Abstract
Lipoblastoma is a rare and benign tumor arising from embryonal fat cells. It is generally diagnosed in children younger than 3 years of age and can occur in the extremities or on the trunk. We present our series of 10 children with lipoblastoma treated at Schneider Children’s Medical Center of Israel between 2011 and 2016. Six boys and four girls underwent tumor resection at a median age of 2 years and 3 months (range 5 months to 5.6 years). Locations were trunk (6), groin (2), perineum (1), and omentum (1). Follow up ranges from 1 to 5 years. Two patients had a local recurrence and required a second resection 2 years (perineal) and 6 years (trunk) after the first surgery without further recurrence at 1.9 and 2.9 years, respectively.
Conclusion: Higher awareness of lipoblastoma enables optimal imaging strategies and resection. Long follow up is required due to local recurrences. The treatment of choice consists of complete, but non mutilating surgical resection.
What is Known: • Lipoblastoma is a rare benign tumor of fatty tissue affecting children • Treatment consists of surgical resection |
What is New: • MRI is the modality of choice for follow up • Ten-year long-term follow up is required due to late recurrence |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipoblastoma is a rare and benign tumor arising from embryonal fat tissue, occurring mostly in children younger than 3 years of age. It was first described in 1926 as a tumor of immature fat cells [8] but only gained acceptance as an entity in its own right in 1958 [19]. It can present with a rapid growth rate, exerting pressure on surrounding structures or be slow-growing and more difficult to detect. Its main differential diagnosis is simple lipoma and myxoid liposarcoma, both calling for different management strategies. In this manuscript, we describe our center’s experience with lipoblastoma in the context of the recent literature, aiming to describe current management paradigms for this uncommon tumor.
Material and methods
We retrospectively reviewed the case notes of all patients with a pathology report stating “lipoblastoma” or “lipoblastomatosis” between 2011 and 2016. Ten cases were identified. The collected data consisted of patient data (age, sex, radiological studies, surgical procedures, complications, follow-up) as well as tumor data (size, location, pathology, recurrence). The study was approved by the Institutional Review Board of Schneider Children’s Medical Center of Israel and of Rabin Medical Center (0431-16-RMC).
Results
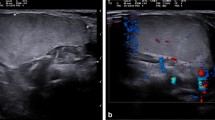
Nine cases of lipoblastoma and one case of lipoblastomatosis were identified. The clinical data is summarized in Table 1. Six males (60%) and four females (40%) (male-to-female ratio 1.5:1) were diagnosed. The average age at surgery was 2.3 years (range 5 months to 5.6 years), with 90% under 3 years. The anatomical distribution was trunk (60%), groin (20%), perineum (10%), and omentum (10%). No extremity lesion was detected in our series. Eight out of 10 patients underwent imaging prior to surgery. Ultrasound sonography (US) only was undertaken in one patient presenting an abdominal wall mass, US followed by magnetic resonance imaging (MRI) in six patients, and computerized tomography (CT) in one patient presenting a vascular ring and discovery of an incidental fatty lesion of the thoracic wall (MRI imaging of patient 1–Fig. 1). Two patients underwent emergency surgery for suspected inguinal hernia and thus escaped imaging prior to surgery.
Patients were followed up with MRI in seven and US in three (superficial tumors in the inguinal region or abdominal wall) cases. Nine patients underwent complete excision with free margins. In one patient, presenting a suspected obturator hernia, an unresectable mass was found at surgery and thus she initially underwent excisional biopsy only. The family refused a secondary complete excision to prevent a mutilating surgery and the patient continues to be monitored closely with an MRI every 6 months. In the last 6 months, the mass has grown clinically and became harder, but is still mobile and painless. Surgery has been proposed but the parents have still not decided.
The resected masses ranged from 1.5 to 18.5 cm in diameter. The largest tumor was intraabdominal with an omental lipoblastoma filling the entire abdominal cavity (Fig. 2). All pathology specimens were examined by two pathologists. Cytogenetic evaluation of the 8q11-13 rearrangement was performed in five patients and found to be positive.
The postoperative follow-up ranges from 6 months to 4 years, using 6–12 monthly US or MRI. Two patients were detected to have local recurrence; 23 months post macroscopic complete resection of a perineal lipoblastoma and 6 years post macroscopic complete resection of a trunk lipoblastomatosis that was previously operated in another institution. At the initial surgery, the lesion involved the resection margins in both cases. The patient with the lipoblastomatosis of the trunk was lost to follow up at the other institution and came back to our institution because of intermittent pain of the left thoracic wall. He presented with a large 10-cm tumor and underwent an MRI before his second procedure showing partial infiltration of the muscles. Both underwent a second complete excision with no evidence of recurrence at 1.9 and 2.9 years, respectively.
Discussion
Adipose tissue tumors occurring during the first decade of life are rare and represent 6% of all soft tissue tumors in children, 5–30% are of the lipoblastomatous type [7].
The term lipoblastoma was first used in 1926 when Jaffe described a tumor of immature fat cells [8]. But it was not until 1958 that Vellios coined the term lipoblastomatosis to describe a benign diffuse tumor in the subcutaneous tissue resembling fetal fat [19]. In 1973, Chung and Enziger described two forms: a localized, superficial, lobulated, encapsulated, or circumscribed form called lipoblastoma and a diffuse, infiltrative form called lipoblastomatosis [4].
Usually, lipoblastomas occur superficially in the subcutis contrary to lipoblastomatosis which tends to infiltrate adjacent soft tissue and muscular planes, involving body compartments (retroperitoneum, abdomen, thorax) [7].
Lipoblastoma is a rare and benign tumor arising from embryonal white fat, occurring predominantly in male children younger than 3 years of age. Our data is consistent with this, showing a male to female ratio of 1.5:1 and 90% younger than 3 years. No metastases are reported, and recurrence rates are quoted between 14 and 25%, requiring re-excisions [9, 17]. Most of these tumors are located in the extremities or trunk, other sites are rare but include the neck, scrotum, axilla, mediastinum, and intraperitoneal. These tumors are generally asymptomatic but in some cases, they can cause morbidity due to the infiltrative nature in lipoblastomatosis and a mass effect on vital structures in the thorax or abdomen [7].
Lipoblastoma in the abdomen are uncommon, but tend to present as large masses at the time of detection [16, 18], as in our intraabdominal tumor, measuring 18.5 cm (Fig. 2). Contrary to previous reports [2, 4, 16], there was no extremity lesion in our series. This might be attributable to referral bias with no orthopedic-oncology services available in our institution.
Less than 250 cases are reported in the literature but recognition of this tumor is important because its treatment and follow-up is different from that of the two lesions which represent its differential diagnosis: simple lipoma and myxoid liposarcoma. Lipoma requires a simple excision and myxoid liposarcoma requires an extensive resection associated with local radiotherapy.
This tumor is rarely suspected before surgery and thus histopathology analyses evoke and generally confirm the diagnosis.
The macroscopic appearance of lipoblastoma consists of a lobulated, soft tissue mass, very often encapsulated, with a cut surface of trabeculated, glistening, yellow-gray parenchyma [1, 3, 5, 7, 9, 15, 18]. Microscopically, lobules of mature and immature fat cells, signet-ring lipoblasts, primitive mesenchymal cells, multivacuolated lipoblasts of varying degrees of differentiation are found [3, 14].
Liposarcoma are rare in patients under 10 years [15]. Usually, histopathologic analysis is sufficient for diagnosis. In cases of difficulty to distinguish between lipoblastoma and myxoid liposarcoma, cytogenetic specimen analysis can supplement the pathological evaluation [6, 7, 11, 14, 16]. Rearrangements of chromosomal region 8q11-33 are found in the majority of lipoblastomas, whereas a distinctive translocation t(12;16) (q13;p11) is found in myxoid liposarcoma [12, 16].
It is not routine practice to perform a cytogenetic analysis if not required by the pathologist. It can be of diagnostic value to differentiate lipoblastoma from liposarcoma [6, 10].
Preoperative imaging is useful to assess the extent of the tumor, but cannot differentiate between the various adipose tissue tumors, as no pathognomic characteristics have been associated with lipoblastoma [14, 16]. Imaging, in particular MRI, is best suited for postoperative surveillance and eventual evaluation of recurrent lesions [3, 14, 16].
The treatment of choice consists of complete surgical resection with free margins to prevent recurrence. Nevertheless, lately a more conservative approach in children with large invasive lesions or lesions in locations like the extremities that would lead to mutilating surgical excision has been described [14]. And for those with incomplete excision, MRI is a useful tool for monitoring tumor progression.
In our study, nine out of ten patients underwent complete excision with free margins. Only one patient presented with an unresectable tumor and is closely followed up.
Regardless of free margins status, all children should be followed for a minimum of 5 years [10, 13, 14]. Recurrences are more common in the lipoblastomatosis form but have also been documented in the lipoblastoma forms [10, 15]. Recurrence has been described up to 10 years, in our series a recurrence occurred 6 years after the initial total resection. US or MRI are helpful for the follow up of these already diagnosed lipoblastoma tumors on a 6 to 12 months basis.
This manuscript aims to generate greater awareness of this pathology among caretakers. An optimal resection, radical but not mutilating, is advised. Contrary to what was described in the literature, we stress the importance of a longer follow up to at least 10 years as recurrences can occur much later, using MRI as preferred imaging tool or US for superficial lipoblastoma tumors.
Abbreviations
- CT:
-
Computerized tomography
- MRI:
-
Magnetic resonance imaging
- US:
-
Ultrasound
References
Armenise T, Gentile O, Orofino A, Leggio S, Lanzillotto MP, Zullino F, Paradies G (2015) Lipoblastoma in infant: our experience. J Ped Surg Case Rep 3(2):63–64. https://doi.org/10.1016/j.epsc.2014.12.001
Chien AL, Song DH, Stein SL (2006) Two young girls with lipoblastoma and a review of the literature. Pediatr Dermatol 23(2):152–156
Chun YS, Kim WK, Park KW, Lee SC, Jung SE (2001) Lipoblastoma. J Ped Surg 36(6):905–907. https://doi.org/10.1053/jpsu.2001.23969
Chung EB, Enziger FM (1973) Benign lipoblastomatosis. An analysis of 35 cases. Cancer 32:482–492
Collins MH, Chatten J (1997) Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 25 tumors. Am J Surg Pathol 21(10):1131–1137. https://doi.org/10.1097/00000478-199710000-00002
Fletcher CD, Akerman M, Dal Cin P, de Wever I, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Sciot R, Tallini G, van den Berghe H, van de Ven W, Vanni R, Willen H (1996) Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol 148(2):623–630
Hicks J, Dilley A, Patel D, Barrish J, Zhu SH, Brandt M (2001) Lipoblastoma and lipoblastomatosis in infancy and childhood: histopathologic, ultrastructural, and cytogenetic features. Ultrastruct Path 25(4):321–333. https://doi.org/10.1080/019131201753136359
Jaffe RH (1926) Recurrent lipomatous tumors of the groin: liposarcoma and lipoma pseudomyxomatodes. AMA Arch Pathol 1:381–387
Jimenez JF (1986) Lipoblastoma in infancy and childhood. J Surg Oncol 32(4):238–244. https://doi.org/10.1002/jso.2930320413
Jung SM, Chang PY, Luo CC, Huang CS, Lai JY, Hsueh C (2005) Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 16 cases in Taiwan. Pediatr Surg Int 21(10):809–812. https://doi.org/10.1007/s00383-005-1502-x
Kerkeni Y, Sahnoun L, Ksia A, Hidouri S, Chahed J, Krichen I, Mekki M, Belghith M, Nouri A (2014) Lipoblastoma in childhood: about 10 cases. Afr J Paed Surg 11(1):32–34. https://doi.org/10.4103/0189-6725.129210
Kloboves-Prevodnick VV, Us-Krašovec M, Gale N, Lamovec J (2005) Cytological features of lipoblastoma: a report of three cases. Diagn Cytopathol 33(3):195–200. https://doi.org/10.1002/dc.20322
Kok KYY, Telisinghe PU (2010) Lipoblastoma: clinical features, treatment, and outcome. World J Surg 34(7):1517–1522. https://doi.org/10.1007/s00268-010-0466-8
McVay MR, Keller JE, Wagner CW, Jackson RJ, Smith SD (2006) Surgical management of lipoblastoma. J Ped Surg 41(6):1067–1071. https://doi.org/10.1016/j.jpedsurg.2006.02.025
Mentzel T, Calonje E, Fletcher CDM (1993) Lipoblastoma and lipoblastomatosis: a clinicopathological study of 14 cases. Histopathology 23(6):527–533. https://doi.org/10.1111/j.1365-2559.1993.tb01238.x
Speer AL, Schofield DE, Wang KS, Shin CE, Stein JE, Shaul DB, Mahour GH, Ford HR (2008) Contemporary management of lipoblastoma. J Ped Surg 43(7):1295–1300. https://doi.org/10.1016/j.jpedsurg.2007.10.068
Stringel G, Shandling B, Mancer K, Ein SH (1982) Lipoblastoma in infants and children. J Ped Surg 17(3):277–280. https://doi.org/10.1016/S0022-3468(82)80012-2
Tang XB, Zhang T, Bai YZ, Wang WL (2009) Giant mesenteric lipoblastoma in a 4-year-old child. J Ped Surg 44(4):859–861. https://doi.org/10.1016/j.jpedsurg.2009.01.052
Vellios F, Baez JM, Shumacker MB (1958) Lipoblastomatosis. A tumor of fetal fat different from hibernoma: report of a case with observations on the embryogenesis of human adipose tissue. Am J Pathol 34:1149–1153
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study conception and design: Dr. A. Baazov, Dr. E. Seguier-Lipszyc, Pr. E. Freud.
Data acquisition: Dr. E. Seguier-Lipszyc, Dr. S. Fichman.
Analysis and data interpretation: Dr. E. Seguier-Lipszyc, Dr. A. Baazov.
Drafting of the manuscript: Dr. E. Seguier-Lipszyc,
Critical revision: Dr. S. Ash, Pr. E. Freud.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Piet Leroy
Emmanuelle Séguier-Lipszyc is the First author
Rights and permissions
About this article
Cite this article
Séguier-Lipszyc, E., Baazov, A., Fichman, S. et al. Current management of lipoblastoma. Eur J Pediatr 177, 237–241 (2018). https://doi.org/10.1007/s00431-017-3059-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-3059-9