Abstract
We aimed to investigate the role of hypoxia-ischemia in the pathophysiology of early NEC/NEC like disease (ENEC) and classic NEC/NEC like disease (CNEC) in preterm infants. In this pilot study, preterm infants who developed the clinical symptoms and signs of NEC/NEC like disease were divided into two groups as early (≤ 7 days, ENEC) or late (> 7 days, CNEC) groups. Beside clinical variables, serum L-lactate, endothelin-1 (ET-1), platelet activating factor (PAF), and intestinal fatty acid binding protein (I-FABP) levels were measured from umbilical/peripheric venous blood in the first hour of life and during the clinical presentation in all groups. A total of 86 preterm infants were enrolled in the study. In the ENEC group, the incidences of fetal umbilical artery Doppler velocimetry abnormalities, IUGR, and delayed passage of first meconium were higher. In addition, mean levels of L-lactate, ET-1, PAF, and I-FABP were higher in the first hour of life.
Conclusion: Our study firstly showed that the dominant pathophysiological factor of ENEC is prenatal hypoxic-ischemic event where intestinal injury and inflammation begin in-utero and become clinically apparent in the first week of life. Therefore, we propose a new term “Hypoxic-Ischemic Enterocolitis (HIEnt)” for the definition of ENEC in preterm infants with prenatal hemodynamic disturbances and IUGR. This new sight can provide individualized preventive and therapeutic strategies for preterm infants.
What is Known: | |
• The pathophysiology of early necrotizing enterocolitis (NEC) or NEC-like disease which is seen in the first week of life seems different than classic necrotizing enterocolitis (CNEC) which is always seen after the first week of life. | |
What is New: | |
• This study suggests that perinatal hypoxic-ischemic process with inflammation is the point of origin of fetal intestinal injury leading to ENEC. | |
• We propose a new term “Hypoxic-Ischemic Enterocolitis (HIEnt)” for the definition and differentiation of this unique clinical entity. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Necrotizing enterocolitis (NEC) is the most important acquired gastrointestinal disease of the newborn infants and is characterized by intestinal injury, systemic inflammation, and multisystem organ failure [1]. The combination of multiple risk factors including genetic predisposition, intestinal structural and functional immaturity, hypoxia-ischemia, abnormal microbial colonization, timing of initiation, composition, and rate of enteral feedings causes NEC [2, 3]. The pathophysiologic mechanism and the time of onset of NEC seem to be related. In very low gestational age (GA) preterm infants, classic NEC develops almost always after the first week of life probably related to abnormal intestinal microbiota [4,5,6,7]. In late preterm or term newborn infants, NEC usually develops in the first week of life due to perinatal hypoxic-ischemic events or diseases such as congenital left heart obstructive lesions, polycythemia, and intrauterine growth restriction (IUGR) [8]. Spontaneous intestinal perforation (SIP) which is mostly seen in preterm growth restricted infants in the first week of life has been also related to an intestinal hypoxic-ischemic insult [9, 10].
Beside these entities, there is another group of preterm infants who develop early NEC or NEC-like disease in the first week of life. These infants almost always have IUGR and fetal hemodynamic disturbances such as absent or reversed end-diastolic blood flows (AREDF) in umbilical artery. The clinical presentation varies from feeding intolerance to typical NEC. In these infants, postnatal superior mesenteric artery Doppler studies have showed the continuation of redistribution which has started prenatally [11, 12].
Gordon et al. [13] reported 14 different subsets of NEC and suggested that “day of diagnosis-centric data” can help in understanding the NEC pathophysiology. Timing of onset was the starting point of our study to distinguish NEC pathophysiology. We aimed to investigate the role of hypoxia-ischemia in the pathophysiology of early NEC/NEC like disease (ENEC) and classic (late) NEC/NEC like disease (CNEC) in preterm infants by the biomarkers which are indicative for hypoxia-ischemia and intestinal cell injury[14,15,16].
Methods
This single-center prospective observational study (Hacettepe University Research Grant No: 011 D04 101008) was conducted in the neonatal intensive care unit (NICU) of Hacettepe University Ihsan Dogramaci Children’s Hospital, Ankara, Turkey between 1 July 2010-1 September 2012. The Institutional Ethics Committee approved the study (No: HEK 10/91) and informed consent forms were received from all parents before inclusion in the study.
Study population
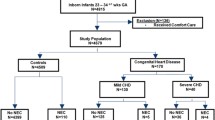
Preterm infants (GA < 37 weeks) who were admitted to NICU for any reason were eligible for study. Infants who developed NEC/NEC like disease in the first 7 days of life (≤ 7 days) constituted “early NEC (ENEC) group,” while preterm infants who developed NEC/NEC like disease after the 7th day of life (>7 days) constituted “classic NEC (CNEC)” group. Preterm infants who did not develop any NEC/NEC like disease during the entire NICU stay served as the control group. The study and control population included only inborn preterm infants whose parents agreed to participate in the study during the study period. Also, after reaching a reasonable number of cases in the control group, we stopped including new cases to the control group. Infants with congenital or chromosomal abnormalities, inherited metabolic diseases, infants with culture positive early neonatal sepsis in the first week of life, infants with SIP, and infants who were hospitalized shorter than 7 days because of discharge or death were excluded. In addition, symptomatic infants without occult/gross blood in stool and infants who were proven to swallow maternal blood by Apt test were not enrolled in order to exclude infants with feeding intolerance.
Definition of NEC/NEC like disease
All infants who developed at least one gastrointestinal sign or symptom such as gastric residuals, vomiting and bilious drainage from enteral feeding tubes, abdominal distention, prominent intestinal loops, abdominal tenderness and discoloration, abdominal mass with radiological features such as dilated intestinal loops, fixed loop, pneumatosis intestinalis, portal air or free air in the abdomen, gasless abdomen + and/or systemic signs mimicking feeding intolerance or NEC such as; temperature instability, hypotension, apnea, respiratory failure, lethargy, were taken into study groups. Infants who had occult or gross blood in stool in addition to mild gastrointestinal/systemic signs were classified as stage I NEC/NEC like disease. Infants with stage II or III NEC were defined according to the modified Bell’s criteria [17]. The diagnosis was confirmed by a senior neonatologist who was blind and not involved in the study.
Nutritional protocol and procedures
According to our NICU enteral nutrition protocol, the first choice was breast milk and the second choice was preterm infant formula in case of complete absence or inadequate amount of maternal breast milk. Minimal enteral nutrition was initiated on the first day of life at a volume of 10–20 ml/kg/day in infants with a birth weight of < 1500 g and 21–40 ml/kg/day in infants with a birth weight of ≥ 1500 g. The enteral nutrition volume was increased by 10–15 ml/kg/day if possible and finally reaching 150–180 ml/kg/day. All infants received enteral nutrition every 3 h (× 8/day). Infants who could not suck bottles adequately were fed by orogastric feeding tubes. All infants were kept in a supine position with their head and back 30° above the horizontal position until the end of the first hour after each enteral feeding. Total parenteral nutrition was initiated according to nursery protocol in very low birth weight (< 1500 g) infants and in those for whom enteral nutrition was not sufficient to achieve an energy supply of 120–150 kcal/kg/day. For these infants, intravenous protein was initiated at a dose of 1.5 g/kg/day on day 1 and reached 3.5 g/kg/day on day 3, while intravenous lipid was initiated at 1 g/kg/day on day 2 and reached 2–2.5 g/kg/day on day 3. In addition, a glucose infusion was initiated at a rate of 6–8 mg/kg/min and increased to 12–14 mg/kg/min as tolerated.
Data collection
Prenatal data included intrauterine growth (as defined by growth curve of Fenton et al. [18]), maternal and obstetric diseases (chronic hypertension, pregnancy induced hypertension, preeclampsia-eclampsia, chorioamnionitis), fetal umbilical artery Doppler flow velocimetry disturbances (defined as the following: (a) AREDF in the umbilical artery seen on at least 50% of waveforms on at least one occasion during pregnancy or (b) cerebral redistribution (umbilical artery pulsatility index> 95th centile and middle cerebral artery pulsatility index < 5th centile for gestation [19])), and prenatal steroid therapy. Natal data including gender, GA, birth weight, mode of delivery, 5th minute Apgar score, and presence of aggressive resuscitation at birth (positive pressure ventilation through bag and mask or endotracheal tube, chest compression, or drug administration) were recorded. Also, postnatal morbidities such as respiratory distress syndrome, congenital pneumonia, surfactant therapy, patent ductus arteriosus, intraventricular hemorrhage, bronchopulmonary dysplasia, durations of parenteral nutrition, mechanical ventilation, supplemental oxygen, and hospitalization were noted. All infants were fed according to our institutional NICU nutritional protocol. In the study, all systemic, gastrointestinal and radiological signs, day of onset of NEC/NEC like disease, duration (day) and volume (ml/kg/day) of enteral nutrition at the onset of NEC/NEC like disease, and the presence of delayed passage of the first meconium (> 48 h) [20] were recorded.
Serological markers
We studied “plasma L-lactate” and “ET-1” levels as indicators of tissue hypoxia-ischemia, “PAF” as a good indicator of inflammation, and I-FABP as a marker for intestinal cell injury [21, 22]. In order to clarify the pathophysiologic and chronologic differences between ENEC and CNEC, levels of these biomarkers were measured from umbilical/peripheral venous blood samples both in the first hour of life and then during the clinically symptomatic period. After admission to the NICU, 2 ml of venous blood was collected in tubes containing EDTA for ELISA analysis and heparin for blood gas analysis (pH and L-lactate) from all preterm infants. In infants who developed gastrointestinal +/- systemic signs and symptoms, second venous blood samples (2 ml) were obtained for the same analysis. All EDTA plasma samples were centrifuged at room temperature, 1500 rpm, for 15 min and stored at − 80 °C until ELISA analysis for I-FABP, PAF, and ET-1 levels. In the control group who did not develop any NEC/NEC like disease, the second blood samples were taken randomly during the 5–14 days of life.
The determination of plasma I-FABP concentrations was performed by HK-406 Human I-FABP Elisa Kit (Hycult Biotech Inc., PA, USA). Plasma PAF levels were measured with Human PAF Elisa Kit (Cusabio Biotech Co. Ltd., PRC), and plasma levels of ET-1 were determined using Human Endothelin Kit (1-21) (Biomedica Medizinprodukte GmbH & Co KG, Austria). All the procedures proceeded according to the manufacturers’ instructions. Plasma L-lactate levels were measured by an automated blood gas analyzer (Radiometer, Copenhagen, Denmark).
Statistical analysis
The statistical data was analyzed using “Statistical Package for Social Sciences” (SPSS for Windows 15.0, Chicago, USA) software on a personal computer. Power analysis was not performed as there was a limitation for the study period as defined by 2 years due to fellowship program. All data were initially controlled for normality of distribution according to the Kolmogorov Smirnov test. The data were presented as a mean ± standard deviation (SD), median (minimum–maximum), frequency, and percentage. For descriptive statistical analysis, mean ± standard deviation (SD) was used for normally distributed data, median was used for non-parametric data and percentage was used for categorical variables. Continuous variables were compared using Mann–Whitney for nonparametrically distributed data. Categorical variables were analyzed using the χ2 test or Fisher’s exact test. Also, ANOVA, Welch ANOVA, Kruskal Wallis, and Conover’s test were used for statistical analysis. P value of < 0.05 was accepted as statistically significant. We performed a multivariate analysis correcting for possible known confounders.
Results
During the study period, a total of 86 infants were enrolled in the study and of these, 24 (27.9 %) were in the ENEC group, 19 (22.1 %) were in the CNEC group, and 43 (50.0%) were in the control group. The demographic and clinical characteristics of the control, ENEC, and CNEC groups were given in Table 1. There were no statistically significant differences between the mean GA and birth weights of the ENEC and CNEC groups (p = 0.391, 0.172, respectively). In spite of similar mean GA, the lower mean birth weight of the ENEC group than the control group was due to the significantly higher incidence of IUGR infants in the ENEC group (p < 0.001).
The incidences of AREDF, IUGR, and delayed first meconium passage were significantly higher in the ENEC group than the CNEC and control groups. There were no significant differences in the incidences of major neonatal morbidities and mortality between ENEC and CNEC groups. Also, NEC stages were similar between the study groups (p = 0.568) (Table 1). The percentage of infants who received no enteral feeding including minimal enteral nutrition (MEN) before the onset of NEC/NEC like disease were significantly higher in the ENEC group than the CNEC group (p = 0.027)(Table 2).
In the first blood samples, mean plasma levels of L-lactate, PAF, I-FABP, and median ET-1 levels were significantly higher in the ENEC group than CNEC and control groups (p = 0.006, 0.000, 0.000, 0.007, respectively) (Table 3). For the first blood samples, multivariate analysis was performed including the following parameters: prenatal Doppler disturbances, maternal hypertension, IUGR, prenatal betametasone, gestational age, birth weight, cord pH, and Apgar score. This analysis revealed that plasma L-lactate level was affected by cord pH and IFABP level was affected by prenatal Doppler disturbances.
In the second blood samples, plasma mean PAF and I-FABP levels were significantly higher in the CNEC group (Table 4). For the second blood samples, multivariate analysis was performed including the following parameters: RDS, PDA, IVH, pneumonia, BPD, C-reactive protein (CRP), and procalcitonin. We detected no effect of these parameters on plasma L-lactate, ET-1, and IFABP levels in the second blood samples. However, plasma PAF level was found to be affected by serum CRP level and PDA. In the second blood samples, plasma L-lactate, ET-1, PAF, and I-FABP levels were significantly higher both in stage I and II/III infants of ENEC and CNEC groups than the control group as expected (p = 0.070, 0.024, 0.000, 0.000, respectively). Although there was no statistical significance, L-lactate, ET-1, and IFABP levels were higher in infants with stage I NEC/NEC like disease than infants with stage II/III NEC (Table 5).
Discussion
For the first time in the literature, this study suggests that prenatal systemic/intestinal hypoxia-ischemia could be a major leading factor to intestinal cell injury and inflammation causing an early NEC or NEC-like disease especially in preterm infants with IUGR. The higher plasma levels of L-lactate, ET-1, PAF, and I-FABP in the first blood samples (which is almost reflecting cord blood levels) and higher plasma L-lactate in the second sample in the ENEC group have supported this pathophysiology.
In our study, the most important characteristics of ENEC group were the significantly higher incidences of AREDF, IUGR, and delayed first meconium passage when compared with CNEC group (79.2% vs 31.6%, 75.0% vs 36.8% and 54.2% vs 21.1% , p = 0.002, 0.012, 0.039, respectively). Previously, it was reported that the risk of NEC increases in infants with IUGR and AREDF with an OR of 2.13 (95% CI 1.49–3.03) [23]. In the IUGR fetus, hypoxemia produces circulatory redistribution toward the brain and away from the viscera (especially gastrointestinal system) and placenta. The prolonged redistribution may cause structural, neuromotor, secretory, and mucosal function alterations of the intestinal tissue so that postnatally it is more susceptible to dismotility, abnormal colonization, and bacterial invasion [24]. After delivery, oxygenation improves but circulatory redistribution persists [12].
Another remarkable point of the ENEC group was the significantly higher frequency of infants who have not received any enteral feeding before the onset of NEC/NEC like disease when compared with the CNEC group (25% vs 0%, p = 0.027). This was due to withholding of even MEN as these infants were seriously symptomatic from the first hours or days of life. Therefore, it seems that enteral nutrition is not a prerequisite for the development of ENEC, and on the contrary, enteral nutrition could be rather preventive [25].
Serum L-lactate is frequently used in adults with acute mesenteric ischemia where it is elevated significantly [26]. However, increased expression of ET-1 has been shown in the removed bowel segments with NEC [27]. In our study, in the first blood samples, mean L-lactate and ET-1 levels were significantly higher in the ENEC group than the CNEC and control groups. Our results suggest that infants in the ENEC group have already been born with ischemic intestinal injury which has started prenatally as a result of fetal hemodynamic disturbances. However, in the control and CNEC groups, mean serum L-lactate and ET-1 levels were similar both in the first and second blood samples. These results support the hypothesis that perinatal (or postnatal) hypoxia-ischemia is not a primary causative factor in the pathophysiology of CNEC.
The increase in plasma I-FABP and PAF levels in NEC have been well demonstrated [15, 28, 29]. In our study, in the first blood samples, mean PAF and I-FABP levels were significantly higher in the ENEC group than CNEC and control groups. It is clear that not only hypoxia-ischemia but also intestinal inflammation leading to intestinal injury have begun in utero in the ENEC group. In the second blood samples, significantly higher levels of I-FABP and PAF were noted in the CNEC group than the ENEC group. This finding suggests that the inflammatory process and the disease severity were more profound in the CNEC group.
The first limitation of our study was being a single center study and having a small sample size, while the second limitation was inclusion of infants with Bell’s stage I NEC/NEC like disease. Recently, there is a consensus on exclusion of Bell’s stage 1 NEC and SIP among research networks [30, 31]. In our opinion, this approach inhibits the inclusion of the real patients who are at the initial “subclinical” phase of a developing disease and decreases patient number. Despite the risk of overlapping clinical pictures of feeding intolerance and stage I NEC, we have to evaluate all stages of NEC as naturally the severity of the disease increases stepwise from stage I to III. Further in our study, plasma levels of serological biomarkers in infants with stage I NEC/NEC like disease were as high as the levels in infants with stage II/III NEC and were significantly higher than the levels in infants of the control group (Table 5). Therefore, infants with stage I NEC/NEC like disease were in fact seriously affected by intestinal ischemia and inflammation. However, the clinical disease severity seems to be affected by other unknown or individual factors. Therefore, we think that selective approach considering only infants with stage II/III NEC in clinical studies should be re-evaluated.
Conclusion
Today “NEC” is a “roof term” which is believed to include more than one disease with different pathophysiological etiologies and clinical presentations. Therefore, there is an obvious need to redefine and re-classify neonatal gastrointestinal diseases presenting like NEC as Bell’s criteria have been developed only for disease severity staging. In our study, prenatal intestinal/mesenteric hypoxia-ischemia seems to be the primary pathophysiological factor leading to ENEC/NEC like disease in the first week of life in preterm infants with AREDF and IUGR. ENEC seems to begin “in utero,” and these infants are born with already injured and “programmed” gastrointestinal system and significant systemic inflammation. Therefore, we would like to propose a new terminology Hypoxic-Ischemic Enterocolitis (HIEnt) for preterm infants presenting with early NEC/NEC like disease and who have prenatal hemodynamic disturbances and IUGR. This new term has been firstly created and used by our colleague Dr. Ayse Korkmaz a few years ago. We believe that this new terminology would raise awareness on the pathophysiology of ENEC and contribute to providing individualized, preventive, and therapeutic strategies for preterm infants after multicenter prospective new studies.
Abbreviations
- AREDF:
-
absent or reversed end-diastolic blood flow
- CNEC:
-
classic necrotizing enterocolitis
- ENEC:
-
early necrotizing enterocolitis
- ET-1:
-
endothelin-1
- I-FABP:
-
intestinal fatty acid binding protein
- IUGR:
-
intrauterine growth restriction
- NEC:
-
necrotizing enterocolitis
- NICU:
-
neonatal intensive care unit
- PAF:
-
platelet activating factor
References
Neu J, Walker A (2011) Necrotizing enterocolitis. N Engl J Med 364:255–264. https://doi.org/10.1056/NEJMra1005408
Gordon PV, Swanson JR (2014) Necrotizing enterocolitis is one disease with many origins and potential means of prevention. Pathophysiology 21:13–19. https://doi.org/10.1016/j.pathophys.2013.11.015
Neu J (2014) Necrotizing enterocolitis: the mystery goes on. Neonatology 106:289–295. https://doi.org/10.1159/000365130
Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, Network CN (2012) Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129:e298–e304. https://doi.org/10.1542/peds.2011-2022
Clark DA, Munshi UP (2014) Feeding associated neonatal necrotizing enterocolitis (primary NEC) is an inflammatory bowel disease. Pathophysiology 21:29–34. https://doi.org/10.1016/j.pathophys.2013.11.006
Chen AC, Chung MY, Chang JH, Lin HC (2014) Pathogenesis implication for necrotizing enterocolitis prevention in preterm very-low-birth-weight infants. J Pediatr Gastroenterol Nut 58:7–11. https://doi.org/10.1097/MPG.0b013e3182a7dc74
Nowicki PT (2005) Ischemia and necrotizing enterocolitis: where, when, and how. Semin Pediatr Surg 14:152–158. https://doi.org/10.1053/j.sempedsurg.2005.05.003
Martinez-Tallo E, Claure N, Bancalari E (1997) Necrotizing enterocolitis in full-term or near-term infants: risk factors. Biol Neonate 71:292–298. https://doi.org/10.1159/000244428
Tarrado X, Castanon M, Thio M, Valderas JM, Aparicio LG, Morales L (2005) Comparative study between isolated intestinal perforation and necrotizing enterocolitis. Eur J Pediatr Surg 15:88–94. https://doi.org/10.1055/s-2004-821255
Gordon PV (2009) Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res 65:138–144. https://doi.org/10.1203/PDR.0b013e31818c7920
Bozzetti V, Tagliabue PE (2017) Enteral feeding of intrauterine growth restriction preterm infants: theoretical risks and practical implications. Pediatr Med Chir 39:160. https://doi.org/10.4081/pmc.2017.160
Bora R, Mukhopadhyay K, Saxena AK, Jain V, Narang A (2009) Prediction of feed intolerance and necrotizing enterocolitis in neonates with absent end diastolic flow in umbilical artery and the correlation of feed intolerance with postnatal superior mesenteric artery flow. J Matern Fetal Neonatal 22:1092–1096. https://doi.org/10.3109/14767050903029600
Gordon PV, Swanson JR, MacQueen BC, Christensen RD (2017) A critical question for NEC researchers: can we create a consensus definition of NEC that facilitates research progress? Semin Perinatol 41:7–14. https://doi.org/10.1053/j.semperi.2016.09.013
Block T, Nilsson TK, Björck M, Acosta S (2008) Diagnostic accuracy of plasma biomarkers for intestinal ischemia. Scand J Clin Lab Invest 68:242–248. https://doi.org/10.1080/00365510701646264
Young C, Sharma R, Handfield M, Mai V, Neu J (2009) Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatr Res 65:91R–97R. https://doi.org/10.1203/PDR.0b013e31819dba7d
Evennett N, Alexander N, Petrov M, Pierro A, Eaton S (2009) A systematic review of serologic tests in the diagnosis of necrotizing enterocolitis. J Pediatr Surg 44:2192–2201. https://doi.org/10.1016/j.jpedsurg.2009.07.028
Walsh MC, Kliegman RM (1986) Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33:179–201. https://doi.org/10.1016/s0031-3955(16)34975-6
Fenton TR (2003) A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr 3:13. https://doi.org/10.1186/1471-2431-3-13
Bahado-Singh RO, Kovanci E, Jeffres A, Oz U, Deren O, Copel J, Mari G (1999) The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol 180:750–756. https://doi.org/10.1016/s0002-9378(99)70283-8
Arnoldi R, Leva E, Macchini F, Di Cesare A, Colnaghi M, Fumagalli M, Mosca F, Torricelli M (2011) Delayed meconium passage in very low birth weight infants. Eur J Pediatr Surg 21:395–398. https://doi.org/10.1055/s-0031-1291301
Frost BL, Jilling T, Caplan MS (2008) The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol 32:100–106. https://doi.org/10.1053/j.semperi.2008.01.001
Mannoia K, Boskovic BS, Slater L, Plank MS, Angeles DM, Gollin G (2011) Necrotizing enterocolitis is associated with intestinal injury. J Pediatr Surg 46:81–85. https://doi.org/10.1016/j.jpedsurg.2010.09.069
Dorling J, Kempley S, Leaf A (2005) Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch Dis Child Fetal Neonatal Ed 90:F359–F363. https://doi.org/10.1136/adc.2004.060350
Bozzetti V, Tagliabue PE, Visser GH, van Bel F, Gazzolo D (2013) Feeding issues in IUGR preterm infants. Early Hum Dev 89(Suppl 2):S21–S23. https://doi.org/10.1016/j.earlhumdev.2013.07.006
Kirtsman M, Yoon EW, Ojah C, Cieslak LSK, Shah PS (2015) Nil-per-os days and necrotizing enterocolitis in extremely preterm infants. Am J Perinatol 32:785–794
Demir IE, Ceyhan GO, Friess H (2012) Beyond lactate: is there a role for serum lactate measurement in diagnosing acute mesenteric ischemia? Dig Surg 29:226–235. https://doi.org/10.1159/000338086
Nowicki PT, Dunaway DJ, Nankervis CA, Giannone PJ, Reber KM, Hammond SB, Besner GE, Caniano DA (2005) Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr 146:805–810. https://doi.org/10.1016/j.jpeds.2005.01.046
Caplan MS, Simon D, Jilling T (2005) The role of PAF, TLR and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 14:145–151. https://doi.org/10.1053/j.sempedsurg.2005.05.002
Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL et al (2010) Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg 251:1174–1180. https://doi.org/10.1097/SLA.0b013e3181d778c4
Neu J, Modi N, Caplan M (2018) Necrotizing enterocolitis comes in different forms: Historical perspectives and defining the disease. Semin Fetal Neonatal Med 23:370–373. https://doi.org/10.1016/j.siny.2018.07.004
Markiet K, Szymanska-Dubowik A, Janczewska I, Domazalska-Popadiuk I, Zawadzka-Kepczynska A, Bianek-Bodzak A (2017) Agreement and reproducibility of radiological signs in NEC using the duke abdominal assessment scale (DAAS). Pediatr Surg Int 33:335–340. https://doi.org/10.1007/s00383-016-4022-y
Acknowledgment
We thank Professor Pinar Erkekoglu for performing ELISA analysis.
Funding
This study was supported by Hacettepe University (Research Grant No: 011 D04 101008)
Author information
Authors and Affiliations
Contributions
Dr. Surmeli Onay organized the data collection, carried out the initial analyses, drafted the initial manuscript.
Dr. Korkmaz conceptualized and designed the study, proposed the new terminology, controlled the analyses, drafted the first and final manuscripted as submitted.
Drs Yigit and Yurdakok reviewed the manuscript and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Institutional Ethics Committee approved the study (No: HEK 10/91).
Informed consent
Informed consent forms were received from all parents before inclusion in the study.
Additional information
Communicated by Patrick Van Reempts
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Surmeli Onay, O., Korkmaz, A., Yigit, S. et al. Hypoxic-ischemic enterocolitis: a proposal of a new terminology for early NEC or NEC-like disease in preterm infants, a single-center prospective observational study. Eur J Pediatr 179, 561–570 (2020). https://doi.org/10.1007/s00431-019-03539-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03539-w