Abstract
To estimate the levels of malondialdehyde (MDA) and 8-hydroxy-2-deoxyguanosine (8-OH-dG) in cord blood plasma of newborns born through meconium-stained amniotic fluid (MSAF) and also to find out the correlation between their levels with birth weight and gestation, we measured the cord blood plasma levels of MDA and 8-OH-dG in 59 newborns born through MSAF and 50 newborns born through clear liquor. The levels of cord blood plasma MDA and 8-OH-dG were significantly higher in full-term and late-preterm newborns born through MSAF. On further comparison, it was found that both full-term and late-preterm intrauterine growth restricted (IUGR) neonates had higher levels of these markers as compared to babies born as appropriate for gestational age (AGA) through MSAF. Plasma levels of MDA and 8-OH-dG were significantly correlated with birth weight even after controlling the relationship with gestational age for all cases as well as all full-term cases. These markers are also significantly correlated to each other.
Conclusions: The present study suggest that the neonates born through MSAF experience higher degrees of oxidative stress, as evidenced by increased levels of cord blood plasma MDA and 8-OH-dG.
What is known: • Aspirated meconium has been found to induce free radical generation and cellular damage in animal studies. • Its role in free radical generation and oxidative damage in human neonates is scarce. |
What is new: • Neonates born through meconium-stained amniotic fluid experience significant oxidative stress. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meconium is a sterile, thick, blackish green, odorless material observed in the fetal intestine as early as the third month of gestation composed of water (approximately 80%), mucopolysaccharides, bilirubin, intestinal enzymes, hair, and squamous cells [2]. Meconium-stained amniotic fluid (MSAF) complicates deliveries in about 12 to 16% of cases [21], primarily in situations of advanced fetal maturity or in utero fetal distress. It is associated with higher rate of cesarean delivery, increased need for resuscitation, and meconium aspiration syndrome (MAS) [39]. The risk factors for MSAF are both maternal and fetal. The maternal factors are hypertension, gestational diabetes mellitus, maternal chronic respiratory or cardiovascular diseases, post-term pregnancy, preeclampsia, and eclampsia. The fetal factors include oligohydramnios, intrauterine growth restriction, and poor biophysical profile [14].
The rates of MAS have shown a dramatic decline in developed countries largely attributed to the reduction in post-term deliveries, more frequent detection of non reassuring fetal heart rate patterns, and increased use of amnioinfusion [47]. However, it still remains a serious problem in developing countries with incidence from India reported to be as high as 13% [31]. MAS is associated with increased risk of respiratory failure, air leaks, persistent pulmonary hypertension [11], mortality, and long-term pulmonary and neurodevelopmental sequelae [45]. Inflammation and damage to lung tissue can be caused by the aspirated meconium due to the presence of both hydrophilic (consisting of gastrointestinal enzymes including pancreatic phospholipase A2 or bile acids) and hydrophobic (consisting of cholesterol, free fatty acids, etc.) fractions of meconium. In addition, meconium contains variable amounts of cytokines (IL-1b, IL-6, IL-8, TNFɑ) and heme [12]. Consequently, components of meconium enhance chemotactic activity of polymorphonuclears (PMNs), particularly of neutrophils, and stimulate their leak through the alveolocapillary membrane [46]. It also indirectly induces inflammation, as it stimulates expression of cytokines (e.g., TNFɑ, IL-1, IL-6, IL-8), pro-inflammatory enzymes (phospholipase A2, proteases), and other bioactive substances such as derivatives of arachidonic acid, endothelin-1, and platelet activating factor from the activated cells. Furthermore, the cells generate large amounts of reactive oxygen species (ROS) leading to oxidative stress [10, 41]. All the mentioned substances predispose to subsequent surfactant dysfunction, parenchymal damage, edema, pulmonary vasoconstriction, and bronchial smooth muscle contraction [22].
Oxidative stress is an imbalance between the systemic manifestation of reactive oxygen species and a biological system’s ability to readily detoxify it or to repair the resulting damage. It is a physiological event during the normal intrauterine- to extrauterine transition, which greatly increases the production of free radicals and must be controlled by the antioxidant defense system, the maturation of which follows the course of the gestation. Adequately mature and healthy infants are able to tolerate this drastic change in the oxygen concentration. Problem arises when the intrauterine development is incomplete or abnormal. Preterm and intrauterine growth-retarded (IUGR) neonates are typically of this kind [26, 27]. Oxygen free radicals are extremely reactive chemical species that react with several living cell contents, e.g., phospholipids, aminoacids, and nucleic acids leading to lipid peroxidation, DNA strand breaks, and base methylation. Prime targets of reactive oxygen species are the PUFAs (polyunsaturated fatty acids) in cell membranes causing lipid peroxidation, which may lead to damage of the cell structure and function [19]. Additionally, decomposition of lipid hydroperoxides yields a wide variety of end products, including malondialdehyde (MDA) which can be assessed biologically as a measure of lipid peroxidation. Reactive oxygen species such as superoxide radical (O−·) and hydrogen peroxide (H2O2) do not directly react with DNA [16].
But the H2O2 crosses the nuclear membrane and reacts with ions of iron or copper to form highly toxic hydroxyl radicals (OH·) which directly react with DNA bases [15]. Among the oxidative bases, 8-OH-dG is the most abundant and accepted as a sensitive marker for oxidative DNA damage [17].
Diseases resulting from oxidative damage are grouped and categorized as free radical-mediated diseases (FRD) [33]. FRD in neonates include retinopathy of prematurity, bronchopulmonary dysplasia, periventricular leukomalacia, intraventricular hemorrhage, necrotizing enterocolitis, and patent ductus arteriosus [32–34]. In a study by Arjmand et al. measuring prooxidant-antioxidant balance in umbilical cord blood of neonates born through MSAF versus clear liquor showed that the former are exposed to oxidative stress as compared to the latter [3].
The identification of reliable biomarkers is essential for the characterization of oxidative stress (OS) and probably for the early discovery of OS-associated diseases. Biomarkers evaluate host susceptibility to OS by measuring proteins, lipids, and DNA damage. They can be used as “intermediate endpoints or early-outcome predictors” of disease development [7, 30]. The biomarkers that have been studied in various disorders of oxidative stress in neonates include advanced oxidation protein products [8, 9], total hydroperoxide [7–9], carbonylated proteins [38], acrolein-lysine adduct [42, 43], MDA [25, 28, 29], and 8-OH-dG [25, 28, 29].
There is a paucity of literature on markers of oxidative stress in neonates delivered through MSAF. There is just one study that showed that neonates delivered through MSAF have high concentration of 8-iso-prostaglandin F2alpha in neonatal cord blood, as a marker of lipid oxidation, and suggests that these infants were exposed to oxidative stress [20]. We hypothesized that delivery through MSAF is associated with oxidative stress in infants which is responsible for the acute and long-term complications. The present study was conducted to evaluate the oxidative stress markers (MDA, 8-OH-dG) in neonates delivered through MSAF and thus to ascertain their role in this condition.
Methods
This cross-sectional study was conducted in the Division of Neonatology, Department of Pediatrics, Institute of Medical Sciences and the Department of Biophysics, Banaras Hindu University, Varanasi, India, between September 2009 and April 2011. The study was approved by the Institute Ethics Committee, and informed consents were taken from parents before inclusion in the study.
Study population
Inclusion criteria
All term (≥37 weeks) and late-preterm neonates (35–36 weeks) born through MSAF were included as cases, whereas those born through clear liquor were included as controls.
Exclusion criteria
Difficulty in collecting sample within 30 min of delivery, severe congenital malformation, and mothers suffering from severe illnesses were part of the exclusion criteria.
In the absence of previous studies in the same topic, a convenient sample size of 109 babies was chosen.
Clinical data
Detailed base line information of maternal and neonatal variables were recorded including number of antenatal visits (booked pregnancy was defined as ≥4 and unbooked as <4 antenatal visits), gestational age at delivery, maternal medical and reproductive history, use of continuous electronic fetal monitoring for detection of fetal distress during active labor, presenting part of the baby at the time of delivery, mode of delivery and Apgar scores at 1 and 5 min. Weight of the newborn was recorded immediately after delivery in Seca weighing scale with an accuracy within 5 g.
IUGR status was defined as discrepancy between gestational age assessment by clinical examination and antenatal scans of >3 weeks and/or estimated fetal weight <10th centile as per standards using antenatal scan.
In the absence of blood gases, we defined perinatal asphyxia as Apgar score of ≤6 at 1 min which is in accordance with the National Neonatal-Perinatal Database of India [24].
Meconium aspiration syndrome was defined as the presence of any two of the following: [24]
-
(a)
Meconium staining of liquor or of nails or umbilical cord or skin
-
(b)
Respiratory distress soon after birth, within 1 h of birth
-
(c)
Radiological evidence of aspiration pneumonitis (atelectasis and or hyperinflation)
Laboratory analysis
Ten-milliliterS of free flowing cord blood of all the neonates (cases and controls) were collected in a sterile, heparinized, deionized polyethylene vials within 30 min of delivery. Plasma was separated from the blood samples immediately by centrifugation at 1500g for 10 min and stored in separate deionized vials at −20 °C.
Malondialdehyde—marker of lipid peroxidation
Plasma malondialdehyde (MDA) levels in the samples were assayed by thiobarbituric acid reactive substances (TBARS) technique of Philpot [35]. The plasma (1 ml) was mixed thoroughly with 2 ml of TCA–TBA–HCl (15% w/v TCA and 0.375% w/v TBA in 0.25 N HCl). The mixture was heated in a boiling water bath for 15 min. Thereafter, samples were centrifuged at 750g for 10 min. The absorbance of the sample was determined at 530 nm in a spectrophotometer against a suitable blank. The MDA concentration of each sample was calculated by using extinction coefficient of 1.56 × 105 M − 1 cm − 1.
8-OH-dG—marker of oxidative DNA damage
Plasma of all the samples was used for the measurement of 8-OH-dG levels using competitive in vitro enzyme linked immunosorbent assay (ELISA) kit obtained from Caymen Chemical Company, U.S.A. [40]. 8-OH-dG measurements were performed using microtiter ELISA plate pre-coated with anti-mouse IgG. Fifty microliters plasma, 50 μl 8-OH-dG AChE (acetylcholinesterase) tracer, and 50 μl 8-OH-dG monoclonal antibody were added to each well and incubated at 4 °C for 18 h. Thereafter, the wells were washed for five times, and 200 μl Ellman’s reagent was added to each well. The wells were incubated at room temperature in the dark for 100 min. The absorbance was read at wavelength of 420 nm. ELISA assay displays IC50 (50% B/B0) and IC80 (80% B/B0) values of approximately 100 and 30 pg/ml, respectively.
Statistical analysis
Statistical analysis was done using the SPSS statistical package (version 16.0). Data were expressed as mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. Student’s t test (unpaired) was used for analysis of continuous variables. Categorical variables were compared by chi-square test or Fisher’s exact test. Correlations between variables were studied by Pearson’s correlation test. A value of p < 0.05 was considered statistically significant.
Results
The flow of participants into the study is presented in Fig. 1. For statistical analysis, the cases were further subclassified into those who were appropriate for gestational age (AGA) and those who were intrauterine growth restricted (IUGR). Table 1 shows the baseline characteristics of study participants. The MSAF group had significantly higher number of unbooked pregnancies, primiparous mothers, neonates having fetal distress during active labor, breech presentation at the time of delivery, and cesarean deliveries. These babies were born at a significantly higher gestational age than the control group (3 babies were post-term in cases and none in control arm). However, the two groups were comparable in birth weight, Apgar score at 1 and 5 min, hypertensive disorders of pregnancy, IUGR status, and IUGR babies born vaginally versus delivered by cesarean section (16 vaginally born vs 17 delivered by cesarean section).
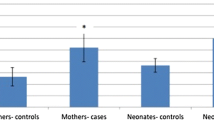
Table 2 shows the concentration of oxidative damage markers in umbilical cord blood of full-term newborn delivered through MSAF as compared to clear liquor including both AGA and IUGR babies. The extent of lipid peroxidation and oxidative DNA damage as evidenced by MDA and 8-OH-dG, respectively, was significantly increased in MSAF group as compared to control subjects (2.43 ± 0.89 vs 0.94 ± 0.24 for MDA, and 55.36 ± 12.4 vs 24.60 ± 13.49 for 8-OH-dG, respectively). Full-term IUGR and AGA MSAF group had significantly higher levels of MDA and 8-OH-dG levels as compared to their respective controls. It was also found that full-term IUGR MSAF group had significantly higher levels of MDA and 8-OH-dG levels as compared to full-term AGA MSAF group (3.24 ± 1.07 vs 2.08 ± 0.52 for MDA, and 67.12 ± 12.21 vs 50.38 + 8.77for 8-OH-dG, respectively). Further, the levels of 8-OH-dG were significantly higher in full-term IUGR control as compared to full-term AGA control (36.73 ± 11.02 vs 21.01 + 12.11). However, the levels of MDA were comparable in both groups.
Table 3 shows the concentration of oxidative damage markers in umbilical cord blood of late-preterm newborn delivered through MSAF as compared to clear liquor including both AGA and IUGR babies. Levels of MDA and 8-OH-dG were significantly higher in MSAF group as compared to control (2.83 ± 0.65 vs 1.11 ± 0.57 for MDA, and 56.63 ± 10.27 vs 39.59 ± 5.39 for 8-OH-dG, respectively). Late-preterm IUGR and AGA MSAF group had significantly higher levels of MDA and 8-OH-dG levels as compared to their respective controls. It was also found that late-preterm IUGR MSAF group had higher means for MDA and 8-OH-dG levels as compared to late-preterm AGA MSAF group (3.38 ± 0.29 vs 2.32 ± 0.52 for MDA and 64.86 ± 7.77 vs 49.36 ± 6.61 for 8-OH-dG, respectively). However, the levels of MDA and 8-OH-dG in late-preterm IUGR control was comparable to late-preterm AGA control.
In the present study, 6 babies (5 full term and 1 late preterm) developed meconium aspiration syndrome. The mean MDA and 8-OH-dG levels in these subgroups of babies were 3.17 ± 0.74 mmol/L and 70.08 ± 12.69 pg/ml, respectively.
Table 4 showed that plasma MDA levels were significantly correlated with birth weight even after controlling the relationship between MDA and gestation, when all MSAF deliveries were considered. Similar trend persisted when all full-term MSAF deliveries were analyzed as a group. The plasma 8-OH-dG levels were significantly correlated with birth weight as well as gestation age; however, the relationship between 8-OH-dG and gestational age became insignificant when relation was controlled for birth weight. All full-term babies delivered through MSAF revealed significant correlation between birth weight and 8-OH-dG even after controlling the relationship for gestation. Correlation between MDA and 8-OH-dG levels in all subjects was also found to be highly significant (r 0.6173 and p < 0.0001). However, in the late-preterm MSAF group, no significant correlation was found between these oxidative stress markers with birth weight or gestational age as well as no significant intercorrelation was found between these markers.
Discussion
Meconium-stained amniotic fluid (MSAF) complicates about 10 to 20% of deliveries in which enzymatic antioxidant defenses fails and oxidative damage to the tissues occurs [3]. The present study evaluated whether babies born through MSAF show higher degree of oxidative stress in the cord blood as compared to babies born through clear liquor as well as to find out the correlation between plasma MDA and 8-OH-dG levels with birth weight and gestation as well as to each other.
In the present study, most of the babies born through MSAF belonged to unbooked primiparous mothers and were born at a higher mean gestational age, which are consistent with the previous studies of Mundhra et al. [23] and Sankhyan N et al. [36].
In our study, the breech presentation was more commonly associated with in utero passage of meconium, which is in agreement with the previous study by Balchin et al. [4].
In the present study, most of the cases had fetal distress during active labor and were born through cesarean section. Saunder et al. also found fetal distress as a significant predictive factor for in utero passage of meconium with cesarean sections being performed twice as frequently which were largely due to increased rates of fetal distress, failure of progression of labor, and aggressiveness of obstetricians in managing a fetus with in utero passage of meconium [37].
Free radical injury has been implicated in the pathogenesis of several neonatal diseases including perinatal asphyxia, but the status of free radical injury in babies born through MSAF remained unexplored. A study on prooxidant-antioxidant balance (PAB) assay in umbilical cord blood of infants delivered through MSAF found significantly increased PAB value in cord blood of these infants suggesting that they are exposed to oxidative stress [3].
We have found significantly higher serum MDA levels in both full-term and late-preterm babies born through MSAF as compared to those born through clear liquor. Even after excluding IUGR from the analysis, serum MDA levels were significantly higher in AGA babies (include both full term and late preterm) born through MSAF as compared to those born through clear liquor. Lipids are the most susceptible to oxidative damage. Estimation of lipid peroxidation products in cord blood has been proposed as a reliable marker of ROS activity in the fetus and as a marker of perinatal outcome [25]. This observation is in agreement with the previous studies by Bhatia et al. [5] and Wang et al. [44]. However, the sample size of both the previous studies was small, and subgroup comparison (IUGR and AGA) was not done.
Our study have also shown that IUGR babies (include both full term and late preterm) born through MSAF have significantly higher levels of MDA as compared to those born through clear liquor suggesting that they are additionally subjected to further oxidative stress due to meconium. It was further found that serum MDA levels in IUGR babies delivered through MSAF were significantly higher as compared to the AGA babies delivered through MSAF. There is sufficient evidence in literature to conclude that IUGR babies have significantly higher levels of markers of lipid peroxidation (MDA) in cord blood as compared to the AGA babies [6, 13, 18]. Whether they are subjected to further elevation in the levels of MDA due to meconium per se is not known.
We have found significantly higher serum 8-OH-dG levels in both full-term and late-preterm babies born through MSAF as compared to clear liquor. Further, it was additionally found that both IUGR and AGA babies (include both full term and late preterm) born through MSAF had significantly higher levels of 8-OH-dG as compared to those born through clear liquor. It was also found that both full-term and late-preterm IUGR babies born through MSAF had significantly higher levels of 8-OH-dG as compared to the AGA born through MSAF. Currently, 8-OH-dG has been used as a sensitive marker for oxidative DNA damage in various studies [25, 26, 28, 29]. But till now, there is no human study related to 8-OH-dG as a marker of oxidative DNA damage in cord blood of neonates delivered through MSAF. There is a related animal study conducted in Finland on newborn piglets born through MSAF by Minna Aaltonen et al. [1] which showed higher levels of 8-OH-dG in hippocampal neurons at 6 h following meconium instillation in the hippocampus of newborn piglets. The present study suggests that babies delivered through MSAF are exposed to higher degree of oxidative stress in cord blood than those delivered through clear liquor.
Further, we examined whether any correlation exists between these oxidative stress markers with birth weight or and gestational age as well as any intercorrelation between these markers. Our result showed that MDA levels were significantly correlated with birth weight even after controlling the relationship between MDA and gestation, when all MSAF deliveries were considered. Similar trend persisted when all full-term MSAF deliveries were analyzed as a group. The plasma 8-OH-dG levels were significantly correlated with birth weight as well as gestation; however, the relationship between 8-OH-dG and gestation became insignificant when relation was controlled for birth weight. All full-term babies delivered through MSAF revealed significant correlation between birth weight and 8-OH-dG even after controlling the relationship for gestation. The correlation between MDA and 8-OH-dG levels in all subjects was also found to be positive and highly significant. This observation is in agreement with the previous studies by Negi et al. [25]. However, in the late-preterm MSAF group, no significant correlation was found between these oxidative stress markers with birth weight or gestational age as well as no significant intercorrelation was found between these markers. This could be explained by the small sample size in late-preterm group (n = 12) in our study and needs further evaluation in a larger study.
On the basis of the present study, we suggest that the levels of oxidative stress markers are elevated in neonates born through MSAF. In addition to meconium, activated leukocytes as well as structural cells of the airway induce production of oxygen radicals, proteases, and cytokines leading to lipid peroxidation and oxidative DNA damage. These ROS have important effect on a variety of lung cells as regulator of signal transduction, activators of key transcription factors, and modulators of gene expression and apoptosis. The levels of these free radicals may be high enough to counteract the effect of normally increased levels of antioxidant system in response to inflammation. Therefore, we suggest that the levels of MDA and 8-OH-dG may also be used as predictive parameters at birth for neonates who subsequently develop meconium aspiration syndrome or perinatal asphyxia, but further research is necessary involving biomarkers as well as its clinical validation. One also needs to establish normative gestational age dependent data, along with their definite cutoff values. Our study is limited by small sample size, failure to establish if similar effect is also seen in neonates who subsequently develop MAS in a large sample of babies.
To conclude, although the incidence of MAS has decreased in developed countries, it poses a significant problem in developing nations. Lack of antenatal care, primiparity, fetal distress during active labor, breech presentation, and advanced gestational age at the time of delivery are the risk factors for in utero passage of meconium. Higher levels of MDA and 8-OH-dG have been documented as markers of oxidative stress in babies born through MSAF. Levels of these oxidative stress markers are higher in IUGR as compared to AGA babies born as either late preterm or full term. Correlation of MDA and 8-OH-dG with birth weight as well as with birth weight even after controlling the relationship with gestation was significantly higher for all cases as well as all full-term cases. These markers are also significantly correlated to each other.
Authors’ contributions
Dr. Tapas Bandyopadhyay collected the data, carried out the initial analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
Dr. B.D. Bhatia conceptualized and designed the study and critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. H.D. Khanna critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Abbreviations
- 8-OH-dG:
-
8-Hydroxy-2-deoxyguanosine
- AGA:
-
Appropriate for gestational age
- IUGR:
-
Intrauterine growth retardation
- MAS:
-
Meconium aspiration syndrome
- MDA:
-
Malondialdehyde
- MSAF:
-
Meconium-stained amniotic fluid
References
Aaltonen M, Soukka H, Halkola L, Jalonen J, Holopainen IE, Kaapa PO (2005) Meconium aspiration induces oxidative injury in the hippocampus of newborn piglets. Early Hum Dev 81:439–447
Ahanya S, Lakshmanan J, Morgan B, Ross M (2005) Meconium passage in utero: mechanisms, consequences and management. Obstet Gynecol Surv 60:45–56
Arjmand MH, Shah FA, Moghadam MS, Tara F, Jalili A, Bazaz MM et al (2013) Prooxidant-antioxidant balance in umbilical cord blood of infants with meconium stained of amniotic fluid. Biochemistry Research International 2013:1–4
Balchin I, Whittaker JC, Lamont RF, Philip J (2011) Maternal and fetal characteristics associated with meconium-stained amniotic fluid. Obstet Gynecol 117:828–835
Bhatia BD, Goel A (2005) Study of free radicals in neonates born through meconium stained amniotic fluid deliveries. Indian Pediatr 42:956–957
Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I (2007) Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Investig 64:187–192
Bonassi S, Au WW (2002) Biomarkers in molecular epidemiology studies for health risk prediction. Mutat Res 511:73–86
Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R (2000) Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr Res 47:221–224
Buonocore G, Perrone S, Longini M, Vezzosi P, Marzocchi B, Paffetti P et al (2002) Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res 52:46–49
Craig S, Lopez A, Hoskin D, Markham F (2005) Meconium inhibits phagocytosis and stimulates respiratory burst in alveolar macrophages. Pediatr Res 57:813–818
Dargaville PA, Copnell B (2006) The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics 117:1712–1721
de Beaufort AJ, Bakker AC, Van Tol MJ, Poorthuis BJ, Schrama AJ, Berger RM (2003) Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured A549 epithelial cells. Pediatr Res 54:491–495
Gupta P, Narang M, Banerjee BD, Basu S (2004) Oxidative stress in term small for gestational age neonates born to undernourished mothers: a case control study. BMC Pediatr 4:14
Hackey WE (1999) Meconium Aspiration. In: Gomella TL (ed) Neonatology, 4th edn. Lange Medical Books, New york, p 507
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon Press, Oxford
Halliwell B, Arouma OI (1991) DNA damage by oxygen-derived species its mechanism and measurement in mammalian systems. Elsevier Science 281:9–20
Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M (2007) Increased testicular 8-hydroxy-2′-deoxyguanosine in patients with varicocele. BJU Int 100:863–866
Karowicz-Bilinska A, Kedziora-Kornatowska K, Bartosz G (2007) Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic Res 41:870–873
Kimmick GG, Bell RA, Bostick RM (1997) Vitamin E and breast cancer: a review. Nutr Cancer 27:109–117
Liu BY, Wang CC, Lau TK, Chu CY, Pang CP, Rogers MS (2005) Meconium-stained liquor during labor is associated with raised neonatal cord blood 8-iso-prostaglandin F2훼 concentration. Am J Obstet Gynecol 192(1):289–294
Maymon E, Chaim W, Furman B, Ghezzi F, Shoham Vardi I, Mazor M (1998) Meconium stained amniotic fluid in very low risk pregnancies at term gestation. Eur J Obstet Gynecol Reprod Biol 80:169–173
Mokra D, Drgova A, Mokry J, Antosova M, Durdik P, Calkovska A (2015) N-acetylcysteine effectively diminished meconium-induced oxidative stress in adult rabbits. J Physiol Pharmacol 66:101–110
Mundhra R, Agarwal M (2013) Fetal outcome in meconium stained deliveries. J Clin Diagn Res 7:2874–2876
National Neonatology Forum NNPD Network. (2005) National Neonatal-Perinatal Database. report 2002–2003. New Delhi
Negi R, Pande D, Kumar A, Basu S, Khanna RS, Khanna HD (2011) In-vivo oxidative DNA damage, protein oxidation and lipid peroxidation as a biomarker of oxidative stress in preterm low birth weight infants. J Med Sci 11:77–83
Negi R, Pande D, Kumar A, Khanna RS, Khanna HD (2012a) In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low birth weight infants. J Trop Pediatr 58:326–328
Negi R, Pande D, Kumar A, Khanna RS, Khanna HD (2012b) Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth neonates. The Journal of Maternal-Fetal and Neonatal Medicine 25:1338–1341
Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD (2014) Association of oxidative DNA damage, protein oxidation and antioxidant function with oxidative stress induced cellular injury in pre-eclamptic/eclamptic mothers during fetal circulation. Chem Biol Interact 208:77–83
Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD (2015) A novel approach to study oxidative stress in neonatal respiratory distress syndrome. BBA Clinical 3:65–69
Ogino K, Wang DH (2007) Biomarkers of oxidative/nitrosative stress: an approach to disease prevention. Acta Med Okayama 61:181–189
Patil KP, Swamy MK, Samatha K (2006) A one year cross sectional study of management practices of meconium stained amniotic fluid and perinatal outcome. J Obstet Gynecol India 56:128–130
Perrone S, Vezzosi P, Longini M, Marzocchi B, Paffetti P, Bellieni CV et al (2009) Biomarkers of oxidative stress in babies at high risk for retinopathy of prematurity. Front Biosci (Elite Ed) 1:547–552
Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F et al (2010) Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum Dev 86:241–244
Perrone S, Tataranno ML, Negro S, Cornacchione S, Longini M, Proietti F et al (2012) May oxidative stress biomarkers in cord blood predict the occurrence of necrotizing enterocolitis in preterm infants? J Matern Fetal Neonatal Med 25(Suppl 1):128–131
Philpot JS (1963) Estimation and identification of organic peroxides. Radiat Res. Suppl 3:55–70
Sankhyan N, Sharma VK, Sarin R, Pathania K (2006) Predictors of meconium stained amniotic fluid: a possible strategy to reduce neonatal morbidity and mortality. J Obstet Gynecol India 56:514–517
Saunders K (2002) Should we worry about meconium? A controlled study of neonatal outcome Trop Doct 32:7–10
Schock BC, Sweet DG, Halliday HL, Young IS, Ennis M (2001) Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 281:L1386–L1391
Shaikh EM, Mehmood S, Shaikh MA (2010) Neonatal outcome in meconium stained amniotic fluid—one year experience. J Pak Med Assoc 60:711–714
Shigenaga MK, Ames BN (1991) Assays for 8-hydroxy-2′-deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med 10:211–216
Soukka HR, Ahotupa M, Ruutu M, Kaapa PO (2002) Meconium stimulates neutrophil oxidative burst. Am J Perinatol 19:279–284
Tsukahara H, Shibata R, Ohshima Y, Todoroki Y, Sato S, Ohta N et al (2003) Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci 72(22):2509–2516
Uchida K (1999) Current status of acrolein as a lipid peroxidation product. Trends in Cardiovascular Medicine 9(5):109–113
Wang CC, Rogers MS (1997) Lipid peroxidation in cord blood: the effect of amniotic fluid volume. Br J Obstet Gynaecol 104:1140–1144
Wiswell TE (2001) Advances in the treatment of the meconium aspiration syndrome. Acta Paediatr 90:28–30
Yamada T, Minakami H, Matsubara S, Yatsuda T, Kohmura Y, Sato I (2000) Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol 46:21–30
Yoder BA, Kirsch EA, Barth WH Jr, Gordon MC (2002) Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol 99:731–739
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Source of support
None
Additional information
Communicated by Patrick Van Reempts
Rights and permissions
About this article
Cite this article
Bandyopadhyay, T., Bhatia, B.D. & Khanna, H.D. A study of oxidative stress in neonates delivered through meconium-stained amniotic fluid. Eur J Pediatr 176, 317–325 (2017). https://doi.org/10.1007/s00431-016-2845-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2845-0