Abstract
Purpose
Since 1971, a statutory early detection programme has operated in Germany which comprises health-insurance-paid annual examinations of the breast, cervix, prostate, rectum, and the skin. Since the programme is conceptualised as opportunistic screening, the attendance rates have been low and only reached about 50% among females and 13% among males by the end of the 1990s. Based on these figures and present knowledge on the efficacy of screening modalities, we assessed past benefits and the future potential of cancer screening in Germany.
Methods
We used published data on the efficacy of screening procedures and German attendance rates, and internationally available data on incidence and mortality in Germany and, for cervical cancer, in other countries. Incidence and mortality rates have been standardised to the world standard, and screening benefit has been given as the population preventable fraction given in percentage.
Results
The past benefits of the statutory early detection programme ranged around 2.0–6.5%. Since the upper limit was due to generous assumptions regarding efficacy or inclusion of treatment effects, the true value might be closer to the estimates of the effect of cervical cancer screening (2.0–4.7%). The achievable future benefit of exploiting the theoretical potential of more exhaustive screening could provide a further mortality reduction of about 3.4% (50% compliance) or 4.7% (70% compliance).
Conclusions
Screening partially requires an expensive medical infrastructure and is not without risks for the participants. The overall benefit is critically dependent upon the quality of the programme and its in-time control. Any benefit may be annulled by poor quality while costs are overflowing. Well-organised high-quality screening may be a sound basis for cancer control. To preserve or increase the impact of screening and control its expenses: (a) further research efforts are needed towards new or better targeted screening tools or modalities; (b) the efficacy of new modalities has to be evaluated carefully in advance; (c) the programme has to be reconceptualised as organised screening; (d) in-time quality control based on the collection of the basic performance data must be an intrinsic part of the programme.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Germany, about 350,000 cancers are diagnosed each year and approximately 210,000 persons die from the disease. Epidemiological considerations indicate that at least 60% of these deaths are related to environmental factors in a broad sense and should be avoidable in principle. Under realistic assumptions, the factually achievable mortality reduction may range between 18–33% within medium-term periods (Willett et al. 1996; Becker 2001; Adami et al. 2001).
These figures have two implications: first, they demonstrate the enormous potential of primary prevention (20,000–60,000 avoidable incident cancers and cancer deaths in Germany annually) which has been insufficiently exploited so far. Second, the main proportion of cancer cases, however, cannot be avoided even if knowledge regarding primary prevention were optimally used. This underlines the great importance of “secondary prevention” with screening as a part of it.
Principles of screening
It has widely been accepted that; (a) a number of preconditions must be fulfilled to make screening for a specific cancer reasonable; and (b) in advance of its introduction as a population-based programme, a screening modality has to be evaluated with regard to its efficacy to reduce mortality from the respective cancer. In short, the preconditions state the circumstances under which early detection of the cancer of interest may be feasible and effective, and the false-positive rate limited and acceptable (Wilson and Jungner 1968; Morrison 1992). Previous evaluation of the efficacy of a screening modality is required because early detection of asymptomatic cancers does not necessarily imply a mortality reduction for the respective cancer site. Randomised trials provide the most reliable evidence, though other designs, e.g., case-control studies, are feasible as well, but may be considerably biased (Miller 1985; Morrison 1992; German readers should also see Becker 2002 for details).
In a series of expert meetings, the UICC has evaluated the evidence regarding screening trials for several cancer sites and published the results and recommendations in a number of publications (Miller 1978; Prorok et al. 1984; Chamberlain et al. 1986; Chamberlain and Miller 1988; Hakama et al. 1985; Hakama et al. 1986; Day et al. 1986; Day and Miller 1988; Miller et al. 1990; Miller et al. 1991).
Finally, there is wide acceptance that if screening is offered and promoted, it should be offered as an organised screening programme with attention to quality control (Hakama et al. 1985).
Established screening modalities
Given these preconditions, in its last evaluation in 1991, the UICC has judged as efficacious/effective only screening for cervix and breast cancers. A specific recommendation was given for Japan regarding stomach cancer due to the particularly high incidence in this country. This is not applicable to European countries which have much lower rates. Screening was not recommended for the skin, but it was stated that health promotion programmes advocating enhanced individual awareness may be beneficial, though data are not available which confirmed this. Meanwhile, the efficacy of the faecal occult blood test (FOBT) has been proven for colorectal cancer screening by three randomised trials (see below).
Cervical cancer
Numerous observational studies have accumulated a large body of evidence for the effectiveness of this screening approach (reported or reviewed in Hakama et al. 1985, 1986) and provided data about different screening modalities and the related mortality reduction.
Despite the simplicity of the test and its wide distribution, the quality of its performance, and thus the effectiveness of the early detection programmes, continues to be a matter of concern (see, for example, Anonymous 1985; Koss 1989; Miller 2002). The cytological smear is considered to be a routine procedure but the individual steps required (taking the smear, fixation, laboratory processing, interpretation) are susceptible to errors, and therefore the effectiveness of early detection is dependent upon a series of steps executed by qualified personel (Koss 1989). Guidelines for quality assurance have been released (Coleman et al. 1993) which have been adopted in different countries to various degrees (see below for Germany). Studies suggest that organised programmes might be superior to ‘opportunistic’ early detection in terms of factually achieved mortality reduction (Nieminen et al. 1999).
Breast cancer
Breast cancer screening by mammography alone or together with physical examination has been evaluated by several large randomised trials in different countries, most of which demonstrated a reduction of breast cancer mortality by up to 30% (Day et al. 1986; Day and Miller 1988). Based on this evidence, many countries introduced mammography screening with or without physical examination and for different age ranges, at least for the minimal age range 50–64 years (Shapiro et al. 1998; Ballard-Barbash et al. 1999; IARC 2002). Recently, the evidence has been reviewed by an international expert meeting which confirmed the conclusiveness of the studies in terms of breast cancer mortality reduction by mammography screening (IARC 2002). The experts concluded that mammography screening in the age range 50–69 years may reduce breast cancer mortality by about 25% and in programmes by 5–20%. Inadequate evidence is available for the efficacy of physical examination and breast self-examination.
Quality issues appear especially critical for breast cancer screening. Less experienced radiologists have a lower sensitivity and specificity of diagnosis (Esserman et al. 2002). Limited specificity implies the risk of false positive results, i.e., further assessment including biopsies which may accumulate over the attended screening rounds to a substantial amount (Christiansen et al. 2000). Guidelines for quality assurance have been released for various countries (for citations see IARC 2002). For the European Community, guidelines which have the character of recommendations have been issued by the European Commission (Perry et al. 2001).
Physical examination (PE) has never been evaluated against ‘no screening’ and no clear evidence about its efficacy is available. However, studies suggest some benefit (Stockton et al. 1997; Kuroishi et al. 2000; IARC 2002). It is used in many countries for early detection including Germany (see below) and needs consideration in the present context.
Colorectal cancer
For colorectal cancer, three studies proved the efficacy of FOBT (Hardcastle et al. 1996; Kronborg et al. 1996; Mandel et al. 1993, 1999). The studies demonstrated mortality reductions of up to 33% with annual screening and up to 21% with biannual screening.
Before FOBT was validated and incorporated into screening recommendations, digital rectal examination (DRE) was partially recommended for rectal screening. This approach was never evaluated by a randomised trial and no evidence about its efficacy is available (Cuzick 1999). A case-control study of deaths from distal rectal cancer found an odds ratio of OR = 0.96 (confidence interval = 0.56–1.7) for a screening history, i.e., a null effect. However, due to the limited size of the study, a modest effect could not be ruled out (Herrinton et al. 1995).
Prostate cancer
The most strongly debated approach is early detection of prostatic cancer by PSA testing. For this potential screening modality, two large randomised studies are under way, but no evidence about a mortality reducing effect yet exists. Since no proper quantitative data about its potential benefit are available and harm is known to be considerable (strong overdiagnosis and overtreatment leading to a high proportion of incontinence and impotence as outcome among the treated subjects), PSA testing is clearly not yet recommended (Auvinen et al. 1996; 2002).
A frequently used screening test is digital rectal examination (DRE) for which, however, no evidence about a mortality reducing effect exists (Bentvelsen and Schröder 1993). A case-control study among men with metastatic prostate cancers found an odds ratio of OR = 0.9 (confidence interval 0.5–1.7) for a screening history of at least one DRE versus none, which provides little support for efficacy. However, a small benefit cannot be ruled out (Friedman et al. 1991).
Skin cancer
No evidence exists regarding the potential efficacy of visual examination of the skin as a screening modality from a randomised trial. A case-control study on skin self-examination provided an odds ratio of OR = 0.37 (confidence interval = 0.16–0.84) suggesting a strong benefit (Berwick et al. 1996). However, due to potential biases in a case-control approach the quite strong effect may also be interpreted non-causally (Elwood 1996).
The German Statutory Early Detection Programme
In 1971, the so-called “statutory early detection programme” was established in West Germany by law. It states that every person who is health-insured with a statutory health insurance company and within a cancer-site and gender-specific age range is eligible to receive a specified annual early detection examination paid by the respective insurance company (Herwig 1975; Schenck and von Karsa 2000). Since about 90% of the German population are covered by statutory health insurances and the remaining 10% by so-called “private” health insurances (membership in a health insurance is mandatory) who joined the programme, the coverage is factually 100%.
The law allows that the programme may be regularly updated and assigns the responsibility for the decision about the target cancers and compensated modes of early detection to the so-called “Federal Board of Physicians and Health Insurances” (Herwig 1975). In the initial years, the programme comprised gynaecological and rectal examinations for females beginning at age 30 years, and digital rectal and prostate examinations for males beginning at 45 years old. Over the years, the board mentioned above expanded the programme to younger women and the coverage to further target sites, that is, the breast, colon, and rectum, including FOBT since 1977 (Table 1). The most recent expansions include full colonoscopy every 10 years from the age of 55 years since 2002 and biannual breast screening by mammography at ages 50–69 years from 2004.
The programme was established relatively early compared to other countries, since decision-makers did not wait until the results of randomised trials confirmed the efficacy of screening modalities for the target cancers of interest. Nevertheless, the principles outlined in Wilson and Jungner (Wilson and Jungner 1968) were taken into account as guidelines for the decisions about the content of the programme leading to pragmatic considerations about technical and personal practicability, acceptability by healthy subjects, and reasonable sensitivity and specificity of the tests (Flatten 1988). However, the problem mentioned above that early detection does not intrinsically imply a benefit in terms of mortality reduction appears to have been ignored.
While the recent inclusion of colorectal screening by full colonoscopy followed this manner of decision-making, the decision about mammography screening was based on the consideration of the evidence from randomised studies, expert evaluations and pre-investigations. After an initial pilot study identified serious shortcomings in existing clinical mammography (Frischbier et al. 1994), model projects have been initiated and are currently running with the purpose of exploring whether organised and quality-controlled mammography screening according to the guidelines of the European Commission (Perry et al. 2001) is feasible under the conditions of the decentralised health care system in Germany (Junkermann et al. 2001). The first data indicate that the pilot studies achieved the required quality from the beginning.
As just mentioned, the statutory early detection programme is adjusted to the decentralised German health care system which is largely based on office-based physicians. It is factually conceptualised as an opportunistic screening programme. The basic shortcomings of this approach are poor compliance and lack of proper quality control.
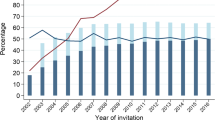
Overall, compliance was low from the very beginning and considered unsatisfactory by the decision-making institutions. Only about 13% of males and 22% of females participated in 1972, the first year of action. The attendance increased slowly to about 50% of females and 30% of males in the late 1990s. However, outside of this programme, increasing numbers of “curative” mammographies developed as factual screening mammographies which are paid by the insurance companies as well but are not covered by the regular statistics of the programme. These peculiar circumstances are addressed in the Results section in the context of the appropriate assumptions on screening prevalence for the effectiveness assessment.
Early cancer detection which is structured in this way creates problems regarding establishing effective quality control. Beginning with the data of 1972, the Central Research Institute of Health Insurance Physicians issued regular annual reports about compliance and the results of the early detection programme (Herwig 1975 and subsequent annual issues). However, these data are not very specific and far from, for example, the requirements outlined in the guidelines for some of the target cancers (Coleman et al. 1993; Perry et al. 2001). Attempts have been undertaken to improve the quality of the existing opportunistic screening gradually (for cervical cancer see Schenk and von Karsa 2000; Miller 2002). Even with mammography screening, gradually upgrading of existing screening has been proposed, avoiding an organised approach. These attempts leave the epidemiological parameters for quality control largely out of consideration and apparently underestimate their relevance as surrogates for the actually interesting target quantity mortality reduction (see also Rittgen and Becker 2001).
In East Germany, screening was not organised in a national programme. Participation at cervical screening was encouraged and facilitated since, in the 1970s, the test was increasingly offered by the occupational health services. With the reunification of Germany, the West German programme was expanded to East Germany (Schenck and von Karsa 2000).
Material and methods
The age-standardised incidence rates for Germany and, if used, for other countries, have been taken from ‘Cancer in Five Continents, Vol I—VIII’ (Doll et al. 1966, 1970; Waterhouse et al. 1976, 1982; Muir et al. 1987; Parkin et al. 1992, 1997, 2002) with the ‘world population’ as standard. The incidence rate for cervical cancer in the USA for the years 1947–1950 was computed as a standardised rate, with the world population as standard, from the age-specific incidence rates of the SEER program presented in Appendix 2 of Devesa et al. (Devesa et al. 1987). The age-standardised incidence rate for 1960 in Germany/East was taken from Möhner et al. (Möhner et al. 1994) and for 1993–1997 communicated from the Common Cancer Registry of the five East German federal states.
The absolute number of cancer deaths and the overall cancer mortality rate for Germany in 1995 were taken from the German Cancer Atlas (Becker & Wahrendorf 1997). The mortality rates are likewise age-standardized with reference to the world population. Despite more recent data being available, the data of 1995 have been used to keep the figures comparable to the article on primary prevention in Germany (Becker 2001).
The effects of screening were computed as population preventable fraction PF = p×(1−RR) given in percent, where p denotes the proportion of the population under exposure (attending the screening) and RR denotes the rate ratio of mortality reduction under screening, i.e., 1−RR the preventable fraction (Rothman and Greenland 1998).
The benefit of cervical cancer screening was directly estimated from the incidence and mortality rates. However, the figures of the mortality statistics are rather unreliable for cervical cancer (ICD-8 180), at least in the years before 1970, due to misclassification of cervical cancer into the category of ‘unspecified uterine cancer’ (ICD-8 179 and 182). Thus, mortality from all uterine cancers (ICD-8 179, 180, and 182) were combined and only mortality among the young age groups of 20- to 44-year-old women was considered, which is likely to be attributable mainly to cervical cancer (Levi et al. 2000). These rates were fitted with piecewise regression (Kim et al. 2000) using a computer program made available in the Internet (Joinpoint Regression Program, Version 2.5, March 2000, National Cancer Institute; http://srab.cancer.gov/joinpoint/). Then the resulting parametric regression lines were used to extrapolate the age-standardised mortality rates for cervical cancer (ICD-8 180) over all age groups to the years before 1970. The parameters of this set of regression lines were kept fixed and a multiplicative adjustment factor was determined by a least squares fit to the observed mortality rates after 1970 (Fig. 2).
Results
In the following, past achievements and future potential are considered for the cancer sites covered by the programme.
Cervical cancer (ICD-9 180)
Initial efforts to offer cervical cancer screening were started in the 1960s. Thus, for the assessment of the presumed benefit of early cervical cancer detection, the data of the 1960s should provide a reasonable picture of cervical cancer incidence before the start of screening. Figure 1a shows the incidence data of East and West Germany in comparison to selected countries of Europe and North America. It demonstrates that: a) in Germany in the early 1960s, the incidence ranged around a level comparable to that of other countries (e.g., USA) or even higher; and b) it shows a strong decline of German cervical cancer incidence from 1960 to 1990 by 73% (see Table 2). Figure 1b shows the secular trend of cervical cancer mortality among young women (age groups 20–44 years) and shows a decline of 74%, consistent with the incidence data. Notice that mortality declined even before the start of screening (explained by increased diagnostic activity outside screening, changes in diagnostic criteria, and improved treatment (Pontén et al. 1995)) so that these figures clearly indicate an upper limit of benefit.
Regarding the consistency of these findings with the performance data of the statutory early detection programme, one has to take into account that despite annual attendance rates ranging around 45%, biannual participation reached about 55%, and less than 20% of women were reported as never attending screening (Robra 1990). For a lower limit, the benefit of a biannual screening (91% mortality reduction) and the observed attendance rate (35%) have been used. Notice that the potentially relevant poor sensitivity of the test (see Discussion section) has been ignored with this procedure.
For the 1950s and 1960s, the cervical cancer mortality data for all age groups are unreliable (Becker and Wahrendorf 1997). Thus, in order to assess the overall benefit of the screening, the data of the young women was fitted with piecewise regression, and the resulting regression lines fitted to cervical cancer mortality (all age groups) after 1970 (Fig. 2) to obtain an extrapolation to the previous decades. The resulting estimated cervical cancer mortality rate for 1960 has been used to assess the proportion of cancer deaths prevented by screening, yielding a range of 3.2–4.7%. Using the mortality data of 1969–1971 would have provided much lower values and underestimated the effect (Table 3).
Regarding future potential, the theoretical benefit of early cervical cancer detection (about 90%) is assumed to be achievable which allows for a further reduction of cervical cancer mortality by about 60%; however, this reduction decreases cancer mortality starting from a baseline which is already very low (about 1% of overall cancer mortality in 1995, see Table 4).
Breast cancer (ICD-9 174)
Physical breast examination has been part of the early detection programme since the very beginning. However, since there is inadequate evidence for a mortality reduction by physical breast examination (IARC 2002), a 0% programme benefit cannot be excluded. In order to obtain an upper limit of a potential programme’s effectiveness, the mortality reduction potentially attributable to increased awareness of breast cancer may be taken into consideration. Increased awareness may shift breast cancers to earlier stages at the time of diagnosis as seen, for example, in the UK after the mid-1980s. Stockton et al. (Stockton et al. 1997), assessed the early diagnosis-related decrease of mortality to about 3.5–5%.
Mammography screening is not officially offered, but it is estimated that about 4–5 million “grey” mammographies are carried out annually (Schultz et al. 2001, page 7). Given a) the unsatisfactory quality of existing mammographic early detection of breast cancer as observed in a demonstration project (Frischbier et al. 1994) and b) the estimation of Blanks et al. (Blanks et al. 2000) that until now only a small fraction of changes in mortality can be attributed even to organised screening (6%), it is unlikely that the use of mammography has led to a considerable reduction of breast cancer mortality so far.
Overall, the programme effectiveness achieved so far may not exceed 6% mortality reduction as a generous upper limit providing an estimated maximal benefit of 0.4% (Table 4).
Regarding future potential, the benefit achievable by organised quality-controlled screening has been assumed to be about 35% among the participants in the age range 50–69 years, as assessed by the recent expert meeting (IARC 2002, p. 179) providing a breast cancer mortality reduction of about 15.7% over all age groups. With a participation rate of 50%, an overall cancer mortality reduction ranges around 0.7%, and with a participation rate of 70% it is around 1.0% (Table 4).
Colorectal cancer (ICD-9 153–154)
Based on the routinely collected data about participation in the early detection programme, Robra and Schwartz (Robra and Schwartz 1986) reported attendance rates of 15% (males) and 25% (females) for the year 1981. Taking the slightly increasing trend of compliance—at least among females—into account, we assumed attendance rates to FOBT of 15% among males and 35% among females (Table 3).
For efficacy, we used the 33% mortality reduction found for annual screening (Mandel et al. 1993) as the upper limit. The lower limit of zero takes into account that subjects may participate with irregular and sometimes long screening intervals and thus lose the benefit arising from regular participation.
DRE will not be taken into account separately, because: a) these cancers may also be found by FOBT; and b) even under a hypothetically assumed mortality reduction of 56% (lower confidence limit of the above-mentioned case-control study on DRE) among the distal rectal cancers (representing about 10% of all colorectal cancers), the benefit specific to DRE would be rather small and is well covered by the above-mentioned upper limit assumption.
Table 3 shows that, due to the different colorectal cancer mortality and attendance rates among males and females, the effect of screening may have been higher among females (0–11.6%) than males (0–5.0%). Since attendance at regular annual screening seems to have been rather low in the past (see Robra and Schwartz (1986)), a future increase of compliance at regular screening would probably provide almost the full effect of mortality reduction (16.5% or 23.1% with 50% or 70% compliance, respectively) implying a reduction of total cancer mortality by about 2.4% or 3.3%, respectively (Table 4).
Prostate cancer (ICD-9 185)
For estimating the past effect of DRE, we assumed a 10% mortality reduction as an upper limit of programme effectiveness assuming that the study of Friedman et al. (Friedman et al. 1991) might have been unable to confirm the observed effect (OR = 0.9, see above) statistically due to a too-small study size. The average participation rate of males was about 15% in the late 1980s. Despite the generous assumption, the potential reduction of mortality from prostate cancer is in the magnitude of 0–1.5% and for total cancer of 0–0.06% (Table 3). If one assumed—as a very extreme and unlikely maximal theoretical efficacy—the lower confidence limit (CL1) of CL1 = 0.5 in the DRE study (Friedman et al. 1991), providing a 50% mortality reduction, the upper limit of programme effectiveness in Table 3 would be 7.5% (prostate cancer mortality) and 0.31% (total cancer mortality), respectively (data not shown). PSA testing did not play a role in Germany in the 1980s which is the most relevant calendar time period for prostate cancer mortality in the mid-1990s. However, notice that for the year 2002 it is estimated that about 1.5 million tests are sold and most likely applied annually (personal communication).
Cancer of the skin (ICD-9 172–173)
Though no evidence about the efficacy of skin examination exists and a 0% effect may not be excluded, as an upper limit of programme effectiveness the OR = 0.37 of the case control study on skin self-examination (Berwick et al. 1996) wase incorporated into the evaluation of the potential past benefit. This is a rather strong assumption, since case-control studies tend to overestimate a screening effect (Begg et al. 1996; Miller 2002). Nevertheless, the mortality reducing effect is in the magnitude of 0–15.8% for cancers of the skin and, due to the small percentage of skin cancers among all cancers, around 0–0.15% for total cancer mortality (Table 3).
Overall evaluation
Taken together, the past benefit of the statutory early detection programme might range in the magnitude of 2.0–6.5%. Since the upper limit results from generous assumptions (breast, prostate, skin cancer) or inclusion of treatment effects (cervical and breast cancer), the true value might most likely be lower than the upper limit of 6.5% and closer to the estimates of the effect of cervical cancer screening (2.0–4.7%). The achievable future benefit, by exploiting the theoretical potential of screening more exhaustively, could provide a further cancer mortality reduction of about 3.4% (50% compliance) or 4.7% (70% compliance). Since the calculations behave additively, it can very simply be extrapolated that with a compliance of 90% the cancer mortality reduction would increase to about 6%.
Discussion
The methods used to assess past benefit and future potential of cancer screening in Germany may be considered to be crude, and they may be refined at some point or another. However, they appear to be sufficiently accurate to quantify the magnitude of the respective target quantities. Similar assessments are in agreement with the range presented above. An assessment carried out in the US in the context of a projection of the future potential of cancer control obtained a mortality reduction of 3% among women and zero among men (Greenland and Sondik 1986). Adami et al. (Adami et al. 2001) assumed a mortality reduction by cancer screening achieved in the past of about 6%, without, however, presenting the underlying quantitative assessment.
The estimated past achievement may be considered disappointing. However, these percentages mean—on the basis of the cancer mortality data of 1994–1996 (Table 4)—about 4,250–13,800 saved lives per year. On the other hand, the data on future potential indicate to what extent the available potential has not been exploited so far and imply that thousands of lives could have been saved annually with the existing screening methods even leaving full colonoscopy, which is part of the current programme, out of consideration.
In view of this partial failure of the programme, three issues must be addressed: compliance, the quality of the programme, and improvement and extension of available screening tools.
Organised screening
The remarkably poor compliance is apparently a characteristic of the opportunistic approach to screening. Other countries report attendance rates of approximately 80% (van Ballegooijen et al. 2000; Kuska 2000; Wang et al. 2001; Olsson et al. 2000) to 90% (Dean et al. 1999; van Ballegooijen et al. 2000) for organised screening programmes.
These rates are achieved on the basis of organised programmes which imply regular written invitations to the subjects eligible for screening. Beyond the purely quantitative aspect of high compliance, the organised approach allows for: a) focussing specifically on the target age range; b) strictly maintaining the screening intervals, i.e., avoiding too few or too many screening tests; and c) inclusion of social groups which have an above-average cancer risk, but are represented as below-average in opportunistic screening. All these topics may increase the effectiveness of the programme and, importantly, decrease misallocation of resources and, thus, save costs. Additionally, a revision of the offered screening methods based on the scientific evidence of their effectiveness would further improve the cost/benefit relationship.
Quality
Much concern has been raised about the quality of the programme. For example, at present about 14 million pap smears are taken annually (Bollmann 2001) and evaluated in about 2,000 cytological laboratories (Schenk and von Karsa 2000), leading to an average of about 7,000 smears per laboratory. This is below the recommended minimum of 20,000–30,000 smears per lab (Miller 2002). Reported values for the sensitivity of cytological screening are in the range of 20–30% (Schneider 1996; 2000), i.e., at the lower limit of internationally reported average values of 30–87% (Nanda et al. 2000). Taking the specificity of 96% (Schneider et al. 1996) to 99% (Schneider et al. 2000) into account, which is in agreement with international data (Nanda et al. 2000), and the fact that annual screening is recommended, the lifelong risk of a false-positive result is 36–84%.
For breast cancer, the estimated 4–5 million annual opportunistic mammographies are carried out by 2,500–3,000 licensed doctors plus a considerable unknown number of physicians in hospitals, leading to an average of less than about 1,670–2,000 mammographies per doctor per year. This is below the recommended minimum of 5,000 annual mammographies per doctor.
In the 1970s, digital rectal examination for prostate cancer was carried out for 77.9% of males by general practitioners and internists (Faul 1982) and provided a detection rate of 0.13% which is below the average of 0.85% in other countries (Bentvelsen and Schröder 1993). Generally, the organisational structures of the programme are unable to generate the data which are needed for regular in-time quality control (Kreienberg 2001; Schenk and von Karsa 2000).
Quality control has turned out to be crucial for the effectiveness of screening programmes. Screening tests may cause harm to the individuals undergoing the test. The only ethically justifiable way to offer screening is to provide it with highest possible quality and to perform routine in-time control. Excellent quality prevents expensive and time-consuming workup of false-positive diagnoses. It is thus not only ethical but additionally saves valuable resources. Again, the organised approach provides a superior frame for collecting the basic data for quality control.
Research on new or more efficacious screening modalities
Cervical cancer
A test would be desirable that indicates whether an oncogenic HPV virus has enhanced cell-cycle dysregulation and rendered infected cells susceptible to transformation, thereby facilitating the development of cancer. Such a test might be available now (Sano et al. 1998a and 1998b) and deserves careful evaluation. It should help to conduct cervical cancer screening more sensitively and more specifically and thus to enhance effectiveness and to reduce the costs of the programme.
Breast cancer
Even among small tumours (TNM stage pT1) found under regular high-quality mammography screening, a considerable portion (in the German pilot projects about 15%) have already disseminated into the lymph nodes implying an advanced stage of disease. Recent findings indicate that variants in genes coding for proteins regulating angiogenesis might affect the risk of early metastasis (Bange et al. 2002). The use of modern molecular biological tools in cancer screening might help to identify subjects who are at risk of early tumour spread and might benefit from a modified screening schedule.
Lung cancer
Currently, 85–90% of subjects diagnosed with lung cancer die of the disease. However, up to 70% of those diagnosed and confirmed as having stage 1a disease will survive 5 years. Recently, uncontrolled studies in Japan and the United States indicated that low-dose helical (spiral) computerised tomography of the lung is capable of detecting approximately four times as many small stage 1 lung cancers as chest X-rays, and that these patients appear likely to have a good prognosis (Henschke et al. 1999; Kaneko et al. 1996; Kakinuma et al. 1999; Sone et al. 1998). Before broad application, the new approach needs careful examination for several reasons: a) it is still unclear whether the technique selectively detects only those cancers with a good prognosis, leaving those with a bad prognosis occurring at about the same time in their natural history as at present, and thus leading to little overall benefit; b) the longer survival generally observed with early detection can be caused by advancing the time of diagnosis and may not be a result of prolonged lifetime as expected from effective screening; and c) a real benefit in terms of mortality reduction has still to be demonstrated. Currently, in Europe and North America, several randomised trials are in preparation and need funding aiming at a conclusive result in 5 year’s time (see, e.g., van Klaveren et al. 2001).
Conclusions
Screening partially requires an expensive medical infrastructure and is not without risks for the participants. The overall benefit is critically dependent upon the quality of the programme and its in-time control. Any benefit may be annulled by poor quality while costs are overflowing.
Well-organised high-quality screening may be a valuable part of cancer control. In order to preserve or to increase the impact of screening and to control its expenses: a) further research efforts are needed towards new or better targeted screening tools or modalities; b) the efficacy of new modalities has to be evaluated carefully in advance of a broad application: c) the programme has to be reconceptualised as organised screening based on scientifically justified characteristics (screening methods, screening frequency, target age groups); and d) in-time quality control based on the collection of the basic performance data must be an intrinsic part of the programme.
References
Adami H-O, Day NE, Trichopoulos D, Willett WC (2001) Primary and secondary prevention in the reduction of cancer morbidity and mortality. Eur J Cancer 37:S118–S127
Anonymous (1985) Cancer of the cervix: death by incompetence. Lancet ii:363–364
Auvinen A, Rietbergen J, Gohagen J, Denis L, Schröder F (1996) Prospective evaluation plan for randomized trials of prostate cancer screening. J Med Screening 3:97–104
Auvinen A, Alexander FE, de Koning HJ, Miller AB (2002) Should we start population screening for prostate cancer? Randomised trials are still needed. Int J Cancer 97:377–378
Ballard-Barbash R, Klabunde C, Paci E, Broeders M, Coleman EA, Fracheboud J, Bouchard F, Rennert G, Shapiro S (1999) Breast cancer screening in 21 countries: delivery of services, notification of results and outcomes ascertainment. Eur J Cancer 8:417–426
Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, Knyazeva T, Müller S, Gärtner S, Sures I, Wang H, Imyanitov E, Häring H-U, Knayzev P, Iacobelli S, Höfler H, Ullrich A (2002) Cancer progression and tumor cell motility are associated with the FGFR4 Arg388 Allele. Cancer Res 62:840–847
Becker N (2001) Epidemiologic aspects of cancer prevention in Germany. J Cancer Res Clin Oncol 127:9–19
Becker N (2002) Screening from the epidemiological point of view. Der Radiologe 42:592–600 (in German)
Becker N, Wahrendorf J (1997) Atlas of cancer mortality in the Federal Republic of Germany (1981–1990), 3rd edn. Springer, Berlin Heidelberg New York Tokyo
Begg CB, Huang Y, Berwick M (1996) Separate estimation of primary and secondary cancer prevention impact: analysis of a case-control study of skin self-examination and melanoma. J Am Assoc 91:1381–1387
Bentvelsen FM, Schröder FH (1993) Modalities available for screening for prostate cancer. Eur J Cancer 29A:804–811
Berwick M, Beg CB, Fine JA, Roush GC, Barnhill RL (1996) Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst 88:17–23
Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ (2000) Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. BMJ 321:665–669
Bollmann R (2001) Diagnostik der CIN und des invasiven Zervixkarzinoms. Zentralbl Gynäkol 123:206–210
Bundesausschuß der Ärzte und Krankenkassen (1996) Richtlinien des Bundesausschusses der Ärzte und Krankenkassen über die Früherkennung von Krebserkrankungen (Krebsfrüherkennungs-Richtlinien). In: Kassenärztliche Bundesvereinigung (Hrsg) Verträge der Kassenärztlichen Bundesvereinigung mit Sozialversicherungs- und anderen Kostenträgern sowie Richtlinien des Bundesausschusses der Ärzte und Krankenkassen Dienstauflage der Kassenärztlichen Bundesvereinigung. Deutscher Ärzte, Köln, Bd 1
Chamberlain J, Day NE, Hakama M, Miller AB, Prorok PC (1986) UICC workshop of the project on evaluation of screening programmes for gastrointestinal cancer. Int J Cancer 37:329–334
Chamberlain J, Miller AB (eds) (1988) Screening for gastro-intestinal cancer. Hans Huber, Toronto
Christiansen CL, Wang F, Barton MB, Kreuter W, Elmore JG, Gelfand AE, Fletcher SW (2000) Predicting the cumulative risk of false-positive mammogramms. JNCI 92:1657–1666
Coleman D, Day N, Douglas G, Farmery E, Lynge E, Philip J, Segnan N (1993) European guidelines for quality assurance in cervical cancer screening Europe Against Cancer Programme. Eur J Cancer 29A [Suppl 4]:S1–S38
Cuzick J (1999) Screening for cancer: future potential. Eur J Cancer 35:1925–1932
Day NE, Baines CJ, Chamberlain J, Hakama M, Miller AB, Prorok P (1986) UICC project on screening for cancer: report of the workshop on screening for breast cancer. Int J Cancer 38:303–308
Day NE, Miller AB (eds) (1988) Screening for breast cancer. Hans Huber, Toronto
Dean PB, Pamilo M for Mammography Working Group, Radiological Society of Finland (1999) Screening mammography in Finland–15 million examinations with 97 percent specificity. Acta Oncologica [Suppl 13]:47–54
Devesa SS, Silverman DT, Young JL, Pollack ES, Brown CC, Horm JW, Percy CL, Myers MH, McKay FW, Fraumeni JF Jr (1987) Cancer incidence and mortality trends among whites in the United States, 1947–84. JNCI 79:701–770
Doll R, Payne P, Waterhouse J (1966) Cancer incidence in five continents: a technical report. Springer, Berlin Heidelberg New York, International Union Against Cancer
Doll R, Muir C, Waterhouse J (1970) Cancer incidence in five continents, vol II. Springer, Berlin Heidelberg New York, International Union Against Cancer
Elwood JM (1996) Skin self-examination and melanoma. J Natl Cancer Inst 88:3–5
Esserman L, Cowley H, Eberle C, Kirkpatrick A, Chang S, Berbaum K, Gale A (2002) Improving the accuracy of mammography: volume and outcome relationships. J Natl Cancer Inst 94:369–375
Faul P (1982) Experience with the German annual preventive checkup examination. In: Jacobi GH, Hohenfellner R (eds) Prostate cancer international perspectives in urology, vol 3. Williams & Wilkins, Baltimore London
Flatten G (1988) Prävention–Eine bewährte Strategie ärztlichen Handelns Zentralinstitut für die kassenärztliche Versorgung in der Bundesrepublik Deutschland. Deutscher Ärzte, Köln
Friedman GD, Hiatt RA, Quesenberry CP, Selby JV (1991) Case-control study of screening for prostatic cancer by digital rectal examinations. Lancet 337:1526–1529
Frischbier H-J Hoeffken W, Robra B-P (eds) (1994) Mammographie in der Krebsfrüherkennung Qualitätssicherung und Akzeptanz Ergebnisse der Deutschen Mammographie-Studie. Enke, Stuttgart
Greenland P, Sondik EJ (eds) (1986) NCI Monographs. Cancer control objectives for the nation: 1985–2000. Public Health Service, National Cancer Institute 2
Hakama M, Chamberlain J, Day NE, Miller AB, Prorok PC (1985) Evaluation of screening programmes for gynaecological cancer. Br J Cancer 52:669–673
Hakama M, Miller AB, Day NE (eds) (1986) Screening for cancer of the uterine cervix. IARC Scientific Publications No 76. IARC, Lyon
Hardcastle JD, Chamberlain JO, Robinson MHE, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM (1996) Randomised controlled trial of fecal occult blood: screening for colorectal cancer. Lancet 348:1472–1447
Herrinton LJ, Selby JV, Friedman GD, Quesenberry CP, Weiss NS (1995) Case-control study of digital-rectal screening in relation to mortality from cancer of the distal rectum. Am J Epidemiol 142:961–964
Herwig E (1975) Krankheitsfrüherkennung–Krebs–Frauen und Männer Aufbereitung und Interpretation der Untersuchungsergebnisse aus den gesetzlichen Früherkennungsmaßnahmen 1972 Zentralinstitut für die kassenärztliche Versorgung in der Bundesrepublik Deutschland. Deutscher Ärzte, Köln
IARC (2002) Breast cancer screening. IARC Handbooks of cancer prevention, Volume 7. International Agency for Research on Cancer World Health Organization. IARC, Lyon
Junkermann H, Becker N, Peitgen H-O (2001) Konzept und Durchführung der Modellprojekte für Mammographiescreening in Deutschland. Radiologe 41:328–336
Henschke CI, McCauley DI, Yankelevitz DF, et al (1999) Early lung cancer action project: overall design and findings from baseline screening. Lancet 354:99–104
Kakinuma R, Ohmatsu H, Kaneko M, et al (1999) Detection failures in spiral CT screening for lung cancer: analysis of CT findings. Radiology 212:61–66
Kaneko M, Eguchi K, Ohmatsu H, et al (1996) Peripheral lung cancer: screening and detection with low-dose spiral CT versis radiography. Radiology 201:798–802
Kim H-J, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335–351
Kuroishi T, Hirose K, Suzuki T, Tominaga S (2000) Effectiveness of mass screening for breast cancer in Japan. Breast Cancer 7:1–8
Kreienberg R (2001) Früherkennung von Karzinomen der Zervix, Vulva, Vagina. Der Gynäkologe 11:1079–1085
Koss LG (1989) The Papanicolaou test for cervical cancer detection: a triumph and a tragedy. JAMA 261:737–743
Kronborg O, Fenger C, Olsen J, Jörgensen OD, Sondergaard O (1996) Randomised study of screening for colorectal cancer with faecal occult-blood test. Lancet 348:14467–14471
Kuska B (2002) Mass breast cancer screening in the Netherlands: 10 years and counting. J Natl Cancer Inst 90:1764–1766
Levi F, Lucchini F, Negri E, Franceschi S, la Vacchia C (2000) Cervical cancer mortality in young women in Europe: patterns and trends. Eur J Cancer 36:2266–2271
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F (1993) Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 328:1365–1371
Mandel JS, Church TR, Ederer F, Bond JH (1999) Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 91:434–437
Miller AB (1978) Screening iN CAncer: a report of a UICC international workshop. UICC Technical Report Series–vol 40, Toronto, Canada, April 24–27. UICC, Geneva
Miller AB (ed) (1985) Screening for cancer. Academic, Toronto Montreal
Miller AB (2002) The (in)efficiency of cervical screening in Europe. Eur J Cancer 38:321–326
Miller AB, Chamberlain J, Day NE, Hakama M, Prorok PC (1990) Report on a workshop of the UICC project on evaluation of screening for cancer. Int J Cancer 46:761–769
Miller AB, Chamberlain J, Day NE, Hakama M, Prorok PC (1991) Cancer screening. UICC, Cambridge New York
Möhner M, Stabenow R, Eisinger B (1994) Atlas of cancer incidence in the GDR 1961–1989 (German/English). Ullstein Mosby, Berlin Wiesbaden
Morrison AS (1992) Screening in chronic disease monographs in epidemiology and biostatistics, vol 19. Oxford University, New York Oxford
Muir C, Waterhouse J, Mack T, Powell J, Whelan S (1987) Cancer incidence in five continents, vol V. IARC Scientific Publications No 88. IARC, Lyon
Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB (2000) Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 132:810–819
Nieminen P, Kallio M, Anttila A, Hakama M (1999) Organised vs spontaneous PAP-smear screening for cervical cancer: a case-control study. Int J Cancer 83:55–58
Olsson S, Andersson I, Karlberg I, Bjurstam N, Frodis E, Hakansson S (2000) Implementation of service screening with mammography in Sweden: from pilot study to nationwide programme. J Med Screening 7:14–18
Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J, Powell J (1992) Cancer incidence in five continents, vol VI. IARC Scientific Publications No 120. IARC, Lyon
Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J (1997) Cancer incidence in five continents, vol VII. IARC Scientific Publications No 143. IARC, Lyon
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB (2002) Cancer incidence in five continents, vol VII. IARC Scientific Publications No 155. IARC, Lyon
Perry N, Broeders M, de Wolf C, Törnberg S (eds) (2001) European guidelines for quality assurance in mammography screening, 3rd edn. Office for Official Publications of the European Communities, Luxembourg
Pontén J, Adami H-O, Bergström R, Dillner J, Friberg L-G, Gustafsson L, Miller AB, Parkine M, Sparén P, Trichopoulos D (1995) Strategies for global control of cervical cancer. Int J Cancer 60:1–26
Prorok PC, Chamberlain J, Day NE, Hakama M, Miller AB (1984) UICC Workshop on the evaluation of screening programmes for cancer. Int J Cancer 34:1–4
Rittgen W, Becker N (2001) Statistical issues in quality control of organized mammography screening. J Epidemiol Biostatistic 6:425–432
Robra B-P, Schwartz FW (1986) Experiences with a nationwide screening program for colorectal cancer in the Federal Republic of Germany. In: Hardcastle JD (ed) (1986) Haemoccult screening for the early detection of colorectal cancer. Schattauer, Stuttgart New York
Robra B-P, Dierks M-L (1990) Entwicklung der Teilnahme an den Krebsfrüherkennungsuntersuchungen der Frau. Gynäkologe 23:308–311
Rothman KJ, Greenland S (1998) Modern epidemiology. Lippincott–Raven, Philadelphia, pp 55 and pp 295
Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T (1998a) Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. Pathol Int 48:580–585
Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T (1998b) Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Path 153:1741–1748
Schenck U, von Karsa L (2000) Cervical cancer screening in Germany. Eur J Cancer 36:2221–2226
Schneider A, Zahm DM, Kirchmayr R, Schneider VL (1996) Screening for cervical intraepithelial neoplasia grade 2/3: validity of cytologic study, cervicography, and human papillomavirus detection. Am J Obstet Gynecol 174:1534–1541
Schneider A, Hoyer H, Lotz B, Leistritza S, Kühne-Heid R, Nindl I, Müller B, Haerting J, Dürst M (2000) Screening for high-grade cervical intra-epithelial neoplasia and cancer by testing for high-risk HPV, routine cytology or colposcopy. Int J Cancer (Pred Oncol) 89:529–534
Schulz K-D, Albert U-S, Kreienberg R, Fischer F (2001) Brustkrebs-Früherkennung in Deutschland. Manual I und II. Konzertierte Aktion Brustkrebs-Früherkennung in Deutschland, Marburg
Shapiro S, Coleman EA, Broeders M, Codd M, de Koning H, Fracheboud J, Moss S, Paci E, Stachenko S, Ballard-Barbash R (1998) Breast cancer screening programmes in 22 countries: current policies, administration, and guidelines. Int J Epidemiol 27:735–742
Sone S, Takashima S, Li F, et al (1998) Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 351:1242–1245
Stockton D, Davies T, Day N, McCann J (1997) Retrospective study of reasons for improved survival in patients with breast cancer in East Anglia: earlier diagnosis or better treatment? BMJ 314:472–475
Van Ballegooijen M, van den Akker-van Marle E, Patnick J, Lynge E, Arbyn M, Anttila A, Ronco G, Dik J, Habbema F (2000) Overview of important cervical cancer screening process values in European Union (EU) countries, and tentative predictions of the corresponding effectiveness and cost-effectiveness. Eur J Cancer 36:2177–2188
Van Klaveren RJ, Habbema JDF, Pedersen JH, de Koning HJ, Oudkerk M, Hoogsteden HC (2001) Lung cancer screening by low-dose computed tomography. Eur Resp J 18:857–866
Wang H, Karesen R, Hervik A, Thoresen SO (2001) Mammography screening in Norway: results from the first screening round in four counties and cost-effectiveness of a modeled nationwide screening. CCC 12:39–45
Waterhouse J, Muir C, Correa P, Powell J (1976) Cancer incidence in five continents, vol III. IARC Scientific Publications No 15. IARC, Lyon
Waterhouse J, Muir C, Shanmugaratnam K, Powell J in collaboration with Peacham D, Whelan S (1982) Cancer incidence in five continents, vol III. IARC Scientific Publications No 42. IARC, Lyon
Willett WC, Colditz GA, Mueller NE (1996) Strategies for minimizing cancer risk. Sci Am 58–63
Wilson JMG, Jungner G (1968) Principles and practice of screening for disease. Public Health Papers 34. WHO, Geneva
Acknowledgements
I thank Roland Stabenow, Gemeinsames Krebsregister, for making East German cancer incidence data available, Anthony B. Miller for many helpful discussions and support in writing the article, Evi Deeg for preparing the graphics, and Heike Weis for text editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becker, N. Epidemiological aspects of cancer screening in Germany. J Cancer Res Clin Oncol 129, 691–702 (2003). https://doi.org/10.1007/s00432-003-0494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0494-y