Abstract
Purpose
Esophageal squamous cell carcinoma (ESCC) in the Indian population exhibits insidious symptomatology, late clinical presentation, aggressive behavior, and high propensity for metastasis. Ets-1, a transcription factor, is expressed in esophageal tumors and associated with poor prognosis. The aim of the present study was to determine the relationship between Ets-1 expression, tumor angiogenesis [vascular endothelial growth factor (VEGF) and microvessel density (MVD)] and the biological behavior of ESCCs.
Methods
In a prospective study the expression of Ets-1, VEGF, and PECAM-1 (CD-31) was determined in 55 ESCCs, by immunohistochemical analysis, correlated with clinicopathological parameters and outcome of the patients.
Results
Overexpression of Ets-1 and VEGF proteins was observed in 44/55 (80%) and 38/55 (69%) of ESCCs, respectively. VEGF immunopositivity was associated with lymph node metastasis (P=0.002). Analysis of mRNA isoforms using RT-PCR revealed increased expression of VEGF 121 transcripts in ESCCs and MVD was correlated with de-differentiation status of the tumors (P=0.049). Kaplan-Meier survival analysis showed significant correlation between poor disease-free survival and tumor stage (P=0.02) and with nodal metastasis (P=0.05). Concomitant expression of VEGF, Ets-1 proteins, and high MVD was correlated with poor disease-free survival (P=0.004).
Conclusion
Significant association of Ets-1 and VEGF proteins with tumor angiogenesis (MVD), lymph node invasion, and poor disease-free survival underscores their relevance regarding aggressive tumor behavior and highlights their potential utility as adverse prognostic factors in esophageal carcinomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma (ESCC) ranks among the six most common cancers worldwide, with a five-year survival rate as low as 5% (Landis et al. 1999). There are more than 300,000 new cases of esophageal cancer (EC) annually; 80% of these occur in the developing countries (Hart and Saini, 1992). The disease is characterized by late clinical presentation, rapid progression, and poor survival (Kohn and Liotta, 1995). Esophageal tumors show extensive local invasion and frequent spread to metastatic sites, posing a major challenge to effective management of the disease. Prediction of tumor angiogenesis by analysis of novel molecular prognostic markers might aid in developing more effective therapeutic strategies for better patient care (Shimada et al. 1999; Hosch et al. 2001). However, none of the molecular markers yet identified has shown unequivocal prognostic utility in EC. Thus, there is an urgent need to identify new indicators of biological malignant potential of ESCCs. In view of the pivotal role of lymph node metastasis in patients with EC, precise knowledge of regulators of lymphatic spread is imperative. Angiogenesis is necessary not only for malignant solid tumor growth, but also for tumor invasion (Folkman 1991; Thompson 2001).
Ets-1 belongs to a distinct class of sequence specific DNA-binding transcription factors, implicated in the transcriptional regulation of proteins mediating angiogenesis, invasion, and metastasis (Vandenbunder et al. 1994; Wernert et al. 1994; Pande et al. 1999). In invasive carcinomas, Ets-1 is found to be overexpressed in stromal fibroblasts surrounding invasive tumors and in the endothelial cells of stromal capillaries. Invasive processes and angiogenesis require degradation of extracellular matrix-by-matrix metalloproteases (MMPs). MMPs have Ets binding domains in their promoters, suggesting that there could be a link between cytokines and angiogenic factors released from tumors, Ets-1, and MMPs. In a variety of human tumors the expression patterns of Ets-1, collagenase, and urokinase suggest their involvement in tumor invasion and metastasis (Bolon et al. 1995). In a recent study, Ets-1 expression has been correlated with penetrating tumor progression in ESCC patients (Saeki et al. 2000). Intratumoral microvessel density (MVD) is the most accurate, independent prognostic factor for disease-free survival; high MVD has been reported to be independently correlated with poor disease-free survival or overall survival in several cancers (Dunphy et. al. 2002; Poon et. al. 2002; Ogawa et. al. 2002). Among the angiogenic factors, vascular endothelial growth factor (VEGF) is currently arousing much interest for its potential as a key regulator of angiogenesis and as a putative target for molecular therapeutics.
VEGF is a potent, multifunctional cytokine that exerts several important and possibly independent effects on the vascular endothelium (Senger et al. 1993). Around the tumor cells, VEGF is proposed to play an important role by directly stimulating the endothelial cells to proliferate and migrate. It also activates many proteases that degrade the surrounding matrix. Recently, VEGF expression has been shown to predict outcome and lymph node metastasis in ESCC (Shin et al. 2000; Millikan et al. 2000).
Taken together, the aforesaid reports suggest a crucial role for Ets-1, VEGF, and intratumoral microvessel density as isolated factors in esophageal tumor progression. However, to our knowledge, the relationship between expression of Ets-1 and angiogenic determinants—namely VEGF and MVD—in human ESCCs remains to be determined. The present study was undertaken to analyze the expression of Ets-1 and VEGF proteins in ESCCs and determine their correlation with intratumoral MVD, clinicopathological parameters, risk factors, and disease prognosis, with the goal of evaluating their potential for predicting tumor aggressiveness and prognosis of esophageal cancer.
Patients and methods
Patients and tissue specimens
This study was approved by the ethics committee of the All India Institute of Medical Sciences, New Delhi, India. Informed consent was obtained from all patients before inclusion in the study. Fifty-five surgically resected specimens of ESCCs were obtained from Gastrointestinal Surgery O.T., All India Institute of Medical Sciences, New Delhi, India. Specimens of 27 paired normal esophageal tissues were obtained from a distal site from the esophageal lesions of cancer patients during curative esophagectomy. Specimens were fixed in 10% formaldehyde solution and embedded in paraffin. Sections, 5-μm thick, were cut, mounted on glass slides, and air dried. The clinical and pathological data were recorded in a predesigned proforma including TNM staging (Sobin et al. 1997), histopathological differentiation, age, gender, and site of the lesion (Table 1). The age of the patients ranged from 35 years to 75 years; 33 were male and 22 were female. The patients were grouped based on the tumor stage pT1/pT2 (19 patients); pT3/pT4 (36 patients), and nodal metastasis pN0 (24 patients); pN1 (31 patients) distant organ metastasis pM0 55 patients. The various subsites of esophageal tumors included: upper and middle one-third (25 patients) and lower one-third (30 patients).
Follow-up studies
Fifty-five primary ESCC patients were enrolled in the follow-up study for whom the expression of Ets-1, VEGF, and MVD was analyzed prior to treatment, to investigate the prognostic utility of these markers. The primary tumors were surgically resected and the patients were followed up periodically (at 3-month intervals) in the cancer clinic. Esophageal cancer patients underwent complete curative tumor resection (resection margins free of tumor cells), thus achieving an R0 situation. Ten patients were lost during the follow-up due to poor patient compliance and socioeconomic restraints. Most of these patients were referred to the All India Institute of Medical Sciences from different parts of the country. After surgery these patients returned to their native place and were lost to follow-up. Forty-five of these 55 patients could be monitored up to a period of 44 months. The mean follow-up period of the patients was 18.7 months. These 45 patients received postoperative radiotherapy (RT, five cycles of 5 Gy over a period of 30 days, total dose 25 Gy). Tumor recurrence was observed in 17 patients monitored in this study and six patients died due to the disease during the follow-up. Twenty-two patients did not show recurrence of the disease and were alive at the end of the follow-up period. Disease-free survival was expressed as the number of months from date of surgery to the date of detection of local recurrence of the disease or systemic metastasis. Consecutive tissue sections were used for the immunohistochemical analysis of Ets-1, VEGF, and MVD; to assess the prognostic relevance of these potential biomarkers on clinical outcome, univariate analysis was carried out.
Immunohistochemical staining of Ets-1, VEGF proteins, and microvessels
Paraffin-embedded sections (5-μm thick) were used for immunostaining of Ets-1, VEGF, and PECAM-1 (marker for microvessels) proteins. Briefly, the slides were deparaffinized in xylene (three times for 5 min each) and absolute alcohol (twice for 2 min each) and hydrated. Hematoxylin and eosin staining was carried out for histopathological analysis. Immunostaining was performed in serial sections. Endogenous peroxidase activity was blocked by incubating the sections for 30 min in methanol containing hydrogen peroxide (0.3% v/v). Slides were then washed with 0.01 M Tris buffered saline pH 7.4 (TBS) and heated for 15 min at 100 °C (microwave treatment) in 10 mM sodium citrate buffer (pH 6.0) for antigen retrieval. The slides were cooled to 37 °C. Non-specific binding was blocked by incubating in 1% BSA in 0.01 M PBS pH 7.4 for 30 min. After washing with TBS, the tissue sections were incubated with primary antibodies, anti-Ets-1 polyclonal antibody (C-20), anti-VEGF monoclonal antibody (C-1), anti-PECAM-1 (CD 31, C-20) monoclonal primary antibody for microvessel counting (Santa Cruz Biotechnology, Santa Cruz, Calif., USA) at a dilution of 1:100 (final concentration 1 μg/ml) at 4 °C overnight (o/n) as described previously (Pande et al. 1999). Thereafter, slides were washed with TBS and incubated with biotinylated secondary antibody (rabbit anti-mouse IgG for monoclonal antibodies and goat anti-rabbit IgG for polyclonal antibodies) for 45 min at 37 °C. Following TBS washes, the slides were incubated with the avidin-biotin-horseradish peroxidase complex (LSAB plus kit, Dakopatts, Denmark) for 45 min at 37 °C. Color was developed with 0.06% (w/v) 3,3′-diaminobenzidine tetrahydrochloride (DAB) and hydrogen peroxide (0.03% v/v) in 10 mM PBS, pH 7.5. In negative controls the primary antibody was replaced by PBS or nonimmune mouse IgG of the same isotype to ensure specificity. Human oral squamous cell carcinoma tissue sections with known immunopositivity for Ets-1, VEGF, and PECAM-1 were used as the positive controls in each batch of sections analyzed (data not shown).
Positive criteria for immunohistochemical staining
Tumors were regarded as Ets-1 immunopositive if the tumor cells showed cytoplasmic and/or and nuclear immunoreactivity. Ets-1 immunostaining was classified into four categories defined by the percentage of tumor cells displaying positive staining as follows: <10%= negative, 10–30%= +1, 30–50%= +2, and >50%= +3. The VEGF expression in the tumor cells was evaluated using a semi-quantitative scoring system: 0 for absence of immunostaining, +1 for <20% positive tumor cells, +2 for 20–50% positive tumor cells, and +3 for >50% positive tumor cells. The immunostaining was evaluated in five areas of the slide sections for correlation and confirmation of the tissue analysis. The matched distal histologically normal esophageal tissues were regarded as immunopositive for Ets-1 or VEGF protein if the epithelial cells showed cytoplasmic staining. The immunostaining was classified as described above taking into account the percentage of stained epithelial cells.
Microvessel counting
Microvessel density (MVD) was assessed in tumor areas showing the highest density of staining as determined by an initial scan at low-power magnification (×100) to identify the areas with the highest density of microvessels (Bossi et al. 1995). In each case, the three most vascularized areas were selected, and the microvessels in these three areas were counted in a ×200 field (field diameter, 0.9 mm). The average counts of the three ×200 fields were recorded for analysis. The areas with the highest density of staining were predominantly localized at the invading edge of the tumor mass. MVD was determined by two of us independently (T.M, M.M). The histopathological grading and immunostaining for assessment was carried out in a blind study using coded slides, without any prior knowledge of the clinicopathological parameters or clinical outcome of the patients.
Immunoblotting
Total cellular protein extracts (100 μg protein/lane) prepared from ESCCs and normal esophageal tissues—following the procedures previously described from our laboratory—were resolved by SDS-PAGE using the Bio-Rad mini gel electrophoresis apparatus, and proteins were transferred to nitrocellulose membranes. The membranes were treated with a blocking solution (5% non-fat milk in phosphate-buffered saline 0.01 M, pH 7.5) overnight at 4 °C, washed, and incubated with anti-Ets-1, anti-VEGF or anti-PECAM-1 (CD31) primary antibody (final concentration 0.1 μg/ml) overnight at 4 °C. The anti-VEGF antibody used is specific for VEGF and does not cross react with any of the other VEGF isoforms. Membranes were washed with tris-buffered saline-Tween 20 five times after each incubation step. Thereafter, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody at a dilution of 1:400 for 2 h at 37 °C and washed. Proteins were detected by the enhanced chemiluminescence method.
Statistical analysis
Statistical analysis of the data was performed using the Microstat software and SPSS software (version 9.0). The relationship between expression of Ets-1, VEGF, and MVD and patient variables were analyzed using Fisher's exact test or Chi square test. Independent t-test was performed for determining the relationship between MVD and clinicopathological parameter and Ets-1 and VEGF proteins using Statistic version 4.0. The survival curves were obtained using the Kaplan-Meier method and the difference in survival time for tumors with high or low expression of VEGF, Ets-1, and MVD was analyzed using the log-rank test. P values less than 0.05 were considered to be significant.
Results
Ets-1 protein expression in esophageal squamous cell carcinomas
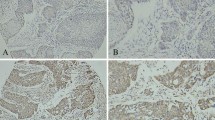
The results of immunohistochemical analysis of Ets-1, VEGF, and PECAM-1 expression in serial sections of 55 untreated primary ESCCs are summarized in Table 1. Intense Ets-1 staining was observed in 44 of 55 (80%) ESCCs. Ets-1 protein was localized either in the cytoplasm and/or nucleus of tumor cells in the deep margin areas of the invasive layer (Fig. 1a), while the histologically normal esophageal tissues showed low basal level of Ets-1 staining (Fig. 1b). Immunoblotting analysis in a subset of ESCCs (ten patients) showed a 54 kDa band (Fig. 2a). Analysis of Ets-1 mRNA by reverse transcription polymerase chain reaction (RT-PCR) revealed a 500 bp PCR product in ESCCs and esophageal carcinoma cell line TE 13 (Fig. 3a).
Expression of Ets-1, VEGF, and PECAM-1 proteins in esophageal squamous cell carcinomas. a Representative photomicrograph showing immunostaining of Ets-1 protein in cytoplasm and nucleus of tumor cells in the invading layer of the epithelium of ESCC; b Matched histopathologically normal epithelia showing basal Ets-1 expression; c Expression of VEGF protein in the invading layer of the epithelium of ESCC; d Matched histopathologically normal epithelia showing basal VEGF expression in the cytoplasm; e Moderately differentiated ESCC showing positively stained blood vessels; f Matched histopathologically normal epithelium showing positively stained blood vessels. (a–f, original magnification ×200)
Immunoblotting analysis of Ets-1 protein expression in esophageal tissues. Lane 1 shows a 54 kDa band of Ets-1 protein in ESCC cell line, TE-13 (positive control); Lanes 2 and 4 show no detectable expression of Ets-1 in normal esophageal tissues, while the corresponding ESCC tissues show 54 kDa band of Ets-1 in Lanes 3 and 5; b Immunoblotting analysis of VEGF protein in esophageal tissues. Lane 1 shows a 32 kDa band of VEGF protein in TE-13 ESCC cell line (positive control), Lanes 2 and 4 show expression of VEGF protein in ESCCs, while no detectable expression of VEGF protein was observed in the matched normal esophageal tissues, Lanes 3 and 4; c Immunoblotting analysis of PECAM-1 protein in esophageal tissues. Lane 1 shows a band of 130 kDa depicting basal level of PECAM-1 expression in esophageal tissue; Lane 2 shows increased expression of PECAM-1 protein in ESCC
RT-PCR analysis of Ets-1 mRNA in esophageal normal and tumor tissues. Lane M 1 kb ladder; Lane 1 Negative control, the RNA was replaced by equal amount of water; Lanes 2, 4, 6, and 8 show amplified RT-PCR product of 500 bp; Lane 2 shows 500 bp Ets-1 mRNA transcript in TE-13 esophageal cancer cell line; Lanes 4, 6, and 8 show 500 bp Ets-1 mRNA transcript in ESCCs. In the matched normal esophageal tissues, Lanes 3 and 7 do not show detectable Ets-1 mRNA transcripts, while in Lane 5 a basal level of mRNA transcripts was observed; b RT-PCR analysis of VEGF mRNA levels in esophageal normal and tumor tissues. Lane M 1 kb ladder; Lane 1 Negative control, the RNA was replaced by equal amount of water; Lanes 2, 4, 6, and 8 show amplified RT-PCR product of 408 bp; Lane 2 shows 408 bp and 541 bp VEGF mRNA transcript in TE-13 esophageal cancer cell line; Lanes 4, 6, and 8 show 408 bp VEGF mRNA transcript in ESCCs; Lanes 3, 5, and 7 show no detectable VEGF mRNA transcript in corresponding normal esophageal tissues
VGEF expression and intratumoral microvessel density
Thirty-eight of 55 (69%) ESCCs showed VEGF protein expression. In most of the ESCCs increased expression of VEGF protein was observed in the invading layer of the epithelium (Fig. 1c), while low basal level of VEGF was seen in histologically normal esophageal tissues (Fig. 1d). Multivariate analysis carried out showed VEGF as an independent predictor of lymph node metastasis (P=0.002). Immunoblotting analysis showed 32 kDa band in ESCCs (Fig. 2b). Analysis of VEGF mRNA isoforms by RT-PCR revealed a 408 bp PCR product representing the VEGF 121 isoform in ESCCs (Fig. 3b). To evaluate vascular sprouting, the number of microvessels, endothelial cells stained with anti-PECAM-1(CD31) polyclonal antibody, were counted (Fig. 1e). Intratumoral MVD in ESCCs was compared with that in the paired histopathologically normal esophageal tissues (Fig. 1e and f), respectively. MVD was significantly increased in esophageal tumors as compared to the histologically normal esophageal tissues (P=0.034). Immunoblotting analysis showed an intense 130 kDa band in ESCCs, while basal level of PECAM-1 expression was observed in normal esophageal tissue (Fig. 2c). The MVD was observed to increase with increase in VEGF expression (P=0.062). ESCCs showing increased VEGF immunoreactivity had higher MVD (13.65±9.05, mean±SD/mm2) as compared to cases which did not show detectable levels of VEGF immunostaining, MVD (10.01±6.37 mean±SD/mm2) (Fig. 4a). The MVD based on the mean number of vessels was divided into two categories. Cases with a number of vessels greater than the mean value were taken as positive while cases with MVD less than the mean value were considered negative for analyzing the association between MVD and the various clinicopathological parameters. MVD was significantly correlated with dedifferentiation of the tumors from well- to moderately to poorly differentiated squamous cell carcinoma (P=0.049, (Fig. 4b). Significant correlation was observed between expression of Ets-1 protein, VEGF, and high MVD (P=0.049).
Relationship between VEGF immunostaining and MVD in ESCC. Percentage of cells positive for VEGF protein <10%= negative, 10–30%= +1, 30–50%= +2, and >50%= +3. The MVD for tumors negative for VEGF protein was 10.01±6.37 mm2/HPF (mean±SD); with +1 positivity was 10.46±5.6 mm2/HPF(mean±SD), with +2 positivity was 11.20±6.16 mm2/HPF (mean±SD), and with +3 positivity was 13.65±9.05 mm2/HPF (mean±SD); b Relationship between histopathological grading and MVD in ESCC. In tumors that were well-differentiated (1.0) the MVD was 8.03±4.72 mm2/HPF (mean+SD), while in the moderately differentiated ESCCs (2.0) the MVD was 12.03±6.80 72 mm2/HPF (mean+SD) and in tumors that were poorly differentiated (3.0) the MVD was 16.70±10.40 mm2/HPF (mean+SD)
Survival analysis
The prognostic significance of the proteins was assessed in a follow-up study of ESCC patients. A significant correlation was observed between poor survival and increased tumor stage (P=0.02, Fig. a) as well as with nodal metastasis (P=0.05, Fig. 5b). The median survival time of patients with advanced tumor stage was 9.0 months and was the same for patients with nodal metastasis. Due to the small sample size we analyzed the protein expression with the survival rate using independent-samples t-test. ESCC patients positive for Ets-1 protein showed poorer survival as compared to Ets-1 negative patients (P=0.01). The patients positive for VEGF as well as Ets-1 proteins and having high MVD had significantly poorer rates of survival compared to patients negative for these proteins and having lower MVD (P=0.004). The survival time of patients positive for VEGF, Ets-1, and high MVD was 7.80±5.62 months (mean±S.D.), when compared to patients negative for these proteins (11.045±10.45 months).
Kaplan-Meier estimation of cumulative proportion of tumor recurrence or metastasis or death in relation to tumor stage of the patients with ESCCs. The median survival in the cases with increased tumor stage (T3+T4) was 9.0 months and in cases with early tumor stage (T1+T2) this was more than 40 months (P=0.02); b Kaplan-Meier estimation of cumulative proportion of tumor recurrence or metastasis or death in relation to lymph node involvement of the patients with ESCCs. The median survival in cases with node positive (N1) was 9.0 months and in cases with node negative (N0) it was more than 40 months (P=0.05)
Discussion
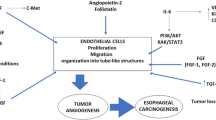
In this study VEGF expression was significantly associated with Ets-1 expression and high MVD in ESCCs. Esophageal tumor cells showed enhanced VEGF expression. VEGF protein was localized in the deep invasive margins of the invading layer of the epithelium. Interestingly, a concomitant increase was also observed in Ets-1 expression in this layer as well as in the surrounding tumor and endothelial cells, suggesting that VEGF may influence Ets-1 expression in these cells. Furthermore, enhanced VEGF and Ets-1 expression was correlated with increased tumor angiogenesis in ESCCs. VEGF has been shown to induce the expression of various angiogenesis related genes in endothelial cells. In vitro studies on human umbilical vein endothelial cell lines (HUVEC) have shown that VEGF elicited induction of Ets-1 in HUVECs. The induction of Ets-1 on the endothelial cells is mediated by the formation of KDR-Flt-1 heterodimer or KDR homodimer in response to the binding of VEGF on endothelial cells (Kuwano et al. 1998; Tanaka et al. 1998; Saeki et al. 2000). In addition, the promoter of VEGFR-1 contains Ets-1 binding motifs and the expression of VEGFR-1 is regulated by Ets-1 (Wakiya et al. 1996). However, concomitant increased expression of VEGFR-1 in turn facilitates binding of VEGF on the endothelial cells. Extrapolating these in vitro studies to our clinical observations we propose that VEGF and Ets-1 may act synergistically during the process of angiogenesis and are correlated with high MVD in ESCCs. Increased expression of VEGF and Ets-1 proteins and high MVD were significantly associated with shorter disease-free survival in ESCC patients (P=0.004), suggesting that these proteins are components of a common mechanistic pathway and act in synergistic manner to attribute aggressive characteristics to the tumors, thereby adversely affecting the clinical behavior of these tumors. Our data demonstrated significant correlation of VEGF expression with lymph node metastasis in ESCC (P=0.002). No significant correlation was observed between reduced disease-free survival and increased expression of VEGF, Ets-1 or high MVD alone suggesting that VEGF may influence the growth of esophageal tumors in transmural invasion, and lymph node metastasis by affecting primary sites. Furthermore, VEGF has been shown to induce urokinase-type plasminogen activator (uPA) and its receptor, tissue-type PA (tPA) and plasminogen activator inhibitor-1 (PAI-1) in vascular endothelial cells and bovine lymphatic endothelial cells (Pepper et al. 1991; Pepper et al. 1994; Mandriota et al. 1995). VEGF might facilitate lymph node metastasis by degrading tumor marginal extracellular matrix through the uPA and uPA receptor system, as well as through matrix metalloproteinases (MMPs), some of which might be activated by plasmin. These connections may facilitate the extravasation of the tumor cells at primary sites and may be a cause of enhanced VEGF expression correlating significantly with increasing lymph node metastasis. Alternatively, VEGF-mediated induction of Ets-1 in the endothelial cells via the KDR/Flt-1 heterodimer or KDR homodimers has been shown to result in the induction of various MMPs (MMP-1, MMP-3, and MMP-9) which are involved in the degradation of the extracellular matrix and the basement membrane required for invasion and metastasis (Yasufumi et al. 2000).
In conclusion, the present study showed the correlation of VEGF protein with Ets-1 expression and high MVD, as well as with lymph node metastasis underscoring their implication in angiogenesis and invasion. Furthermore, the increased angiogenesis in ESCCs accorded aggressive tumor behavior and poor prognosis.
References
Bolon I, Gouyer V, Devouassoux M, Vandenbunder B, Wernert N, Moro D, Bossi P, Viale G, Lee AK, Alfano R, Coggi G, Bosari, S (1995) Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res 55:5049–5053
Brambilla C, Brambilla E (1995) Expression of c-ets-1, collagenase 1, and urokinase type plasminogen activator genes in lung carcinomas. Am J Pathol 147:1298–1310
Dunphy F, Stack BC Jr, Boyd JH, Dunleavy TL, Kim HJ, Dunphy CH (2002) Microvessel density in advanced head and neck squamous cell carcinoma before and after chemotherapy. Anticancer Res 22:1755–1758
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Hart IR, Saini A (1992) Biology of tumor metastasis. Lancet 339:1453
Hosch SB, Stoecklein H, Pichlmeier U, Rehders A, Scheunemann P, Niendorf A, Knoefel WT, Izbicki JR (2001) Esophageal cancer. The mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 19:1970–1975
Kohn EC, Liotta LA (1995) Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res 55:1856–1862
Kuwano H, Saeki H, Kawaguchi H, Sonoda K, Kitamura K, Nakashima H, Toh Y, Sugimachi K (1998) Proliferative activity of cancer cells in front and centre areas of carcinoma in situ and invasive sites of esophageal squamous-cell carcinoma. Int J Cancer 78:149–152
Landis SH, Taylor M, Bolden S, Wingo PA (1999) Cancer statistics. CA Cancer J Clin 49:8–31
Mandriota SJ, Seghezzi G, Vassalli JD, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper MS (1995) Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem 210:298–305
Millikan KW, Mall JW, Myers JA, Hollinger EF, Doolas A, Saclarides, TJ (2000) Do angiogenesis and growth factor expression predict prognosis of esophageal cancer? Am Surg 66:401–405
Ogawa S, Kaku T, Kobayashi H, Hirakawa T, Ohishi Y, Kinukawa N, Nakano H (2002) Prognostic significance of microvessel density, vascular cuffing and vascular endothelial growth factor expression in ovarian carcinoma:a special review for clear cell adenocarcinoma. Cancer Lett 176:111–118
Pande P, Mathur M, Shukla NK, Ralhan R (1999) Role of Ets-1 in oral carcinogenesis; potential involvement in invasion and metastasis. J Pathol 189:40–45
Pepper MS, Ferrara N, Orci L, Montesano R (1991) Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor 1 in Microvascular endothelial cells. Biochem Biophys Res Commun 181:902–906
Pepper MS, Wasi S, Ferrara N, Orci L, Montesano R (1994) In vitro angiogenesis and proteolytic properties of bovine lymphatic endothelial cells. Exp Cell Res 210:298–305
Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J (2002) Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma:a prospective study. J Clin Oncol 20:1775–1785
Saeki H, Kuwano H, Kawaguchi H, Ohno S, Sugimachi K (2000) Expression of Ets-1 transcription factor is correlated with penetrating tumor progression in patients with squamous cell carcinoma of the esophagus. Cancer 89:1670–1676
Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvora AM, Dvorak HF (1993) Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12:303–324
Shih CH, Ozawa S, Ando N, Ueda M, Kitajima M (2000) Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 6:1161–1168
Shimada Y, Imamura M, Watanabe G, Uchida S, Harada H, Makino T, Kano M (1999) Prognostic factors of esophageal squamous cell carcinoma from the perspective of molecular biology. Br J Cancer 80:1281–1288
Sobin LH, Fleming ID (1997) TNM Classification of malignant tumors, 5th edn. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80:1803–1804
Tanaka K, Oda N, Iwasaka C, Abe M, Sato Y (1998) Induction of Ets-1 in endothelial cells during reendothelialization after denuding injury. J Cell Physiol 176:235–244
Thompson D (2001) Tumor versus patient: vascular and tumor survival versus prognosis. J Pathol 193:425–426
Vandenbunder B, Queva C, Desbiens X, Wernert N, Stehelin D (1994) Expression of the transcription factor c-Ets-1 correlates with the occurrence of invasive process during normal and pathological development. Invasion Metastasis 14:198–209
Wakiya K, Begue A, Stehelin D, Shibuya M (1996) A camp response element and an Ets motif are involved in the transcriptional regulation of flt-1 tyrosine kinase (vascular endothelial growth factor receptor 1) gene. J Biol Chem 271:30823–30828
Wernert N, Gille, F, Fafeur V, Bouali F, Raes MB, Pyke C, Dupressoir T, Seitz G, Vandenbunder B, Stehelin D (1994) Stromal expression of c-Ets-1 transcription factor relates with tumor invasion. Cancer Res 54:5683–5688
Yasufumi S, Mayumi A, Katsuhiro T, Chika I, Nobuyuki O, Shinichi K, Manami O, Tohru N, Takayuki I (2000) Properties of two VEGF receptors, Flt-1 and KDR in signal transduction. Annals NY Acad Sci 902:201–207
Author information
Authors and Affiliations
Corresponding author
Additional information
Contract Grant Sponsor: Indian Council of Medical Research
Rights and permissions
About this article
Cite this article
Mukherjee, T., Kumar, A., Mathur, M. et al. Ets-1 and VEGF expression correlates with tumor angiogenesis, lymph node metastasis, and patient survival in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 129, 430–436 (2003). https://doi.org/10.1007/s00432-003-0457-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0457-3