Abstract
The aim of this study was to perform a systematic review and meta-analysis of cut-off points of Homeostasis Model Assessment (HOMA-IR) to determine metabolic syndrome (MetS) in children and adolescents. A literature search was conducted in MEDLINE (via PubMed), EMBASE, Web of Science, Proquest, and Scopus databases from their inception to June 2018. Random effects models for the diagnostic odds ratio (dOR) value computed by Moses’ constant for a linear model and 95% confidence intervals (CIs) were used to calculate the accuracy of the test. Hierarchical summary receiver operating characteristic curves (HSROC) were used to summarize the overall test performance. Six published studies were included in the meta-analysis that included 8732 children and adolescents. The region of HOMA-IR (i.e., dOR) associated with MetS range from 2.30 to 3.54. The pooled accuracy parameters from the studies that evaluated the diagnostic odds ratio of HOMA-IR ranged from 4.39 to 37.67.
Conclusion: the HOMA-IR test may be useful for early evaluating children and adolescents with insulin resistance (IR). Furthermore, they present a good diagnostic accuracy independently of the definition of MetS used. According to the studies, the HOMA-IR cut point to avoid MetS risk ranged from 2.30 to 3.59.
What is Known: • There is no consensus to define the optimal cut-off point of Homeostasis Model Assessment–Insulin Resistance in children and adolescents associated with Metabolic Syndrome. | |
What is New: • The Homeostasis Model Assessment–Insulin Resistance test may be useful for early evaluations in children and adolescents with insulin resistance and presents a good diagnostic accuracy independently of the definition of Metabolic Syndrome used. • The Homeostasis Model Assessment–Insulin Resistance cut point to avoid Metabolic Syndrome risk ranged from 2.30 to 3.59 |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emerging evidence suggests that cardiometabolic risk is initiated in early life, and it is tracked from childhood into adulthood and may predict future diabetes and cardiovascular disease [1, 2]. Measures of cardiometabolic risk among youth include the obesity, dyslipidaemia, elevated glucose, and blood pressure and cluster together in young children [3].
Insulin resistance (IR) has been proposed as the primary mediator of metabolic syndrome (MetS) [4], so that identification of youth with IR has been proposed as a strategy for identifying high-risk subjects for targeting MetS interventions.
Different methods have been proposed to measure insulin sensitivity. Among other methods, the euglycemic-hyperinsulinemic clamp is considered to be the gold standard technique to estimate insulin sensitivity [2, 5]. Although clamp technology has been applied to the study of insulin sensitivity and insulin secretion during childhood, it is too invasive for general epidemiologic studies. In the quest for a non-invasive measurement technique for insulin sensitivity, several fasting or “homeostatic” models have been proposed. The homeostasis model for the assessment of IR (HOMA-IR) is frequently used in clinical research [6], because the HOMA-IR model presents a good correlation with the euglycemic-hyperinsulinemic clamp [7]. HOMA-IR is a set of simple, mathematically derived nonlinear equations. It is an equation which related glucose and insulin dynamics that predicts fasting steady-state glucose and insulin concentrations for a wide range of possible combinations of IR and β cell function [8]. The HOMA-IR has high sensitivity and specificity for measuring IR [9]. There are other methods as the quantitative insulin-sensitivity check index (QUICKI), which is similar to the simple equation form of the HOMA-IR model in many aspects, except that a log transform of insulin and glucose [10], and fasted glucose/insulin ratio (FGIR) [11] that have been described frequently as well.
Despite HOMA-IR was found to be much more reliable than FGIR and QUICKI to determinate IR in obese children [11], there is no consensus to define the optimal cut-off point of HOMA-IR in children and adolescents associated with MetS. Therefore, the aim of this study was to review and meta-analyzed the different HOMA-IR cut-off points used to determine IR in children and adolescents associated with MetS.
Materials and methods
Protocol and registration
We followed the checklist of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [12]. The review was registered in PROSPERO (registration number: CRD42018090076).
Literature search and inclusion criteria
A literature search was conducted in MEDLINE (via PubMed), EMBASE, Web of Science, Proquest, and Scopus databases from their inception to June 2018. The search terms used were “children or childhood or pediatrics” and “HOMA or QUICKI or FGIR” and “AUC or ROC or prevalence or sensitivity or specificity or cut-point or cut-off” and “insulin resistance or insulin sensitivity or metabolic syndrome.” Reference lists of related studies and previous systematic reviews were also screened for eligible studies. Two authors (PAR and AGH) independently screened the search results, determined the eligibility of the studies, and extracted the data. Any discrepancies were resolved via consensus-based discussions with a third reviewer.
We aimed to identify original articles analyzing the optimal HOMA-IR cut-off point for defining IR associated with MetS in children and adolescents. The eligibility of the studies was formulated according to the PICOS criteria (i.e., participants, interventions, comparisons, outcomes, and study design). Eligible studies were cross-sectional studies with participants younger than 18 years and reported the prevalence of MetS. For a study to be included, the cut-off point of IR should have been calculated by using Youden’s index (sensitivity-(1-specificity)) associated with MetS. We included only studies published in English.
Data extraction and quality assessment
The following data were collected from each study: (1) author identification, (2) year of publication, (3) country of the study, (4) year of data collection, (5) age of the participants, (6) number of participants, (7) prevalence of MetS, and (8) parameters summarizing the accuracy of the test (cut-off, sensitivity, specificity, and area under the curve (AUC)).
We also used the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS) tool to evaluate four domains of each study: patient selection, index test, reference standard, flow, and timing. Each domain was evaluated in terms of the risk of bias and in terms of concerns regarding the applicability of the results. The QUADAS-2 was designed to assess the validity of the studies. It assesses the quality of included studies in terms of risk of bias and concerns regarding applicability over four domains, as discussed below [12].
The patient selection could be biased by the method of selection of patients or included patient. The concerns regarding applicability evaluate if the review question matches the included patients. The index test may be biased by the interpretation of the test, and the concerns regarding applicability are based on the interpretation that differs from the review question analyzed. The reference standard could be biased by the election of the reference standard test and its application on the chosen sample. The concerns regarding applicability evaluate if the reference standard matches the review question. The flow and timing describe if the reference standard and the index test were applied at the same time and to all patients [12].
Data extraction and quality assessment were independently performed by PAR and AGH, and inconsistencies were managed by consensus.
Statistical analysis and data synthesis
The diagnostic test requires a cut-off point above the reference from which the test is regarded as positive, in other words, allows it to classify individuals as healthy or sick. The value of threshold depends on two statistical parameters, sensitivity indicates the proportion of sick patients that have a positive result and specificity indicates the proportion of healthy patients that have a negative result, so that the sensitivity and specificity vary depending on the situation which the test is used [13]. This variability is the main source of heterogeneity in a meta-analysis. The heterogeneity of results across studies was evaluated using the I2 statistical parameter, I2 was calculated as 100%*(Q − df)/Q, where Q is the Cochrane heterogeneity statistic [14]. I2 values of < 25%, 25–50%, and > 50% usually corresponds to small, medium, and large heterogeneity, respectively [15].
The dOR is a measure of the accuracy of the test data that combines sensitivity and specificity into a single value. The dOR values range from 0 to infinity, with higher values indicating a better discriminatory test performance. A dOR of 1.0 indicates that a test does not discriminate between patients with the disorder and those without it [16].
Forest plots were used to display the sensitivity, specificity, and dOR for HOMA-IR in the reviewed studies. The sensitivity, specificity, and dOR as well as their corresponding 95% confidence intervals (CI) were calculated for HOMA-IR in each study.
Hierarchical summary receiver operating characteristic curves (HSROC) is a quantitative model that allows to relate sensitivity, specificity, heterogeneity, and threshold effects [13]. The HSROC have been proposed to estimate the performance of diagnostic tests on data from a meta-analysis. HSROC were used to summarize the overall test performance.
Each study was weighted in the analyses according to the inverse variance method. Statistical analyses were performed using StataSE software, version 13 (StataCorp).
Results
Literature search and baseline characteristics
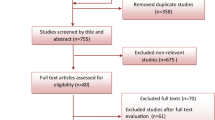
The details of our study selection method are shown in Fig. 1. A total of 5915 articles were retrieved from literature search. After removing 1733 duplicated articles, the titles and abstracts of 4182 studies were screened. We excluded 4148 studies due to at least one exclusion criteria was fulfilled, leaving 34 studies that were reviewed in full. Finally, six studies were included in this systematic review and meta-analysis. All studies had cross-sectional designs.
The six studies comprising this systematic review included 8732 children and adolescents. The age of the participants ranged from 6 to 18 years. In each selected study, the prevalence of MetS was reported according to different criteria Adult Treatment Panel III (ATPIII) [17], International Diabetes Federation (IDF) [18], Cook et al. [19], Cruz et al. [20], Ford [21], de Ferranti et al. [22], and Salas-Fernandez et al. [23]). Among six studies, we found one report, which used three different definitions [24] and another which used six different definitions [18]. All the studies provided information on the MetS prevalence, except for one [5], which was calculated. The prevalence of MetS varied from 1.6 to 29.7% and the HOMA cut-off points ranged from 2.30 to 3.54 (Table 1). Each definition was analyzed as an independent population. The studies were conducted in China [5], Korea [17], Mexico [25], Chile [26], and India [27, 28].
Study quality
All included studies were assessed as minimal risk of bias in terms of the four domains of the QUADAS-2 tool: patient selection, index test, reference standard, flow, and timing (Table 2).
Meta-analysis
Figure 2 shows the dOR forest plot of HOMA-IR. There was heterogeneity across the studies in the dOR of HOMA-IR (I2 = 77%) based on Adult Treatment Panel III (ATP III) definition of MetS and the pooled dOR was 5.45 (95% CIs, 3.59 to 8.28; p = 0.013), whereas the heterogeneity across the studies based on IDF definitions was lower (I2 = 0.0%) and the pooled dOR was 9.58 (95% CIs, 7.10 to 12.91; p = 0.487). The pooled accuracy parameters from the studies that evaluated the diagnostic performance of HOMA-IR ranged from 4.39 to 37.67.
Figure 3 shows the sensitivity forest plot of HOMA-IR. The sensitivity of the optimal cut-off proposed ranged to 0.59 to 0.89. The heterogeneity across the studies based on ATP III and IDF definition was small, and the pooled sensitivity was 0.77 (95% CIs, 0.71 to 0.84; I2 = 0; p = 0.481) and 0.72 (95% CIs, 0.62 to 0.83; I2 = 0; p = 0.533), respectively.
Figure 4 shows the specificity forest plot of HOMA. The specificity of the optimal cut-off points proposed ranged to 0.62 to 0.87. The heterogeneity across the studies based on ATP III and IDF definition was small, and the pooled specificity was 0.65 (95% CIs, 0.63 to 0.68; I2 = 0; p = 0.569) and 0.80 (95% CIs, 0.75 to 0.85; I2 = 53.7; p = 0.090), respectively.
The area under the HSROC estimating the discriminating accuracy of HOMA-IR for identifying IR is show in Fig. 5. The 95% confidence region for the point that summarized the overall test performance included studies in which the cut-off points ranged from 2.30 to 3.54.
Discussion
Our meta-analysis shows that HOMA-IR test as measurement of IR surrogate may be a useful tool for identifying IR associated with MetS among children and adolescents across countries. It presents an enough dOR on diagnostic IR independently of the definition of MetS used.
MetS is a state of chronic inflammation, as a consequence of complex relationship between genetic and environmental factors [29]. It is difficult to establish cut-off points for IR associated with MetS, since the clinical outcomes are observed into adulthood [30]. Some studies demonstrated that HOMA-IR levels increase directly with the numbers of MetS components present in a subject [5]. It has also been proposed that the measure of IR surrogate could be a useful tool to unify the diagnosis criteria of MetS [18]. However, in many studies, cut-off points have been based on the distribution in reference population, instead of using the Youden index.
Our findings show a considerable variation of MetS prevalence, depending on the definition used. We observed that the lowest prevalence was noticed when the IDF definition was used [17,18,19]. The IDF definition requires for diagnosis, the abdominal obesity plus any additional criteria (raised triglycerides levels, reduced high density lipoprotein cholesterol, raised blood pressure or raised fasting plasma glucose). The high prevalence observed for De Ferranti’s definition [22] may be because it uses a lower threshold for waist circumference in comparison with other definitions.
HOMA-IR as a surrogate measure of IR associated with MetS has similar accuracy in terms of the dOR and AUC between different definitions. It also should be noted that when criteria of MetS were adapted to pediatric population, the accuracy diagnostic increased. Ford’s definition [21] reported the best accuracy for HOMA-IR. It could be due to the fact that the cut-off point used to measure fasting glucose is lower in comparison with other definitions. The IDF definition presents better dOR in comparison to ATPIII classification. It could be explained that, for unknown reasons, insulin stimulates hepatic lipogenesis, causing dyslipidemia, which results in ectopic adipose deposition [31] and abdominal obesity is an essential requirement of IDF definition. As is common, all the estimations of the diagnostic accuracy were performed considering the large variability across individual studies, derived from a threshold effect to determine positivity in the tests as the different criteria for defining MetS.
Regarding pooled specificity and sensibility in the meta-analysis, the results show similar values among studies, ranged from 0.59 to 0.89 for sensitivity and from 0.62 to 0.87 for specificity. The number of subjects, the average of age, and the lack of information of maturity sexual among participants could be explained differences among studies that have used the same definition of MetS.
Meta-analysis of diagnostic test synthetizes the performance of a test providing a pooled estimation of diagnostic accuracy parameters and estimates a summary point (a summary sensitivity and specificity estimates) and a HSROC, but does not allow the identification of the optimal cut-off point [32]. However, the cut-off points reported are within the 95% confidence intervals for HOMA-IR, ranged from 2.30 to 3.54; thus, all of them presented a good accuracy diagnosis that could be used in clinical practice.
The present meta-analysis has some limitations: (i) One of the limitations was the insufficient available data prevented us from conducting separate analyses; (ii) we included only population-based studies conducted in apparently healthy children and adolescents aged ≤ 18 years; (iii) the lack of discrimination among children and adolescents is also a limitation, because, during pubertal period, IR increases at the onset of puberty; (iv) the lack of information about nutrition and lifestyle participants was another limitations; (v) due to the lack of available studies, we cannot calculate the AUC and their 95% confidence intervals; (vi) a comparison with gold standard methods was not possible in any study; (vii) the number of studies was too small to allow subgroup analyses by gender and pubertal development; and (viii) the lack of consensus of MetS definition makes difficult the comparison between studies.
Conclusions
Our study determined that the use of HOMA-IR, an indirect marker for IR, seems to be useful for early evaluation among children and adolescents at risk, independently of definition of MetS. Due to the IR plays a main role in the pathogenesis of MetS, the use of these cut-off points early and its proper identification could have benefits of preventive and diagnostic therapeutic intervention.
Abbreviations
- ATP III:
-
Adult Treatment Panel III
- AUC:
-
Area under the curve
- FGIR:
-
Fasted glucose/insulin ratio
- IDF:
-
International Diabetes Federation
- IR:
-
Insulin resistance
- HSROC:
-
Hierarchical summary receiver operating characteristic curves
- HOMA-IR:
-
Homeostasis Model Assessment–Insulin Resistance
- MetS:
-
Metabolic syndrome
- ROC:
-
Receiver operating characteristic curves
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- QUICKI:
-
Quantitative insulin-sensitivity check index
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology.
References
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Phys 237:214–223
Ferrannini E, Mari A (1998) How to measure insulin sensitivity. J Hypertens 16:895–906
Brambilla P, Lissau I, Flodmark CE, Moreno LA, Widhalm K, Wabitsch M, Pietrobelli A (2007) Metabolic risk-factor clustering estimation in children: to draw a line across pediatric metabolic syndrome. Int J Obes 31(4):591–600
Haymond MW (2003) Measuring insulin resistance: a task worth doing. But how? Pediatr Diabetes 4(3):115–118
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Phys 237:214–223
Cutfield WS, Jefferies CA, Jackson WE, Robinson EM, Hofman PL (2003) Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr Diabetes 4(3):119–125
Conwell LS, Trost SG, Brown WJ, Batch JA (2004) Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 27(2):314–319
Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A (2015) Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab 19:160–164
Katz A, Nambi SS, Mather K, Baron AD, Follman DA, Sullivan G (2000) Quantitative insulin sensitivity check index: a simple accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115(4):e500–e503
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15):2008–2012
QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Leeflang MMG (2014) Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect 20(2):105–113
Higgings JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analysis. BMJ 327:557–560
Higgings JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58(10):982–990
Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) (2002) Final Report. Bethesda: National Institutes of Health, National Heart, Lung, and Blood Institute
Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S, IDF Consensus Group (2007) The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes 8:299–306
Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH (2003) Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med 157:821–827
Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI (2004) The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab 89:108–113
Ford ES, Ajani UA, Mokdad AH (2005) National Health and Nutrition Examination (2005) the metabolic syndrome and concentrations of C-reactive protein among U. S youth. Diabetes Care 28:878–881
de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N (2004) Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation 110:2494–2497
Salas-Fernández A, Maldonado-Hernández J, Martínez-Basila A, Martínez-Razo G, Jasso-Saavedra F (2015) The 13C-glucose breath test is a valid non-invasive screening tool to identify metabolic syndrome in adolescents. Clin Chem Lab Med 53:133–138
Kim JW, Park SH, Kim Y, Im M, Han HS (2016) The cutoff values of indirect indices for measuring insulin resistance for metabolic syndrome in Korean children and adolescents. Ann Pediatr Endocrinol Metab 21(3):143–148
Piña-Aguero MI, Zaldivar-Delgado A, Salas-Fernández A, Martínez-Basila A, Bernabe-Garcia M, Maldonado-Hernández J (2018) Optimal cut-off points of fasting and post-glucose stimulus surrogates of insulin resistance as predictors of metabolic syndrome in adolescents according to several definitions. J Clin Res Pediatr Endocrinol 10(2):139–146
Burrows R, Correa-Burrows P, Reyes M, Blanco E, Albala C, Gahagan S (2015) Healthy Chilean adolescents with HOMA-IR≥ 2.6 have increased cardiometabolic risk: association with genetic, biological, and environmental factors. J Diabetes Res 2015:1–8. https://doi.org/10.1155/2015/783296
Singh Y, Garg MK, Tandon N, Marwaha RK (2013) A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. J Clin Res Pediatr Endocrinol 5(4):245–251
Garg MK, Tandon N, Marwaha RK, Singh Y (2014) Evaluation of surrogate markers for insulin resistance for defining metabolic syndrome in urban Indian adolescents. Indian Pediatr 51(4):279–284
Mameli C, Zuccotti GV, Carnovale C, Galli E, Nannini P, Cervia D, Perrotta C (2017) An update on the assessment and management of metabolic syndrome, a growing medical emergency in paediatric populations. Pharmacol Res 119:99–117
Henderson M, Rabasa-Lhoret R, Bastard JP, Chiasson JL, Baillargeon JP, Hanley JA, Lambert M (2011) Measuring insulin sensitivity in youth: how do the different indices compare with the gold-standard method? Diabetes Metab 37(1):72–78
Singer K, Lumeng CN (2017) The initiation of metabolic inflammation in childhood obesity. J Clin Invest 127(1):65–73
Charoensawat S, Böhning W, Böhning D, Holling H (2014) Meta-analysis and meta-modelling for diagnostic problems. BMC Med Res Methodol 14(1):1
Author information
Authors and Affiliations
Contributions
A-R conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
C-R, P-C, and S-M designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript.
G-H and M-V conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arellano-Ruiz, P., García-Hermoso, A., Cavero-Redondo, I. et al. Homeostasis Model Assessment cut-off points related to metabolic syndrome in children and adolescents: a systematic review and meta-analysis. Eur J Pediatr 178, 1813–1822 (2019). https://doi.org/10.1007/s00431-019-03464-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03464-y