Abstract
This prospective cohort study aimed to assess the association of admission hypothermia (AH) with death and/or major neonatal morbidities among very low birth weight (VLBW) preterm infants based on the relative performance of 20 centers of the Brazilian Network of Neonatal Research. This is a retrospective analysis of prospectively collected data using the database registry of the Brazilian Network on Neonatal Research. Center performance was defined by the relative mortality rate using conditional inference trees. A total of 4356 inborn singleton VLBW preterm infants born between January 2013 and December 2016 without malformations were included in this study. The centers were divided into two groups: G1 (with lower mortality rate) and G2 (with higher mortality rate). Crude and adjusted relative risks (RR) and 95% confidence intervals (95%CI) were estimated by simple and multiple log-binomial regression models. An AH rate of 53.7% (19.8–93.3%) was significantly associated with early neonatal death in G1 (adjusted RR 1.41, 95% CI 1.09–1.84) and G2 (adjusted RR 1.29, 95%CI 1.01–1.65) and with in-hospital death in G1 (adjusted RR 1.29, 95%CI 1.07–1.58). AH was significantly associated with a lower frequency of necrotizing enterocolitis (adjusted RR 0.58, 95%CI 38–0.88) in G2.
Conclusion: AH significantly associated with early neonatal death regardless of the hospital performance. In G2, an unexpected protective association between AH and necrotizing enterocolitis was found, whereas the other morbidities assessed were not significantly associated with AH.
What is Known: • Admission hypothermia is associated with early neonatal death. • The association of admission hypothermia with major neonatal morbidities has not been fully established. | |
What is New: • Admission hypothermia was significantly associated with early neonatal and in-hospital death in centers with the lowest relative mortality rates. • Admission hypothermia was not associated with major neonatal morbidities and with in-hospital death but was found to be a protective factor against necrotizing colitis in centers with the highest relative mortality rates. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Admission hypothermia (AH) in preterm infants has been an important problem in neonatal units. Despite the labor room interventions recommended to prevent AH, such as using a cap, a thermal mattress, and a plastic bag and controlling the room temperature, hypothermia prevalence remains high, especially among very low birth weight (less than 1500 g) preterm infants (VLBW) [1].
In a multicenter study, Almeida et al. reported AH occurrence in 51% of preterm newborns (23 to 24 weeks of gestational age) [2]. In Europe, Wilson et al. assessed the neonatal practices of 11 countries participating in the Effective Perinatal Intensive Care in Europe (EPICE) and reported that 53.4% of 5697 preterm newborns (< 32 weeks of gestational age) with hypothermia were admitted to the neonatal units [3]. Moreover, AH has been significantly associated with neonatal death, although the causal relationship remains to be established [2,3,4,5]. Some studies have also investigated the association of AH with neonatal morbidities. In a retrospective study, Chang et al. analyzed the morbidities detected during hospitalization in 341 preterm newborns and demonstrated an association between hypothermia and the occurrence of respiratory distress syndrome requiring the use of a surfactant (relative risk (RR) 2.66, 95%CI 1.27–5.58), although they detected no association between hypothermia and bronchopulmonary dysplasia (RR 0.83, 95%CI 0.38–1.82) [6]. Lyu et al., in a multicenter study involving near 10,000 preterm infants fewer than 33 weeks gestation, showed that neurological injury, severe retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BDP), and nosocomial infection had a U-shaped relationship with admission temperature and some morbidities have the lowest rates at admission temperatures ranging from 36.5 to 37.2 °C [7]. By contrast, Wilson et al. detected no association between hypothermia and neonatal morbidity [3].
According to Raven et al., there is no universally accepted definition of quality of care and there are a few definitions and theoretical models described. Efficient referral system for high-risk newborn infants (pertinent levels of care), employment of appropriate technologies, evaluation of the personal competence, and adherence to internationally recognized good practices are useful for developing quality improvement strategies and activities, and incorporating quality into existing programs. Essentially, quality of health care is an active and continual reevaluation of the workflow process. Based on these previous findings, AH rate reduction has been identified as a marker of the quality of neonatal care and predictor of outcomes, as shown in a recent review [8, 9].
Quality care can be evaluated by health care structure and activities and outcomes measurement (including mortality rates) [8]. As AH has been associated with death, our hypothesis is that AH rates might be an indicator of quality care (or performance) of a hospital.
Thus, this study aimed to assess the association between AH and the relative mortality rate and major neonatal morbidities in VLBW infants. In addition, the study also analyzed whether these associations differed according to the performance of the hospital.
Materials and methods
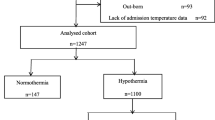
This cohort study is a retrospective analysis of prospectively collected data. Convenience sampling was employed using the database registry of the Brazilian Network on Neonatal Research (abbreviated as RBPN in Portuguese), which consists of 20 Brazilian university tertiary public hospitals. All VLBW preterm inborn infants registered in the database between January 1, 2013, and December 31, 2016, were included in this study. Exclusion criteria, as shown in Fig. 1, were as follows: the presence of major congenital malformations, death in the delivery room, patients transferred to other hospitals, twin births, and patients with no information about temperature at admission.
Inferential decision tree used for the definition of groups of centers using the association of SNAPPE II and mortality in very low birth weight preterm newborns. Node 1, division according to SNAPPE II values < 20 e ≥ 20. Node 2, centers with SNAPPE II < 20. Node 5, centers with SNAPPE II ≥ 20. Node 3, center group 1: lowest mortality rate for SNAPPE II < 20 (17 centers, mortality rate 4.8%). Node 4, center group 2: highest mortality rate for SNAPPE II < 20 (4 centers, mortality rate 13.0%; p < 0.01 in comparison with centers of node 3). Node 6, center group: highest mortality rate for SNAPPE II ≥ 20 (6 centers). Node 7, center group: highest mortality rate for SNAPPE II ≥ 20 (7 centers). The rectangles at the bottom of the figure correspond to the “terminal nodes” and visually show the probabilities of relative mortality for each path through the tree. The first node of the conditional inference tree (the “root node”) splits the data according to categorical values of SNAPPE II < 20 or ≥ 20
AH was defined as axillary temperature of < 36.0 °C recorded within 1 h after admission and for a special circumstance, we have defined hypothermia as temperature < 36.5 °C, as defined by World Health Organization [10]. The following morbidities were evaluated regarding to AH: early neonatal death (< 7 days) and in-hospital death (up to discharge from the hospital), bronchopulmonary dysplasia (BPD; use of any inspired oxygen fraction above 0.21 at the corrected gestational age of 36 weeks), peri-intraventricular hemorrhage (PIVH) classified according to Papille et al. [11], retinopathy of prematurity any grade, stage ≥ II necrotizing enterocolitis [12], cystic or diffuse periventricular leukomalacia diagnosed by ultrasound and/or magnetic resonance image, and a combined outcome of death or BPD or PIVH or NEC. Moreover, the following maternal and neonatal variables were investigated for the description of the population: prenatal use of corticosteroids regardless of the number of doses or the interval between administration and delivery, presence of gestational and/or chronic arterial hypertension, prolonged rupture of fetal membranes (> 18 h), type of delivery, sex, birth weight (subgroups of < and ≥ 1.000 g), gestational age (subgroups of 24–27 weeks/28–32 weeks/33–36 weeks), first- and fifth-minute Apgar score < 7, and SNAPPE II score Richardson [13].
RR and 95% confidence interval (95%CI) were estimated using simple and multiple log-binomial models. The following covariables were considered for the multiple models: sex, birth weight (to remove the effect of collinearity with gestational age), first- and fifth-minute Apgar score < 7, prenatal use of corticosteroids, maternal arterial hypertension, rupture of fetal membranes > 18 h, and cesarean delivery [6, 7, 14,15,16].
The performance of the centers was defined by the relative mortality rate during the study period. Since disease severity is closely related to the mortality risk, we chose the Score for Neonatal Acute Physiology with Perinatal Extension-II (SNAPPE II) as a marker of mortality risk. It has been shown that the discrimination of deaths from survivors by SNAPPE-II is excellent, including for very low birth weight infants [13]. Thus, once the risk of death decreases with decreasing SNAPPE-II values, even for different gestational ages [17], we considered a cut-off point of < 20 to define two groups of comparison once it has been expected that, in centers with score < 20, mortality rates should be the lowest. Thus, to classify the centers according to their performance, we used the statistical models based on conditional inference trees, which are a class of multivariable nonparametric models that incorporates regression models structured as trees and the conditional inference theory. Essentially, this model consists of selecting an attribute (independent variable), named initial (or root) node (in this case SNAPE II), from which individuals are separated in classes known as interests. From each split from interests, new attributes are selected (other independent variables—in this case, mortality rates) and in the end, there are terminal nodes.
Using a SNAPPE score II ≥ 20 (category 1) and < 20 (category 0) [16] as criteria in the initial calculation, two groups of centers according to mortality rate were established: group 1 (G1; 16 centers with a lower mortality rate, 4.8%) and group 2 (G2; four centers with a higher mortality rate, 13.0%) (p < 0.01 (Fig. 1). So, the evaluation of mortality risk was based on relative values for center groups and not for absolute values for each center. Data were analyzed statistically using the SAS statistical software package version 9.4 (SAS Institute, Inc., Cary, NC), with the level of significance set at 5%.
Results
During the study, the RBPN database reported 7519 inborn VLBW infants. After the application of the exclusion criteria, 4397 infants were evaluated (Fig. 2). Forty-one patients (0.9%) were further excluded because of the lack of information on temperature at admission. Thus, the final study population consisted of 4356 newborns (mean gestational age 28.9 ± 2.6 weeks; mean birth weight 1062 ± 272 g). In comparison with G2 group, G1 have shown significantly lower rates of hypertensive mothers (p = 0.002), prolonged rupture of fetal membranes (p = 0.012), vaginal delivery (p < 0.001), male sex (p = 0.036), and 1st Apgar score < 7 (p < 0.001). Other maternal and neonatal characteristics are listed in Table 1.
The overall AH incidence in the 20 RBPN centers was 53.7% (the AH incidence varied significantly among the centers, i.e., 19.8–93.3%). The number of preterm infants in the various centers has ranged from 60 to 991, corresponding to 1.3% and 22.7% of the entire cohort, respectively. High AH rates have been observed both in the centers with the smallest and in those with the largest number of participants (centers 17 and 15, respectively). High AH rates observed both in the centers with the smallest and in those with the largest number of participants (centers 17 and 15, respectively). Median temperature at admission was 35.9 °C (interquartile range 35.2–36.4 °C), only 21.3% of the newborns were admitted with a temperature between 36.5 and 37.5 °C, and two centers reported highest AH indices (center 1, 60.4%; center 2, 46.7%), as shown in Fig. 3. By contrast, hyperthermia (37.6 to 37.7 °C) occurred in 24 newborns only (0.55%).
Frequency distribution (%) of the degrees of hypothermia (mild, moderate, and severe) and of normothermia* according to the centers of the Brazilian Network of Neonatal Research. *According to World Health Organization definitions: normothermia (36.5–37.5 °C), mild hypothermia or cold stress (36.0–36.4 °C), moderate hypothermia (32.0–35.9 °C), severe hypothermia (less than 32 °C). [8]
The temporal trends of AH revealed a fall in the incidence during the study (61.6%–57.1%–49.6%–46.9%), although the rate remained high during the last year of evaluation. Assessment of the association between AH and death and neonatal morbidity outcomes revealed that AH is associated with in-hospital death (adjusted RR 1.29, 95%CI 1.07–1.58) and with early neonatal death (adjusted RR 1.41, 95%CI 1.09–1.84) in G1. For BPD, PIVH, and the combined outcomes of death/BPD/PIVH, an increased risk in the non-adjusted model, with significance disappearing after controlling selected variables, was noted. No significant association with the other outcomes was found (Table 2).
Moreover, in G2, AH was also found to be significantly associated with early neonatal death (adjusted RR 1.34, 95%CI 1.04–1.74) and tended to be associated with hospital death, although the trend was not statistically significant (adjusted RR 1.16, 95%CI 0.95–1.41). The occurrence of necrotizing enterocolitis (NEC) was also statistically significant and was less frequent in the hypothermic group even after adjustment (crude RR 0.64, 95%CI 0.43–0.94; adjusted RR 0.58, 95%CI 0.38–0.8). The non-adjusted model revealed an increased risk of the combined outcome of death/BPD/PIVH and retinopathy of prematurity in the hypothermic group, although statistical significance was lost after adjustment. No association was observed for the remaining outcomes, as shown in Table 2.
Discussion
Despite the measures to maintain normothermia in the delivery room in RBPN centers, which were established since 2011 according to the recommendations of the Brazilian Society of Pediatrics and of the American Academy of Pediatrics, AH rates remained high [18]. Based on the RBPN database, from 2014 to 2016, approximately 92 to 100% of infants had received preventive measures in the delivery room, such as the use of radiant warmers and porous plastic bag and cap. The high AH rates observed could be attributed to extremely long transportation time from delivery room to intensive care unit, inappropriate temperature of transport incubators, and failure to keep the plastic bag sealed and/or the cap in loco. However, appropriate evaluation of these possible reasons was a challenge. Another possible explanation for the high AH rates would be the unavailability of a thermal or chemical mattress that is especially indicated for newborns weighing < 1000 g and/or for extremely preterm babies, which corresponded to 42% and 31% of the study population, respectively. Moreover, the absence of heated humidified gases in the delivery room and during transportation to the admission unit could also be a reason, although this is a controversial topic in the literature because of the short time of exposure to cold gas mixture [19, 20].
Although all the hospitals included in this study have implemented interventions recommended by pediatric societies, effective reduction in AH with occurred in two centers only, with the implementation of several standardized preventive measures [21]. This finding is consistent with Pinheiro et al. study, which has described a project for quality improvement over a period of 70 consecutive months using plan-do-check-act (PDCA) cycles. It has been demonstrated that the analysis of the results and offering feedback to the team permitted to reach the objective of maintaining normothermia at admission in > 90% of VLBW preterm infants [22]. An interesting point of this study could be that all temperature measurements were performed in the axillary region. In some related studies, temperature was measured in the axillary region [2], axillary and rectal regions [3, 7], skin, axillary or rectal region [14], or rectal region only [15], depending on the routine of the centers involved. This aspect is essential because a difference between rectal and axillary temperatures has been demonstrated in newborns. Several studies had shown that axillary measurements tend to be lower than rectal assessment [23,24,25,26]. In consequence, this difference in temperature measurements could affect the definition of hypothermia, thereby hampering the comparisons of AH studies.
A recent review has hypothesized that mortality associated with hypothermia is related to the quality of neonatal care [9]. According to Donabedian [27], the quality of medical care is based on three factors: infrastructure, the work process, and the outcomes. Unfortunately, in our study, we did not have access to the data of physical infrastructure or staff resources of the centers evaluated. Thus, our study is unique in its evaluation of the association between AH and the performance of the centers. We chose the assessment of outcome as a marker of performance. Mortality rate satisfies the criteria of a good measurement of performance/quality as it is easy to define and to observe, is important for the patients and for health care providers, favors changes in attitudes, and is easy to obtain [28, 29]. Moreover, although mortality could be predicted using various indicators, we opted to use the SNAPPE II score, which, together with SNAP-II, is an instrument used for the measurement of neonatal disease severity worldwide and is easy to apply in neonatal units [30]. The score has an excellent discriminating power for in-hospital death. For example, in infants of the same gestational age, the risk of death decreases with lower scores [13, 17]. In our study, we aimed to characterize the neonatal care performance of the centers in patients with good chances of survival (as shown by a lower score). Thus, a high mortality rate in these patients may be interpreted as a marker of inadequate neonatal care.
However, this study did not confirm the hypothesis that mortality and AH are associated with the quality of neonatal care or with clinical outcomes, which could be because the criterion chosen (mortality rate) might not allow an adequate characterization of the performance of the centers, especially given that the definition of assistance quality is complex. We also speculate that the Brazilian hospitals in this study have no differences in the quality of neonatal care that could be considered enough to support the difference in mortality rates. Moreover, AH may be associated with outcomes not measured in this study, such as hemodynamic instability at admission and the management of hypothermic newborn reheating at RBPN centers. Rapid reheating is traditionally considered deleterious for newborns, although Rech Morassuti et al. did not detect significant differences in mortality rates or other neonatal morbidities when comparing rapid and slow reheating [31]. However, the RBPN database does not contain relevant information.
In this study, the outcome variables (morbidities) were carefully selected according to literature recommendations to permit comparison of the findings [6, 7, 14,15,16]. Except for the significant association of NEC with the morbidities evaluated in G2, no association with the remaining morbidities was noted. This result differs from the data of Lyu et al., who reported that the rates of severe neurological damage, severe prematurity retinopathy, NEC, BPD, and late sepsis were lower when the temperature at admission ranged from 36.5 to 37.2 °C, with an increase in the mortality rate and in the frequency of major morbidities when hypothermia and hyperthermia occurred, taking on a U-shaped curve [7]. Nonetheless, other studies also did not demonstrate an association of AH with morbidities after confounder adjustment [3, 5, 15]. One study of a Danish cohort of preterm newborns (< 32 weeks of gestational age) did not detect an association between AH and combined outcomes of respiratory distress syndrome or death (adjusted OR 1.36, 95%CI 0.89–2.08), or BPD or death (adjusted OR 1.03, 95%CI 0.64–1.68). However, hypothermia was defined as a core temperature of < 36.5 °C on admission [15]. In the EPICE study, Wilson et al. also detected no significant difference in the temperature reference value for grade III/IV PIVH, grade II/III NEC, or BPD after adjustment for any temperature range assessed [3]. Furthermore, in an extensive study of 8782 infants with a mean weight of 1072 g and a gestational age of 28 weeks, Miller et al. detected an association between AH and severe retinopathy, late sepsis, PO2 at 36 weeks, and severe PIVH using a non-adjusted analysis. After adjustment of the variables, only severe PIVH remained to be significantly associated with AH (OR 1.3, 95%CI 1.1–1.6) [5].
The significant protective association between AH and NEC in G2 was an uncommon finding. Although hypothermia is a factor for NEC induction in experimental animals [32], some evidence showed that mild induced therapeutic hypothermia may have some protective effect as a rescue therapy for NEC in preterm infants [33, 34]. Thus, this was an unexpected finding where we could perhaps apply the theory of biological plausibility of Bradford Hill, which states that it would be extremely useful if the suspected cause (or, in this case, the association) were biologically plausible, which depends on our current knowledge. Hence, this finding may be considered a statistical phenomenon but without biological significance or could be a finding that warrants further investigation, considering that biological plausibility changes along time [35]. Another two possibilities are that, in high mortality centers, only healthier infants remained alive, resulting in less likelihood to occurrence of NEC, and finally, differences in performances or policies centers might result in this finding. For example, it is possible that enteral intake of fresh mother’s milk or bank human milk, a well protective factor for NEC, varies widely among RBPN centers. Unfortunately, we did not have this information in RBPN database. Horbar et al. [36] have also found large differences in performance across Vermont Oxford Network centers, and, by contrast, Profit et al. have described that their findings of absence of substantial difference between hospital performance in 22 California regional neonatal intensive care units could indicate that quality of care among them was similar [37].
An implication of our study aims to report to Central Council of RBPN our results and discuss how to inform all the centers in respect to managing this serious health problem for VLBW infants and propose educational and quality improvement initiatives in order to reduce HA. Thereby, in the future, a reassessment may be possible to verify the effect of such improvement measures in neonatal care.
In conclusion, the AH rate was high and significantly associated with early neonatal death regardless of the hospital performance, which is based on mortality rate. In the group with the worst performance, an unexpected protective association between AH and NEC was found, whereas the other morbidities assessed were not significantly associated with AH.
Abbreviations
- AH:
-
Admission hypothermia
- BDP:
-
Bronchopulmonary dysplasia
- CI:
-
Confidence intervals
- EPICE:
-
Effective Perinatal Intensive Care in Europe
- NEC:
-
Necrotizing enterocolitis
- PIVH:
-
Peri-intraventricular hemorrhage
- RR:
-
Relative risk
- SNAPPE II:
-
Score for Neonatal Acute Physiology with Perinatal Extension-II
- VLBW:
-
Very low birth weight
References
Bhatt DR, White R, Martin G, Van Marter LJ, Finer N, Goldsmith JP, Ramos C, Kukreja S, Ramanathan R (2007) Transitional hypothermia in preterm newborns. J Perinatol 27(Suppl 2):S45–S47
de Almeida MF, Guinsburg R, Sancho GA, Rosa IR, Lamy ZC, Martinez FE, da Silva RP, Ferrari LS, de Souza Rugolo LM, Abdallah VO, Silveira Rde C, Brazilian Network on Neonatal Research (2014) Hypothermia and early neonatal mortality in preterm infants. J Pediatr 164:271–5.e1
Wilson E, Maier RF, Norman M, Misselwitz B, Howell EA, Zeitlin J, Bonamy AK, Effective Perinatal Intensive Care in Europe (EPICE) Research Group (2016) Admission hypothermia in very preterm infants and neonatal mortality and morbidity. J Pediatr 175:61–67.e4
Laptook AR, Salhab W, Bhaskar B, Neonatal Research Network (2007) Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics 119:e643–e6e9
Miller SS, Lee HC, Gould JB (2011) Hypothermia in very low birth weight infants: distribution, risk factors and outcomes. J Perinatol 31(Suppl 1):S49–S56
Chang HY, Sung YH, Wang SM, Lung HL, Chang JH, Hsu CH, Jim WT, Lee CH, Hung HF (2015) Short- and long-term outcomes in very low birth weight infants with admission hypothermia. PLoS One 10:e0131976
Lyu Y, Shah PS, Ye XY, Warre R, Piedboeuf B, Deshpandey A, Dunn M, Lee SK, Canadian Neonatal Network (2015) Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks’ gestation. JAMA Pediatr 169(4):e150277
Raven JH, Tolhurst RJ, Tang S, van den Broek N (2012) What is quality in maternal and neonatal health care? Midwifery. 28(5):e676–e683
Trevisanuto D, Testoni D, de Almeida MFB (2018) Maintaining normothermia: why and how? Semin Fetal Neonatal Med 23:333–339
World Health Organization (WHO). Safe Motherhood Unit. Division of Reproductive Health http://apps.who.int/iris/bitstream/10665/63986/1/WHO_RHT_MSM_97.2.pdf>. Accessed 10 Mar 2017. (Technical Support). Thermal protection of the newborn: a practical guide
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92:529–534
Walsh MC, Kliegman RM (1986) Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am 33(1):179–201
Richardson DK, Corcoran JD, Escobar GJ, Lee SK (2001) SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr 138:92–100
Laptook AR, Bell EF, Shankaran S, Boghossian NS, Wyckoff MH, Kandefer S, Walsh M, Saha S, Higgins R, Generic and Moderate Preterm Subcommittees of the NICHD Neonatal Research Network (2018) Admission temperature and associated mortality and morbidity among moderately and extremely preterm infants. J Pediatr 192:53–59.e2
Jensen CF, Ebbesen F, Petersen JP, Sellmer A, Bach CC, Henriksen TB (2017) Hypothermia at neonatal intensive care unit admission was not associated with respiratory disease or death in very preterm infants. Acta Paediatr 106(12):1934–1939
Lee SK, Lee DS, Andrews WL, Baboolal R, Pendray M, Stewart S (2003) Higher mortality rates among inborn infants admitted to neonatal intensive care units at night. J Pediatr 143:592–597
Dammann O, Shah B, Naples M, Bednarek F, Zupancic J, Allred EN, Leviton A, for the ELGAN Study Investigators (2009) Interinstitutional variation in prediction of death by SNAP-II and SNAPPE-II among extremely preterm infants. Pediatrics. 124(5):e1001–e1006
American Academy of Pediatrics (2011) Resuscitation of babies born preterm. In: Kattwinkel J (ed) Neonatal resuscitation, 6th edn. American Academy of Pediatrics, Itasca, pp 272–282
McGrory L, Owen LS, Thio M, Dawson JA, Rafferty AR, Malhotra A, Davis PG, Kamlin COF (2018) A randomized trial of conditioned or unconditioned gases for stabilizing preterm infants at birth. J Pediatr 193:47–53
Carlo WA, Chatburn RL (2018) Is it necessary to heat and humidify respiratory gases for resuscitation in preterm infants? J Pediatr 193:10–11
Caldas JPS, Millen FC, Camargo JF, Castro PAC, Camilo ALDF, Marba STM (2018) Effectiveness of a measure program to prevent admission hypothermia in very low-birth weight preterm infants. J Pediatr 94:368–373
Pinheiro JM, Furdon SA, Boynton S, Dugan R, Reu-Donlon C, Jensen S (2014) Decreasing hypothermia during delivery room stabilization of preterm neonates. Pediatrics 133:e218–e226
Morley CJ, Hewson PH, Thornton AJ, Cole TJ (1992) Axillary and rectal temperature measurements in infants. Arch Dis Child 67:122–125
Falzon A, Grech V, Caruana B, Magro A, Attard-Montalto S (2003) How reliable is axillary temperature measurement? Acta Paediatr 92:309–313
Mayfield SR, Bhatia J, Nakamura KT, Rios GR, Bell EF (1984) Temperature measurement in term and preterm neonates. J Pediatr 104:271–275
Craig JV, Lancaster GA, Williamson PR, Smyth RL (2000) Temperature measured at the axilla compared with rectum in children and young people: systematic review. BMJ 320:1174–1178
Donabedian A (1979) The quality of medical care: a concept in search of a definition. J Fam Pract 9(2):277–284
Sprague AE, Dunn SI, Fell DB, Harrold J, Walker MC, Kelly S, Smith GN (2013) Measuring quality in maternal-newborn care: developing a clinical dashboard. J Obstet Gynaecol Can 35:29–38
Janakiraman V, Ecker J (2010) Quality in obstetric care: measuring what matters. Obstet Gynecol 116:728–732
Morse S, Groer M, Shelton MM, Maguire D, Ashmeade T (2015) A systematic review: the utility of the revised version of the score for neonatal acute physiology among critically ill neonates. J Perinat Neonatal Nurs 29:315–344
Rech Morassutti F, Cavallin F, Zaramella P, Bortolus R, Parotto M, Trevisanuto D (2015) Association of rewarming rate on neonatal outcomes in extremely low birth weight infants with hypothermia. J Pediatr 167:557–561.e1-2
Ginzel M, Feng X, Kuebler JF, Klemann C, Yu Y, von Wasielewski R, Park JK, Hornef MW, Vieten G, Ure BM, Kaussen T, Gosemann JH, Mayer S, Suttkus A, Lacher M (2017) Dextran sodium sulfate (DSS) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS One 12:e0182732
Athalye-Jape G, More K, Patole S (2013) Progress in the field of necrotising enterocolitis–year 2012. J Matern Fetal Neonatal Med 26:625–632
Hall NJ, Eaton S, Peters MJ, Hiorns MP, Alexander N, Azzopardi DV, Pierro A (2010) Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics 125:e300–e308
Hill AB (1965) The environment and disease: association or causation? Proc R Soc Med 58:295–300
Profit J, Zupancic JAF, Gould JB, Pietz K, Kowalkowski MA, Draper D, Hysong SJ, Petersen LA (2013) Correlation of neonatal intensive care unit performance across multiple measures of quality of care. JAMA Pediatr 167(1):47–54
Horbar JD (1999) The Vermont Oxford Network: evidence-based quality improvement for neonatology. Pediatrics. 103(1 Suppl E):350–359
Author information
Authors and Affiliations
Contributions
Jamil Pedro de Siqueira Caldas, Walusa A. G. Ferri, Sérgio T.M.Marba, Davi C. Aragon, Ruth Guinsburg and Maria F.B. de Almeida have participated in the concept and design, analysis and interpretation of data, and drafting and revising the manuscript. All other authors have participated in the design of the study, collection and interpretation of data and revising the manuscript. All authors listed on the manuscript approved the submission of this version of the manuscript and take full responsibility for the manuscript.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Ethical approval number: 1.903.783 - Federal University of São Paulo, São Paulo.
Informed consent
All centers agreed to have their data included in this study, and the Research Ethics Committee of the coordinating institution approved the project without needing patients’ informed consent: 1.903.783.
Additional information
Communicated by Patrick Van Reempts
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Siqueira Caldas, J.P., Ferri, W.A.G., Marba, S.T.M. et al. Admission hypothermia, neonatal morbidity, and mortality: evaluation of a multicenter cohort of very low birth weight preterm infants according to relative performance of the center. Eur J Pediatr 178, 1023–1032 (2019). https://doi.org/10.1007/s00431-019-03386-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03386-9