Abstract
Purpose
The aim of this retrospective study was to comparatively investigate the expression of the three drug-resistance genes P-glycoprotein (P-gp), multidrug-resistance protein 1 (MRP1), and lung resistance protein (LRP), in non-small cell lung cancer (NSCLC) tissues, and to assess possible associations with clinicopathologic features.
Methods
Tumor specimens from 126 patients were analyzed by immunohistochemistry and, in selected cases, by reverse transcriptase polymerase chain reaction (RT-PCR), and data were statistically analyzed by SPSS.
Results
The mean expression levels of tumor tissues in the case of P-gp and LRP did not exceed the one of normal epithelia, while MRP1 was significantly enhanced in NSCLC. A weak association was observed between higher grading and P-glycoprotein expression ( p <0.08) as well as lower grading and MRP1 expression in the case of adenocarcinoma ( p <0.05). MRP1 levels were highest in TNM stage I and declined with advanced stage ( p <0.03). A significant association was found between high MRP1 levels and longer overall survival ( N =115, p <0.04), which was highly significant in the patient group never treated with chemotherapy ( N =77; p <0.007). P-gp expression was enhanced in those patients who had received chemotherapy before surgery ( p <0.05).
Conclusions
Our data point towards a major role of MRP1 in the intrinsic treatment resistance of NSCLC and suggest, in addition, a significant activation of P-gp expression during chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost all non-small cell lung cancer (NSCLC), representing approximately 85% of all lung cancers (Fry et al.1996), display intrinsic multidrug resistance (MDR), generally limiting the chance of successful chemotherapy (Ihde and Minna1991). This is one reason why lung cancer is currently a leading cause of cancer death worldwide. As the enhanced resistance of NSCLC cells affects a diverse range of antineoplastic drugs currently in clinical use, it is believed that several protection mechanisms are cooperatively active in NSCLC cells. This multi-factorial in vivo MDR phenotype is also reflected by a distinct chemoresistance of NSCLC cells in vitro (Scagliotti et al.1999; Doyle1993). Members of the ATP-binding cassette (ABC)-transporter family are able to confer MDR by transporting drugs in an energy-dependent manner out of the cell (Tan et al.2000). P-glycoprotein (P-gp)—the archetype of an MDR molecule (Lehne2000)—has been suggested to be of minor importance in the intrinsic chemoresistance of NSCLC cells (Doyle1993). In contrast, a predictive value of P-gp for therapy response to paclitaxel in the case of advanced NSCLC has been suggested recently (Yeh et al.2003). We and others have shown that the multidrug-resistance protein 1 (MRP1) is intrinsically expressed and functionally active in NSCLC cells and correlates inversely with chemosensitivity against diverse antineoplastic drugs (Young et al.2001; Giaccone et al.1996; Narasaki et al.1997; Berger et al.1997). Also, a correlation between MRP3 and MRP1, as well as of both transporter molecules with chemoresistance, has been described in unselected NSCLC cell lines (Young et al.2001).
The lung resistance-related protein (LRP), another marker for chemoresistance in vitro and in vivo, does not belong to the ABC-transporter family. It recently turned out to be the main component of the vault, the largest ribonucleoparticle known so far (Scheffer et al.1995). Despite an overexpression in several drug-selected cell models (Kickhoefer et al.1998), the precise cellular role of vaults is currently unclear. We have shown that LRP is differently expressed in NSCLC cell lines and correlates with resistance to cisplatin but not to several other drugs (Berger et al.2000).
In order to further characterize the intrinsic, multi-factorial MDR phenotype of NSCLC cells in vivo we comparatively investigated the expression of the MDR proteins P-gp, MRP1 and LRP in clinical samples derived from 126 patients with lung cancer, predominantly at early and operable stages, and compared the data with clinicopathologic features and patient survival.
Materials and methods
Patient materials and cell lines
Tissue sections derived from 126 lung tumor patients who underwent surgical resection due to proven NSCLC at the Department of Thoracic Surgery, Otto Wagner Hospital, Vienna, were retrospectively investigated. Thirty-six patients had received chemotherapy before surgery. Chemotherapy regimen always included a platinum compound as well as vinorelbine, vincristine, gemcitabine or Taxol. Four patients were treated with EPICO in an adjuvant setting. Formalin-fixed and paraffin-embedded tissues, archived at the Institute for Pathology and Bacteriology, Otto Wagner Hospital, Vienna, were investigated by immunohistochemistry. The pathological stage of each tumor was classified according to the WHO classifications. The lung tumors analyzed for MDR protein expression were 116 NSCLC and ten neuroendocrine carcinoids and small-cell lung cancer (SCLC) (stage I: 3; stage II: 3; stage III: 4). NSCLC included tumors stages I ( N =67), II ( N =23), and III ( N =34). All patients had given informed consent. Several cell lines were used to establish sensitive detection methods for the three investigated drug-resistance proteins, including drug-sensitive KB-3–1 cells together with P-gp-overexpressing KBC-1 cells (kindly provided by Dr. I. Pastan, NCI, Bethesda, MD), the GLC4 small cell lung cancer cell line together with the MRP1- and LRP-overexpressing, doxorubicin-selected subline GLC4-ADR (kindly donated by Dr. deVries, University of Groningen, The Netherlands) and the LRP-overexpressing NSCLC cell lines A549 and VL-6 (Berger et al.2000).
Immunohistochemistry
Paraffin-embedded samples were cut in 8 µm sections, mounted on silane-coated slides, deparaffinized, rehydrated and pretreated in a microwave at 650 W for 30 min in citrate puffer pH 6.0. Nonspecific binding was blocked with 5% swine or rabbit serum, respectively, in phosphate buffer solution (PBS). Sections were incubated in the primary antibodies at the given working dilutions overnight at 4°C. Antibody binding was visualized by use of the streptavidin biotinylated alkaline phosphatase system from DAKO (Copenhagen, Denmark). As a second antibody, a biotinylated multilink swine anti-goat, mouse, rabbit immunoglobulin (Ig) biotin and a biotinylated rat Ig at dilutions of 1:50 were applied for 30 min. Fuchsin substrate was used as a chromogen, according to the manufacturer’s suggestions. Sections were counterstained with haemalaun. Staining was evaluated independently by two of the authors (U.S., W.B.). Results were scored in a qualitative and a quantitative way. Qualitative score (QLS) was always evaluated relative to the bronchial epithelium, which stained positive in all cases analyzed for all three markers investigated, and graded: 0=below the normal epithelium or negative; 1=resembling the normal epithelium with weak cytoplasmic staining; 2=distinctly enhanced cytoplasmic staining; 3=strong staining in cytoplasm and cell membrane. When different subgroups of tumor cells gave different scores, the percentages of the cells/group were counted and the QLS calculated by multiplying the grading (1–3) with the percentage of cells/100. Thus, QLS levels were continuously distributed from 0 to 3. The quantitative analysis determined the percentage of cells staining positively and was expressed as quantitative score (QNS). At least 1,000 cells/sample in four different parts of the tumor were analyzed and scored: 0=0–5% positive; 1=6–25% positive; 2=26–50% positive; 3=51–100% positive. For statistical analysis the expression index (EI) for each sample was calculated from both scores by the equation: QLS×QNS and thus gave values from 0 to 9.
RT-PCR
Expression levels of MDR1, MRP1 and LRP transcripts in NSCLC tissues were determined by semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) procedure as described previously (Berger et al.2000; Spiegl Kreinecker et al.2002) using glyceraldehyde-3-phosphate-dehydrogenase cDNA (GAPDH) as housekeeping gene. Dynamics of PCR amplification were evaluated at different PCR cycle numbers. For data presentation, 30 cycles were chosen for MDR1, MRP1 and LRP mRNA, because at lower cycle numbers no products were detected in the case of MDR1 and LRP in multiple NSCLC samples. Several negative controls were included in each experiment.
Statistics
Analysis of data was performed using the computer software SPSS for Windows (version 10.0). Nonparametric comparison between independent groups was done using the Kruskal-Wallis test. For survival analysis, Kaplan-Meier curves were calculated, and tests for statistical significance were based on log-rank statistics. Additionally, the prognostic effect on overall survival was evaluated using the univariate and multivariate Cox models. P -values <0.05 are considered statistically significant.
Results
Patients
Characteristics of the 126 NSCLC patients and tumors are summarized in Table 1. For 115/126 NSCLC patients clinical follow-up data could be evaluated. Median follow up was 24 months, ranging 4–80 months.
Expression of P-gp, MRP1, and LRP in normal and tumor tissue
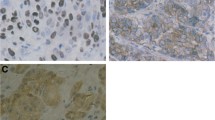
In the non-malignant superficial bronchial epithelium of lung cancer patients P-gp was generally found to be expressed at low levels. Also, immunoreactivity in the malignant tissue was relatively weak and comparable with the normal bronchial epithelium in 60/126 of NSCLC patients. In 24/126 samples the tumor tissue was, in contrast to the normal bronchial epithelium, decreased or completely negative for P-gp immunostaining, indicating a loss of P-gp during malignant progression. In 27/126 patients a distinct up-regulation of P-gp in >10% of tumor cells was detectable. In some other cases (9/126), including both adenocarcinomas (AC) and squamous cell carcinomas (SCC), a small subgroup of tumor cells (3–6%) distinctly overexpressed P-gp (Fig. 1A). When scored for an expression index (EI) (as described above) the mean expression level for P-gp in tumor tissue did not significantly differ from the normal bronchial epithelium (Fig. 2A). In normal as well as malignant cells, P-gp was detected as diffuse cytoplasmic staining and only in strongly positive cells concentrated at the plasma membrane (Fig. 1). The relatively low expression of P-gp in NSCLC samples corresponded with the detection of limited amounts of MDR1 mRNA by RT-PCR (selected cases in Fig. 3).
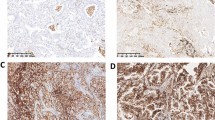
Detection of the investigated drug-resistance proteins in NSCLC specimens by immunohistochemistry. A Consecutive sections of representative tumor specimens of the indicated histology ( AC adenocarcinoma, SCC squamous cell carcinoma) were stained for P-gp, MRP1 and LRP as described in “Materials and methods.” B MRP1 expression in the indicated histological tumor types. Note the strong MRP1 overexpression at the leading edge of the tumor in the left and the right panels
Mean expression levels of the investigated drug-resistance proteins in NSCLC specimens. Expression indices ( EI) were calculated from immunohistochemistry data as described under “Materials and methods.” Mean and SD of all A NSCLC specimens and in the indicated histological subgroups B are shown. ( AC adenocarcinoma, SCC squamous cell carcinoma, LC large cell carcinoma, BAC bronchioloalveolar carcinoma, NE small cell carcinomas and carcinoids). A mean EI of 3 represents the expression levels of normal bronchial epithelium
Expression of mRNA of the investigated drug-resistance genes in selected NSCLC samples analyzed by RT-PCR. Amplification conditions ( left panel) were tested using the indicated MDR cell models and oligonucleotide primers specific for MDR1 (187 bp product), MRP1 (328 bp) and LRP (176 bp). Amplification products at 25 ( column 1), 30 ( column 2) and 35 ( column 3) PCR cycles are opposed to GAPDH amplification products (356 bp, column 4) at 25 cycles. ( Right panel) mRNA derived from central parts of selected lung cancer samples were analyzed by RT-PCR for presence of MRP1 ( column 1), MDR1 ( column 2) and LRP ( column 3) transcripts. ( LRP lung resistance protein, MDR multidrug resistance, RT-PCR reverse transcriptase polymerase chain reaction, AC adenocarcinoma, SCC squamous cell carcinoma, LC large cell carcinoma, BAC bronchioloalveolar carcinoma, NE small cell lung cancer and carcinoids, Others metastases derived from a kidney [ left], colon [ middle] and clear cell cancer [ right]). Results derived from 30 PCR cycle experiments are shown as differences between the three investigated genes, and were most obvious at this cycle number. Due to the high amount of MRP1 mRNA in NSCLC, the respective PCR reactions were already saturated at 30 cycles. Glyceraldehyde-3-phosphate-dehydrogenase cDNA ( GAPDH, 25 cycles, column 4) is shown as housekeeping control for the presence of intact mRNA
In contrast to the relatively restricted expression pattern of P-gp in tumor cells, MRP1 was detected very abundantly in NSCLC cells, whereas the surrounding connective tissue was widely MRP1-negative (Fig. 1). The normal bronchial and bronchiolar epithelium, however, always stained positively for MRP1, as did defined cells within seromucous glands (data not shown). In most of the cases, the tumor tissue displayed a distinct up-regulation of MRP1 immunoreactivity as compared with the normal cell counterpart. Thus the mean expression levels expressed by EI were significantly higher for the tumors, as compared with the normal cells (Fig. 2A). The immunostaining was mainly localized cytoplasmic, which might be an artifact caused by the paraffin embedding. However, in some cases, MRP1 staining was distinctly concentrated at the cell membrane (Fig. 1). Generally within the tumor tissues, cells at the outside of tumor nodules adjacent to connective tissue or vessels displayed more intense MRP1 expression as compared with the inner cells. This was especially obvious in squamous cell carcinoma (SCC) (Fig. 1B). The results of immunohistochemistry were confirmed in selected samples by use of a second MRP1 antibody (QRCL-1). Additionally, RT-PCR analysis (Fig. 3) confirmed a general and intense expression of the MRP1 gene in NSCLC samples. RT-PCR products were mostly detectable already at 22 PCR cycles and were in saturation at the 30 cycles as shown in Fig. 3. Lower amounts of MRP1 were only observed in the case of carcinoids and SCLC, while some lung metastases of other tumor types were MRP1 mRNA negative.
Immunoreactivity for the major vault protein LRP was detectable in the normal bronchial epithelium (data not shown). Generally, only a few lung tumor samples (20/126) were characterized by an intense up-regulation of LRP expression as compared with the normal epithelium (Fig. 1A). Comparable with P-gp, several tumors had lost LRP expression in comparison with adjacent normal epithelial tissue (19/126). The strongest LRP immunoreactivity was detected in alveolar macrophages, especially of tissue samples derived from heavily smoking patients (not shown).
When comparing the staining levels of the three investigated drug resistance markers, it turned out that the expression of P-gp and LRP in the investigated tumor specimen was highly correlated (Pearson r =0.4829, p <0.0001; linear regression 0.483, p <0.0001) while MRP1 expression showed no significant relation to either of the two other proteins.
Comparison of P-gp, MRP1 and LRP expression with clinicopathologic parameters
Expression levels of the investigated proteins were analyzed in relation to patient and tumor characteristics (Table 1, Fig. 2B). Significant differences were not observed with regard to either age or gender of the patients. P-gp but not MRP1 and LRP was expressed significantly higher ( p <0.05) in tumor tissues for patients who had undergone non-adjuvant chemotherapy before surgery. With regard to the histological subtypes (Fig. 2B), no significant association of P-gp and LRP expression could be detected. Only in the few bronchioloalveolar carcinoma (BAC) samples analyzed, P-gp levels were generally lower as compared with the respective normal bronchial epithelia (Fig. 2B). The expression level of MRP1 appeared to be independent of the histological subtype in the case of NSCLC (Fig. 2B), but was significantly lower in SCLC and carcinoids ( p <0.05; NE in Fig. 2B).
With regard to tumor-node-metastasis (TNM) staging, MRP1 was highest in stage I tumors and progressively and significantly decreased in advanced tumor stages (Table 1), while neither P-gp nor LRP were related to staging. With regard to grading, P-gp-expression tended to be enhanced in highly dedifferentiated tumors ( p =0.08), while neither MRP1 nor LRP displayed a significant relation to grading. However, when AC were analyzed separately, MRP1 expression correlated positively with the grade of differentiation ( p <0.05).
Survival analysis
Correlations between the expression of the three investigated drug-resistance proteins and overall survival of NSCLC patients were set up by Kaplan-Meier curves with log rank test (Fig. 4) and univariate Cox regression analysis. As expected, tumor stage ( p <0.0001), tumor (T) status ( p <0.01), presence of local metastases ( p <0.0001), and grade (G1 vs G2+3; p <0.05) but not gender and age predicted survival of NSCLC patients. Neither P-gp nor LRP expression displayed a significant association with patient survival. MRP1 overexpression, however, correlated with a significantly increased overall survival (hazard ratio 0.454, p <0.05) of NSCLC patients. This relationship was even more pronounced when only untreated NSCLC patients were included (hazard ratio 0.262, p <0.005 in regression analysis; Fig. 4) but was absolutely lacking in the NSCLC patient group treated with chemotherapy before surgery (data not shown). MRP1 expression did not reach statistical significance as an independent prognostic marker for overall survival (hazard ratio 0.607) due to its close association with TNM stage.
Overall survival analysis and expression of the investigated drug-resistance genes. Kaplan Mayer blots were generated for all patients ( upper left panel) and for different TNM stages as indicated ( upper right panel). In the lower panels, patients grouped with respect to the expression of the indicated drug-resistance genes are compared. The median expression levels of the respective proteins were used as cut-off values. In the case of the lower right panel, only patients who had not received chemotherapy before surgery (untreated patients) were included
Discussion
Chemotherapy resistance is a major problem in the clinical management of lung cancer. While SCLC initially responds readily to several chemotherapeutic regimens, NSCLC is generally characterized by intrinsic resistance to many antineoplastic drugs (Scagliotti et al.1999). This distinct resistance to treatment is one reason for the persisting unfavorable prognosis of NSCLC patients. The underlying cellular mechanisms are still quite unclear, but intrinsic resistance is believed to be due to multi-factorial causes (Volm et al.2002). In the present study we comparatively investigated the in vivo expression of three well-known drug-resistance proteins, i.e., P-gp, MRP1, and LRP, which all have been associated with the drug insensitivity of NSCLC cells in vitro (Abe et al.1994; Berger et al.1997; Berger et al.2000; Doyle1993). We demonstrate that all these resistance proteins are expressed in normal lung epithelium at detectable levels. P-gp and LRP were overexpressed in tumor tissues of a small subgroup of patients only; P-gp was enhanced mainly in those patients who had undergone chemotherapy before surgery. In contrast, intrinsic MRP1 overexpression in the tumor tissue was an almost general feature of NSCLC. In summary our data suggest that MRP1 is a major cause of the intrinsic drug resistance of NSCLC cells. Moreover, exposure to chemotherapy might be accompanied by activation of the MDR1 gene, leading to an additional acquired resistance phenotype.
P-gp, the archetype of a drug-resistance ABC-transporter encoded by the MDR1 gene, causes chemoresistance of diverse cancer types (Gottesman et al.2002). In our series of lung cancer tissues, we only scarcely found P-gp to be overexpressed as compared with the normal bronchial epithelium. This relative limited expression of P-gp especially in chemo-naive patients supports previous studies suggesting that P-gp overexpression is of minor relevance for intrinsic therapy resistance of NSCLC (Doyle1993; Scagliotti et al.1999). In contrast, in recent reports a predictive value of P-gp expression for the therapy with taxanes has been demonstrated (Chiou et al.2003; Yeh et al.2003), implying that the role of P-gp in NSCLC chemoresistance might have been underestimated. We observed significantly higher P-gp expression levels in those NSCLC patients who had been treated with neoadjuvant chemotherapy, suggesting that P-gp might be of importance in therapy-induced resistance in NSCLC. Correspondingly, an up-regulation of P-gp expression by chemotherapy in NSCLC xenografts has been shown (Abe et al.1996). A major drawback of the present study is that, based on the clinical practice, only a limited number of treated patients ( N =36) could be included who had not all received identical therapeutic regimens. Considering these limitations, the observed up-regulation of P-gp expression by different chemotherapy regimens suggests that activation of the MDR1 gene might be based more generally on cellular stress responses than on a specific therapeutic agent (Shtil2001). Thus, further studies monitoring P-gp expression during application of different forms of chemotherapy in lung cancer are warranted.
When comparing the expression levels of P-gp with the other investigated drug-resistance proteins we found a striking association with LRP. A coordinate overexpression of P-gp and LRP was also part of the most frequent, multi-factorial resistance phenotype of NSCLC samples developed in a recent hierarchical cluster analysis by Volm and colleagues (Volm et al.2002). With regard to in vitro data, LRP has been shown to be overexpressed in a subgroup of NSCLC cell lines correlating with resistance against cisplatin (Berger et al.2000). While recently LRP was reported as a predictive marker for treatment response in NSCLC (Harada et al.2003), another study failed to detect any such association (Dingemans et al.1996). In our investigation LRP expression was relatively low and comparable with that of the normal bronchial epithelium in most patients. In contrast to P-gp, the mean LRP level was not enhanced in those patients treated with chemotherapy. This argues against a central role of LRP in intrinsic or acquired chemoresistance of NSCLC in vivo. In contrast to tumor cells, macrophages within lung tissues derived from smokers expressed very high levels of LRP, suggesting a possible function of LRP in associated detoxification mechanisms (Dingemans et al.1996).
MRP1 was first cloned from a drug-selected SCLC cell line (Cole and Deeley1993). In the case of NSCLC, MRP1 overexpression was not only detected in cells with acquired MDR but has also been found in untreated NSCLC cell cultures (Berger et al.1997; Chuman et al.1996; Young et al.2001). Expression of MRP1 in vitro correlated with enhanced resistance to a broad spectrum of MRP1 substrate drugs (Berger et al.1997; Young et al.2001). Blockade of MRP1 by pharmacological agents including probenecid and benzbromarone up-regulated drug sensitivity of unselected NSCLC cells (Berger et al.1997; Giaccone et al.1996). These in vitro data suggested a substantial contribution of MRP1 to the intrinsic MDR phenotype of NSCLC cells in vitro.
In the present investigation we detected an almost general overexpression of MRP1 in NSCLC tissues in vivo. Also, SCLC and carcinoids expressed MRP1, however, at lower levels as compared with NSCLC. A prevalence of MRP1 overexpression in NSCLC has been found in other studies as well (Nooter et al.1996; Sugawara et al.1995; Wright et al.1998), while lower rates of MRP1 expression in NSCLC were also reported (Dingemans et al.1996; Ota et al.1995). These differences might be explained by the detection methods used (such as mRNA vs protein assays) and the investigated patient collective (early vs advanced stage NSCLC). The patients included in our investigation mainly suffered from early stage NSCLC. Remarkably, MRP1 expression in the malignant tissues was highest in early-stage tumors and declined with progression of disease. These data suggest that MRP1 overexpression is likely to be an inherited feature of the original epithelial cells that is further activated during malignant transformation and then down-regulated during disease progression. In line with this hypothesis, MRP1 expression was associated with a better prognosis, especially in the patient group not pretreated with chemotherapy. Correspondingly, in this and other reports, MRP1 overexpression correlated with a higher grade of differentiation, especially in AC (Nooter et al.1996; Sugawara et al.1995; Wright et al.1998). The association of MRP1 with enhanced overall survival was completely absent or even tending to the opposite in patients treated with neoadjuvant chemotherapy. This suggests that, although MRP1 overexpressing tumors are less aggressive and tend to be more differentiated, they are especially resistant to chemotherapy. A worse prognosis of NSCLC patients expressing high levels of MRP1 and treated by MRP1-related chemotherapy has been reported before (Oshika et al.1998; Ota et al.1995).
With regard to histology, both a predominant expression of MRP1 in SCC (Chuman et al.1996; Ota et al.1995) and in AC (Sugawara et al.1995; Wright et al.1998) have been demonstrated. In our series of patients no significant differences of MRP1 expression between different histological subtypes could be detected. However, especially in the case of SCC, a distinct overexpression of MRP1 was present at the outer cell layer of the tumor nodules in contact with stromal cells and small vessels. A comparable MRP1 overexpression at the tumor borders was also observed by others (Nooter et al.1996; Thomas et al.1994) and suggested that MRP1 expression might be up-regulated due to paracrine signals derived from the surrounding normal tissues. The enhanced expression of MRP1 in those tumor cells located in close proximity to microvessels additionally suggests that MRP1 represents major intrinsic defense mechanisms of NSCLC tissues against poisons delivered by blood circulation.
Summarizing our study further supports a central role of ABC-transporter proteins in the chemotherapy resistance of NSCLC and places emphasis on the necessity for new treatment modalities that are not limited by these drug-resistance mechanisms.
References
Abe Y, Nakamura M, Ota E, Ozeki Y, Tamai S, Inoue H, Ueyama Y, Ogata T, Tamaoki N (1994) Expression of the multidrug resistance gene (MDR1) in non-small cell lung cancer. Jpn J Cancer Res 85:536–541
Abe Y, Ohnishi Y, Yoshimura M, Ota E, Ozeki Y, Oshika Y, Tokunaga T, Yamazaki H, Ueyema Y, Ogata T et al. (1996) P-glycoprotein-mediated acquired multidrug resistance of human lung cancer cells in vivo. Br J Cancer 74:1929–1934
Berger W, Elbling L, Hauptmann E, Micksche M (1997) Expression of the multidrug resistance-associated protein (MRP) and chemoresistance of human non-small-cell lung cancer cells. Int J Cancer 73:84–93
Berger W, Elbling L, Micksche M (2000) Expression of the major vault protein LRP in human non-small-cell lung cancer cells: activation by short-term exposure to antineoplastic drugs. Int J Cancer 88:293–300
Chiou JF, Liang JA, Hsu WH, Wang JJ, Ho ST, Kao A (2003) Comparing the relationship of Taxol-based chemotherapy response with P-glycoprotein and lung resistance-related protein expression in non-small cell lung cancer. Lung 181:267–273
Chuman Y, Sumizawa T, Takebayashi Y, Niwa K, Yamada K, Haraguchi M, Furukawa T, Akiyama S, Aikou T (1996) Expression of the multidrug-resistance-associated protein (MRP) gene in human colorectal, gastric and non-small-cell lung carcinomas. Int J Cancer 66:274–279
Cole SP, Deeley RG (1993) Multidrug resistance-associated protein: sequence correction. Science 260:879
Dingemans AM, van Ark Otte J, van der Valk P, Apolinario RM, Scheper RJ, Postmus PE, Giaccone G (1996) Expression of the human major vault protein LRP in human lung cancer samples and normal lung tissues. Ann Oncol 7:625–630
Doyle LA (1993) Mechanisms of drug resistance in human lung cancer cells. Semin Oncol 20:326–337
Fry WA, Menck HR, Winchester DP (1996) The National Cancer Data Base report on lung cancer. Cancer 77:1947–1955
Giaccone G, van Ark Otte J, Rubio GJ, Gazdar AF, Broxterman HJ, Dingemans AM, Flens MJ, Scheper RJ, Pinedo HM (1996) MRP is frequently expressed in human lung-cancer cell lines, in non-small-cell lung cancer and in normal lungs. Int J Cancer 66:760–767
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58
Harada T, Ogura S, Yamazaki K, Kinoshita I, Itoh T, Isobe H, Yamashiro K, Dosaka Akita H, Nishimura M (2003) Predictive value of expression of P53, Bcl-2 and lung resistance-related protein for response to chemotherapy in non-small cell lung cancers. Cancer Sci 94:394–399
Ihde DC, Minna JD (1991) Non-small cell lung cancer. Part II: Treatment. Curr Probl Cancer 15:105–154
Kickhoefer VA, Rajavel KS, Scheffer GL, Dalton WS, Scheper RJ, Rome LH (1998) Vaults are up-regulated in multidrug-resistant cancer cell lines. J Biol Chem 273:8971–8974
Lehne G (2000) P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr Drug Targets 1:85–99
Narasaki F, Oka M, Fukuda M, Nakano R, Ikeda K, Takatani H, Terashi K, Soda H, Yano O, Nakamura T et al (1997) A novel quinoline derivative, MS-209, overcomes drug resistance of human lung cancer cells expressing the multidrug resistance-associated protein (MRP) gene. Cancer Chemother Pharmacol 40:425–432
Nooter K, Bosman FT, Burger H, van Wingerden KE, Flens MJ, Scheper RJ, Oostrum RG, Boersma AW, van der Gaast A, Stoter G (1996) Expression of the multidrug resistance-associated protein (MRP) gene in primary non-small-cell lung cancer. Ann Oncol 7:75–81
Oshika Y, Nakamura M, Tokunaga T, Fukushima Y, Abe Y, Ozeki Y, Yamazaki H, Tamaoki N, Ueyama Y (1998) Multidrug resistance-associated protein and mutant p53 protein expression in non-small cell lung cancer. Mod Pathol 11:1059–1063
Ota E, Abe Y, Oshika Y, Ozeki Y, Iwasaki M, Inoue H, Yamazaki H, Ueyama Y, Takagi K, Ogata T et al (1995) Expression of the multidrug resistance-associated protein (MRP) gene in non-small-cell lung cancer. Br J Cancer 72:550–554
Scagliotti GV, Novello S, Selvaggi G (1999) Multidrug resistance in non-small-cell lung cancer. Ann Oncol 10:83–86
Scheffer GL, Wijngaard PL, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC, Scheper RJ (1995) The drug resistance-related protein LRP is the human major vault protein. Nat Med 1:578–582
Shtil AA (2001) Signal transduction pathways and transcriptional mechanisms as targets for prevention of emergence of multidrug resistance in human cancer cells. Curr Drug Targets 2:57–77
Spiegl Kreinecker S, Buchroithner J, Elbling L, Steiner E, Wurm G, Bodenteich A, Fischer J, Micksche M, Berger W (2002) Expression and functional activity of the ABC-transporter proteins P-glycoprotein and multidrug-resistance protein 1 in human brain tumor cells and astrocytes. J Neurooncol 57:27–36
Sugawara I, Yamada H, Nakamura H, Sumizawa T, Akiyama S, Masunaga A, Itoyama S (1995) Preferential expression of the multidrug-resistance-associated protein (MRP) in adenocarcinoma of the lung. Int J Cancer 64:322–325
Tan B, Piwnica Worms D, Ratner L (2000) Multidrug resistance transporters and modulation. Curr Opin Oncol 12:450–458
Thomas GA, Barrand MA, Stewart S, Rabbitts PH, Williams ED, Twentyman PR (1994) Expression of the multidrug resistance-associated protein (MRP) gene in human lung tumours and normal tissue as determined by in situ hybridisation. Eur J Cancer 30a:1705–1709
Volm M, Koomagi R, Mattern J, Efferth T (2002) Protein expression profiles indicative for drug resistance of non-small cell lung cancer. Br J Cancer 87:251–257
Wright SR, Boag AH, Valdimarsson G, Hipfner DR, Campling BG, Cole SP, Deeley RG (1998) Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res 4:2279–2289
Yeh JJ, Hsu WH, Wang JJ, Ho ST, Kao A (2003) Predicting chemotherapy response to paclitaxel-based therapy in advanced non-small-cell lung cancer with P-glycoprotein expression. Respiration 70:32–35
Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH (2001) Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res 7:1798–1804
Acknowledgements
The work has been supported by the Jubiläumsfonds der Österreichischen Nationalbank Bank, Grant 7258 and the Herzfelder’schen Familienstiftung
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berger, W., Setinek, U., Hollaus, P. et al. Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: prognostic implications. J Cancer Res Clin Oncol 131, 355–363 (2005). https://doi.org/10.1007/s00432-004-0653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-004-0653-9