of 3q27, 6q23-q25, and 7cen-p14 and losses of 1p34.2-35 and 17p12 were predominantly observed in the differentiated-type, and gains of 2p23-pter, 9p22-pter, and 13q31-qter and a loss of 6p21.3 were predominantly observed in the undifferentiated-type. They suggested that these changes, predominantly observed for their histological type, might be associated with the cell growth and differentiation of gastric carcinomas (Koizumi et al. 1997). Kokkola et al. (1997) reported that the most common gains in the differentiated-type gastric carcinomas were found at 20pq, 8q and 17q, with the minimal common regions at 20q, 8q21.3-23 and 17q12-21, and the most common losses involved 18q and 4q. They also showed that the most frequent gains were at 20q and 6p in the undifferentiated-type of gastric carcinomas. Furthermore, high-level amplifications were detected at 2p, 17q, 18q and 20q in the differentiated type and on 13q in the undifferentiated type. However, the relation between chromosomal changes and phenotypic marker expression of the tumour in gastric differentiated-type carcinomas remains unclear. In the present study, HGM-negative tumours were significantly associated with higher frequencies of gains of 19q13.2 and 19q13.3, compared with HGM-positive tumours. MUC6-positive tumours were significantly associated with a higher frequency of gain of 20q13.2, compared with MUC6-negative tumours. MUC2-positive tumours were significantly associated with a higher frequency of gain of 19p13.3, compared with MUC2-negative tumours. Furthermore, I-phenotype tumours were significantly associated with higher frequencies of gains of 5p15.2, 7p21 and 13q33-34, compared with G- and GI-phenotype tumours. Therefore, the findings in our present study suggest that the phenotypic marker expression of the tumour is related to chromosomal changes detected by CGH in gastric differentiated-type carcinomas.
Obvious differences in the biological behaviour of tumours with different phenotypic marker expressions have been reported. Kabashima et al. (2002) reported that G-phenotype tumours could potentially degrade the extracellular matrix through the overexpression of matrix metalloproteinases, compared with I-phenotype tumours. Shibata et al. (2003) reported that the apoptotic index/proliferative index ratio was significantly lower in G-phenotype tumours than in I-phenotype tumours. Tajima et al. (2001) previously reported that patients with G-phenotype tumours have a poorer prognosis than those with I-phenotype tumours among patients with advanced gastric carcinoma. We recently reported that the majority of peritoneal recurrences after surgery for gastric carcinoma occurred in G-phenotype tumours, especially HGM-positive tumours, while haematogenous recurrence occurred more frequently in MUC2-negative tumours and CD10-positive tumours (Tajima et al. 2004). These differences in the biological behaviour of tumours with different phenotypic marker expressions suggest the action of different genetic alterations, depending on the phenotypic marker expression of the tumour. Several studies have shown significant correlation between microsatellite instability and gastric foveolar phenotypic expression in the stomach cancer (Endoh et al. 2000a, 2000b; Takahashi et al. 2002). Tamura et al. (1995) have shown that the majority of differentiated adenocarcinomas of the stomach may develop through a de novo pathway from the viewpoint of the microsatellite alterations. In the present study, we demonstrated that gastric differentiated-type carcinomas had different characteristics according to the phenotypic marker expression in terms of not only histological findings and E-cadherin expression but also in the pattern of chromosomal changes detected by CGH. Therefore, there could be a possibility of unknown gene groups being present in the regions of these genes associated with the phenotypic marker expression of the tumour. Previous molecular genetic studies have shown that gastric tumourigenesis is a multistep process with an accumulation of genetic alterations (Stadtlander and Waterbor 1999; Tahara et al. 1996). Therefore, the findings in our present study suggest that different genetic pathways, according to the phenotypic marker expression of the tumour, could exist in the tumourigenesis of the gastric differentiated-type carcinoma, leading to their different biological behaviour (Fig. 4).
DNA copy number alterations in chromosomes 5, 7 and 13 analysed by CGH in 34 gastric carcinomas. The regions of DNA copy number gains and losses are shown as the bars on the right and left sides, respectively. The thick bars indicate amplifications. I-phenotype tumours are significantly associated with higher frequencies of gains of 5p15.2 and 13q33-34, compared with G-phenotype tumours and also associated with higher frequencies of gain of 7p21, compared with GI-phenotype tumours
In conclusion, our present results show that gastric differentiated-type carcinomas have different characteristics according to the phenotypic marker expression of the tumour in terms of histological findings, E-cadherin expression and pattern of chromosomal changes.
References
Baldus SE, Zirbes TK, Engel S, Hanisch FG, Monig SP, Lorenzen J, Glossmann J, Fromm S, Thiele J, Pichlmaier H, Dienes HP (1998) Correlations of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis. Int J Cancer 79:133–138
Bara J, Chastre E, Mahiou J, Singh RL, Forgue-Lafitte ME, Hollande E, Godeau F (1998) Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer 75:767–773
Dean FB, Nelson JR, Giesler TL et al (2001) Rapid amplification of plasmid and phage DNA using Phi29 DNA polymerase and multiply-primed rolling circle amplification. Genome Res 11:1095–1099
De Bolos C, Garrido M, Real FX (1995) MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology 109:723–734
Egashira Y, Shimoda T, Ikegami M (1999) Mucin histochemical analysis of minute gastric differentiated adenocarcinoma. Pathol Int 49:55–61
El-Rifai W, Harper JC, Cummings OW, Hyytinen ER, Frierson HF, Knuutila S, Powell SM (1998) Consistent genetic alterations in xenografts of proximal stomach and gastro-esophageal junction adenocarcinomas. Cancer Res 58:34–37
Endoh Y, Tamura G, Motoyama T, Ajioka Y, Watanabe H (1999a) Well-differentiated adenocarcinoma mimicking complete-type intestinal metaplasia in the stomach. Hum Pathol 30:826–832
Endoh Y, Tamura G, Watanabe H, Ajioka Y, Motoyama T (1999b) The common 18-base pair deletion at codons 418–423 of the E-cadherin gene in differentiated-type adenocarcinomas and intramucosal precancerous lesions of the stomach with the features of gastric foveolar epithelium. J Pathol 189:201–206
Endoh Y, Sakata K, Tamura G, Ohmura K, Ajioka Y, Watanabe H, Motoyama T (2000a) Cellular phenotypes of differentiated-type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol 191:257–263
Endoh Y, Tamura G, Ajioka Y, Watanabe H, Motoyama T (2000b) Frequent hypermethylation of the hMLH1 gene promoter in differentiated-type tumours of the stomach with the gastric foveolar phenotype. Am J Pathol 157:717–22
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma-2nd English ed. Gastric Cancer 1:10–24
Jawhari A, Jordan S, Poole S, Browne P, Pignatelli M, Farthing MJG (1997) Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology 112:46–54
Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M (2002) Relationship between biologic behaviour and phenotypic expression in intramucosal gastric carcinomas. Hum Pathol 33:80–86
Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D (1992) Comparative genomic hybridization for molecular cytogenetic analysis of solid tumours. Science 258:818–821
Kim YS, Gum JR (1995) Diversity of mucin genes, structure, function and expression. Gastroenterology 109:999–1013
Knuutila S, Autio K, Aalto Y (2000) Online access to CGH data of DNA sequence copy number changes. Am J Pathol 157:689
Kokkola A, Monni O, Puolakkainen P, Larramendy ML, Victorzon M, Nordling S, Haapiainen R, Kivilaakso E, Knuutila S (1997) 17q12–21 amplicon, a novel recurrent genetic change in intestinal type of gastric carcinoma: a comparative genomic hybridization study. Genes Chromosomes Cancer 20:38–43
KoizumiY, Tanaka S, Mou R, Koganei H, Kokawa A, Kitamura R, Yamauchi H, Ookubo K, Saito T, Tominaga S, Matsumura K, Shimada H, Tsuchida N, Sekihara H (1997) Changes in DNA copy number in primary gastric carcinomas by comparative genomic hybridization. Clin Cancer Res 3:1067–1076
Koseki K, Takizawa T, Koike M, Ito M, Nihei Z, Sugihara K (2000) Distinction of differentiated type early gastric carcinoma with gastric type mucin expression. Cancer 89:724–732
Kushima R, Mukaisho K, Tsukashita S, Peng DF, Sugihara H, Vieth M, Hattori T, Stolte M (2003) Molecular biological characteristics of early stomach adenocarcinomas of the completely gastric phenotype revealed by laser capture microdissection and comparative genomic hybridization (in Japanese with English abstract). Stomach and Intestine 38:707–721
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 64:122–129
Machado JC, Nogueira AM, Carneiro F, Reis CA, Sobrinho-Simoes M (2000) Gastric carcinoma exhibits distinct types of cell differentiation: an immunohistochemical study of trefoil peptides (TFF1 and TFF2) and mucins (MUC1, MUC2, MUC5AC, and MUC6). J Pathol 190:437–443
Morohara K, Nakao K, Tajima Y, Nishino N, Yamazaki K, Kaetsu T, Suzuki S, Tsunoda A, Kawamura M, Aida T, Tachikawa T, Kusano M (2005) Analysis by comparative genomic hybridization of gastric cancer with peritoneal dissemination and/or positive peritoneal cytology. Cancer Genet Cytogenet 161:57–62
Nakamura K, Sugano H, Takagi K (1968) Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann 59:251–258
Nakao K, Shibusawa M, Ishihara A, Yoshizawa H, Tsunoda A, Kusano M, Kurose A, Makita T, Sasaki K (2001) Genomic changes in colorectal carcinoma tumours with liver metastases analyzed by comparative genomic hybridization and DNA ploidy. Cancer 91:721–726
Nessling M, Solinas-Toldo S, Wilgenbus KK, Borchard F, Lichter P (1998) Mapping of chromosomal imbalances in gastric adenocarcinoma revealed amplified protooncogenes MYCC, MET, WNT2, and ERBB2. Genes Chromosomes Cancer 23:307–316
Noguchi T, Wirtz HC, Michaelis S, Gabbert HE, Mueller W (2001) Chromosomal imbalances in gastric cancer. Am J Clin Pathol 115:828–834
Nollet S, Forgue-Lafitte ME, Kirkham P, Bara J (2002) Mapping of two new epitopes on the apomucin encoded by MUC5AC gene: expression in normal GI tract and colon tumours. Int J Cancer 99:336–343
Ochiai A, Akimoto S, Shimoyama Y, Nagafuchi A, Tsukita S, Hirohashi S (1994) Frequent loss of α-catenin expression in scirrhous carcinomas with scattered cell growth. Jpn J Cancer Res 85:266–273
Oda T, Kanai Y, Oyama T, Yoshioka K, Shinoyama Y, Birchmeier W, Sugimura T, Hirohashi S (1994) E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA 91:1858–1862
Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukita S, Shibamoto S, Ito F (1994) A truncated β-catenin disrupts the interaction between E-cadherin and α-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res 54:6282–6287
Reis CA, David L, Correa P, Carneiro F, De Bolos C, Garcia E, Mandel U, Clausen H, Sobrinho-Simoes M (1999) Intestinal metaplasia of human stomach displays distinct pattern of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res 59:1003–1007
Ronco P, Allegri L, Melcion C, Pirotsky E, Appay MD, Bariety J, Pontillon F, Verroust P (1984) A monoclonal antibody to brush border and passive nephritis. Clin Exp Immunol 55:319–332
Peng DF, Sugihara H, Mukaisho K, Tsubosa Y, Hattori T (2003) Alterations of chromosomal copy number during progression of diffuse-type gastric carcinomas: metaphase- and array-based comparative genomic hybridization analyses of multiple samples from individual tumours. J Pathol 201:439–450
Saito K, Shimada T (1986) Histogenesis and early invasion of gastric cancer. Acta Pathol Jpn 36:1307–1318
Saito A, Shimoda T, Nakanishi Y, Ochiai A, Toda G (2001) Histologic heterogeneity and mucin phenotypic expression in early gastric cancer. Pathol Int 51:165–171
Sakakura C, Mori T, Sakabe T, Ariyama Y, Shinomiya T, Date K, Hagiwara A, Yamaguchi T, Takahashi T, Nakamura Y, Abe T, Inazawa J (1999) Gains, losses, and amplifications of genomic materials in primary gastric cancers analyzed by comparative genomic hybridization. Genes Chromosomes Cancer 24:299–305
Sasaki I, Yao T, Nawata H, Tsuneyoshi M (1999) Minute gastric carcinoma of differentiated type with special reference to the significance of intestinal metaplasia, proliferative zone, and p53 protein during tumour development. Cancer 85:1719–1729
Sakamoto H, Yonezawa S, Utsunomiya T, Tanaka S, Kim YS, Sato E (1997) Mucin antigen expression in gastric carcinomas of young and old adults. Hum Pathol 28:1056–1065
Shibata N, Watari J, Fujiya M, Tanno S, Saitoh Y, Kohgo Y (2003) Cell kinetics and genetic instabilities in differentiated type early gastric cancers with different mucin phenotype. Hum Pathol 34:32–40
Stadtlander CT, Waterbor JW (1999) Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis 20:2195–2208
Tahara E (1995) Genetic alterations in human gastrointestinal cancers. Cancer 75:1410–1417
Tahara E, Semba S, Tahara H (1996) Molecular biological observations in gastric cancer. Semin Oncol 23:307–315
Tajima Y, Shimoda T, Nakanishi Y, Yokoyama N, Tanaka T, Shimizu K, Saito T, Kawamura M, Kusano M, Kumagai K (2001) Gastric and intestinal phenotypic marker expression in gastric carcinomas and their prognostic significance: immunohistochemical analysis of 136 lesions. Oncology 61:212–220
Tajima Y, Yamazaki K, Nishino N, Morohara K, Yamazaki T, Kaetsu T, Suzuki S, Kawamura M, Kumagai K, Kusano M (2004) Gastric and intestinal phenotypic marker expression in gastric carcinomas and recurrence pattern after surgery–immunohistochemical analysis of 213 lesions. Br J Cancer 91:1342–1348
Takahashi H, Endo T, Yamashita K, Arimura Y, Yamamoto H, Sasaki S, Itoh F, Hirata K, Imamura A, Kondo M, Sato T, Imai K (2002) Mucin phenotype and microsatellite instability in early multiple gastric cancer. Int J Cancer 100:419–424
Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455
Tamura G, Sakata K, Maesawa C, Suzuki Y, Terashima M, Satoh K, Sekiyama S, Suzuki A, Eda Y, Satodate R (1995) Microsatellite alterations in adenoma and differentiated adenocarcinoma of the stomach. Cancer Res 55:1933–1936
Tatematsu M, Furihata C, Katsuyama T, Miki K, Honda H, Konishi Y, Ito N (1986) Gastric and intestinal phenotypic expressions of human signet ring cell carcinomas revealed by their biochemistry, mucin histochemistry, and ultrastructure. Cancer Res 46:4866–4872
Tatematsu M, Ichinise M, Miki M, Hasegawa R, Kato T, Ito N (1990) Gastric and intestinal phenotypic expression of human stomach cancers as revealed by pepsinogen immunohistochemistry and mucin histochemistry. Acta Pathol Jpn 40:494–504
Tatematsu M, Tsukamoto T, Inada K (2003) Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci 94:135–41
Trejdosiewicz LK, Malizia G, Oakes J, Losowsky MS, Janossy G (1985) Expression of common acute lymphoblastic leukaemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol 38:1002–1006
Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E (1998) Expression of MUC1 and MUC2 mucins in gastric carcinoma: its relationship with the prognosis of the patients. Clin Cancer Res 4:2605–2614
van Dekken H, Geelen E, Dinjens WN, Wijnhoven BP, Tilanus HW, Tanke HJ, Rosenberg C (1999) Comparative genomic hybridization of cancer of the gastroesophageal junction: deletion of 14q31–32.1 discriminates between esophageal (Barrett’s) and gastric cardia adenocarcinomas. Cancer Res 59:748–752
Vidgren V, Kokkola A, Monni O, Puolakkainen P, Nordling S, Kallioniemi A, Kivilaakso E, Knuutila S (1999) Concomitant gastrin and ERBB2 gene amplifications at 17q12-q21 in intestinal type of gastric cancer. Genes Chromosomes Cancer 24:24–29
Wu CW, Chen GD, Fann CSJ, Lee AFY, Chi CW, Liu JM, Weier Ulli, Chen JY (2002) Clinical implications of chromosomal abnormalities in gastric adenocarcinomas. Genes Chromosomes Cancer 35:219–231
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morohara, K., Tajima, Y., Nakao, K. et al. Gastric and intestinal phenotypic cell marker expressions in gastric differentiated-type carcinomas: association with E-cadherin expression and chromosomal changes. J Cancer Res Clin Oncol 132, 363–375 (2006). https://doi.org/10.1007/s00432-005-0062-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-005-0062-8