Abstract
Perthes disease is one of the most common forms of pediatric femoral head osteonecrosis with an unknown etiology. Coagulation factors were the first genetic factors suspected to have a role in the pathogenesis of this disease, but studies showed inconsistent results. It is described that inflammation is present during early stages of Perthes disease, but its genetic aspect has not been studied extensively. Little is known regarding the status of apoptotic factors during the repair process that leads to the occurrence of hip deformity in patients. Therefore, the aim of this study was to analyze major mediators involved in coagulation, inflammation, and apoptotic processes as possible causative factors of Perthes disease. The study cohort consisted of 37 patients. Gene variants of TNF-α, FV, FII, and MTHFR genes were determined by PCR-RFLP, while IL-3 and PAI-1 were genotyped by direct sequencing. The expression level of Bax, Bcl-2, Bcl2L12, Fas and FasL was analyzed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) technique. Our results showed a significantly increased level of expression of pro-apoptotic factor Bax along with significantly higher Bax/Bcl-2 ratio in the patient group.
Conclusion: The results presented indicate that apoptosis could be one of the factors contributing to the lack of balanced bone remodeling process in Perthes patients.
What is Known: •The etiology of Perthes disease is unknown. The role of genetic factors involved in the coagulation process has been studied, showing inconsistent results so far. •Genetic factors involved in inflammation and apoptotic processes that could contribute to development of hip deformity have not been studied extensively. |
What is New: •Our results show significantly increased level of expression of the pro-apoptotic factor Bax as well as significantly higher Bax/Bcl-2 ratios in patient group, indicating that apoptosis could be one of the factors contributing to the lack of a balanced bone remodeling process in Perthes patients. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Legg–Calve–Perthes’ disease (LCPD; Perthes disease) is an idiopathic osteonecrosis of the immature, developing femoral head [21, 35]. It is a rare disease with a great variation of annual incidence, from 1/250,000 in Hong Kong and 1/18,000 in the UK to 1/3,500 in the Faroe Islands (www.orpha.net). It occurs approximately five times more commonly in boys than in girls [31].
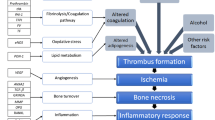
Despite nearly 100 years of detailed characterization of its clinical and radiological features, as well as a research devoted to the pathophysiology of this disease, its etiology remains essentially unknown [26]. Various possible causes have been proposed, including repetitive microtrauma, skeletal retardation and vascular insufficiency [17, 26]. The prevailing view is that both environmental and genetic factors contribute in varying degrees to the onset and development of Perthes disease [14].
It has been shown in experimental models that disrupted blood supply and infarction of the femoral head cause changes similar to those in Perthes patients [19]. Genes related to the coagulation process were the first genetic factors suspected to have a role in the pathogenesis of Perthes disease, causing the impaired local circulation. Previous studies related to the role of coagulation factors in the etiology of Perthes disease provided controversial results, as described in recent meta-analysis [37].
It is well documented that inflammatory cytokines inhibit bone formation and stimulate resorption [9, 20, 36]. There are four well-accepted stages of the pathogenesis of Perthes disease: synovitis, avascular (condensation), fragmentation (resorption) and residual (healed) phase [18, 21]. Inflammation is present during the development of the disease, and inflammatory changes are most marked during the fragmentation phase [7]. We have recently shown that the IL-6 promoter gene variant, which influences expression of the IL-6 gene [11], is associated with Perthes disease [34]. TNF-α has been recognized as a skeletal catabolic agent that stimulates osteoclastogenesis while simultaneously inhibiting osteoblast function [25]. This cytokine is crucial to the pathogenesis of the bone and joint destructions that occur in various inflammatory and rheumatic diseases [10, 22, 24]. Interleukin-3 has important functions with regard to bone turnover [40], and gene variants in IL-3 were shown to be associated with rheumatoid arthritis, Graves’ disease, asthma and atopy [5, 29, 39].
It is known that apoptosis of osteoblasts and osteoclasts is strictly regulated and plays an important role in both the maintenance of physiologic bone turnover and in the development of pathological skeletal conditions [23], but none of the studies on Perthes disease have questioned this mechanism so far. One of the most important factors in the pathophysiology of Perthes disease, which greatly contributes to the development of severe deformity of the affected hip, is an imbalance in bone remodeling, which takes place during the fragmentation phase [16]. Impaired apoptosis is one of the factors that could affect the repair process, which takes place during the fragmentation phase as well as during the avascular phase that precedes it. The inner apoptotic pathway is regulated by the BCL-2 family of proteins, in which the balance between pro-apoptotic and anti-apoptotic molecules is the main determinant of the apoptotic status of the cell. It has been shown that an increased BAX/BCL-2 ratio accelerates the apoptotic process [27]. The Bcl2L12 gene is a new member of the BCL-2 family, with anti- and pro- apoptotic function [33]. The extrinsic apoptotic pathway, where the FAS/FASL system plays a crucial role, is involved in the regulation of bone turnover and may represent a critical link between the immune system and bone remodeling in physiological and pathological processes [38].
Here we analyzed the association of genetic variants of genes involved in coagulation, FV, FII, MTHFR and PAI-1, with Perthes disease. Also, we have examined genetic variants of inflammatory mediators relevant for bone remodeling, TNF-α and IL-3 genes, as susceptibility factors for Perthes disease. In order to address the question about the role of apoptotic mechanism in the formation of hip deformity in Perthes disease, we examined the expression pattern of main apoptotic factors, Bax, Bcl-2, Bcl2L12, Fas and FasL, in our patient group.
Materials and methods
Patients and control subjects
Our study was approved by the Ethics Committee of the Institute for Orthopedic Surgery “Banjica,” Belgrade, Serbia. Written informed consent was obtained for all patients.
This study enrolled 37 patients, 29 males and 8 females with a mean age of 9.10 years (SD—5.31). For all 37 patients, DNA analyses of gene variants involved in coagulation and inflammation processes were performed. As a control, for the comparison of gene variant frequencies for PAI-1, TNF-α, and IL-3 genes, a group of 50 healthy donors was used. This control group was sex matched and included 35 male and 15 female voluntary donors, with a mean age of 29.92 years (SD—12.74). For FV Leiden, FII, and MTHFR genes, a new control group was used, which included another 100 healthy voluntary donors, 46 male and 54 female, with a mean age of 9.5 years (SD—0.5) [8].
For the analysis of mRNA expression, samples of 31 patients (out of 37) were used, since RNA samples were not available for 6 of them. This group consisted of 25 males and 6 females, with a mean age of 7.52 years (SD—2.83). An additional healthy control group was included for expression analysis. It consisted of 11 voluntary donors, 6 males and 5 females with a mean age of 6.39 years (SD—3.86).
All patients were diagnosed with Perthes disease between 1998 and 2012, at the Institute for Orthopedic Surgery “Banjica,” Belgrade. The diagnosis was established according to standard clinical criteria (onset of groin pain, disturbed stance on the affected leg and waddling gait, limitation of hip joint movements, especially internal rotation, absence of clinical signs suggesting trauma or infection), ultrasonographic examination (verification of homogenous hip joint effusion), and radiographic signs (condensation or fragmentation of the epiphyseal ossification center, loss of femoral head sphericity). Two patients had bilateral disease.
Blood sampling, DNA, RNA and cDNA preparation
Venous blood was collected into two 4.5-ml sodium citrate anticoagulant tubes (Vacutainer, Becton-Dickinson, Plymouth, UK) and stored at +4 °C until processing. Within 4 h after sampling, 1 ml of whole blood was separated for DNA isolation and stored at −20 °C, while the rest of the sample was used for peripheral blood mononuclear cell (PBMNC) isolation by Ficoll-Histopaque density gradient (GE Healthcare, Sweden).
Genomic DNA was isolated from whole peripheral blood with QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany) and stored at −20 °C. The isolated PBMNC were stored at −80 °C in TRI Reagent solution (Ambion, USA) until the RNA extraction was done, according to the manufacturer protocol. Complementary DNA (cDNA) was prepared from 1 μg of RNA using RevertAid Reverse Transcriptase (Thermo Scientific, USA) and random hexamer primers.
Detection of coagulation- and inflammation-related gene variants
The detection of gene variants of coagulation factors FV 1691G > A (rs6025), FII 20210G > A (rs1799963), and MTHFR 677C > T (rs1801133) was performed using a PCR-RFLP method described elsewhere [8]. PAI-1 4G/5G (rs1799889) gene variant was genotyped by direct sequencing using primers described elsewhere [15]. The PCR products were directly sequenced using the BigDye Terminator Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA), and sequencing was performed on ABI PRISM 310 Genetic Analyzer using Sequencing Analysis Software (Applied Biosystems). Gene variant TNF-α-308G > A (rs1800629) was detected by PCR-RFLP technique, while IL-3-16C > T (rs31480) and C132T (rs40401) gene variants were detected by direct sequencing (details included in Supplementary material).
Relative quantification of apoptosis-related gene expression
All analyzed genes, primers, probe and assays used for the quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) are shown in Supplementary Table 1.
qRT-PCR analyses for all analyzed genes were performed on 7500 Real-Time PCR System (Applied Biosystems). Melting curve analyses was performed for reactions in which SYBR Green chemistry was used, to ensure the specificity of the products. In all qRT-PCR experiments, the GAPDH gene was used as the endogenous control, in order to normalize the obtained results. All expression analyses were performed in duplicate. In the case of discordant results, the analyses were repeated in duplicate. Relative quantification analyses were performed by a comparative ddCt method, using a median value of normalized target gene expression level of the healthy control group as the calibrator.
The expression of Bax, Bcl-2, Bcl2L12 and GAPDH genes was analyzed by qRT-PCR using KAPA SYBR FAST Universal 2× qPCR Master Mix (KAPA Biosystems, USA) with primers that were taken from listed publications [1, 12, 28]. Fas and FasL expression levels were determined by qRT-PCR using the TaqMan Gene Expression Assays and KAPA PROBE FAST Universal 2× qPCR Master Mix (KAPA Biosystems, USA). To normalize expression of these genes, GAPDH expression level was determined using primers and probes described elsewhere [6].
Statistical analysis
The differences in genotype and allele distributions between patient and control groups were analyzed by Fisher’s exact test. The consistency of genotype distributions with Hardy–Weinberg equilibrium was tested using Pearson chi-square test, for each cohort. Differences in expression levels were analyzed using the Mann-Whitney U test. All tests were two-sided, and differences were considered to be significant in all cases when P < 0.05. Data was analyzed using the SPSS for Windows 20.0 software (SPSS, Inc, Chicago, IL, USA), and the power of the test was performed using the calculator from http://powerandsamplesize.com. Linkage disequilibrium values (D′, r 2) between gene variants were tested using Haploview software, Version 4.2.
Results
Contribution of genetic markers involved in coagulation and inflammation processes in Perthes disease
Coagulation factors FV, FII, MTHFR and PAI-1
The results of the analysis of the FV, FII, MTHFR and PAI-1 gene variants in patients with Legg–Calve–Perthes disease and controls are shown in Table 1. All genotype distributions were in Hardy–Weinberg equilibrium. When genotype and allele frequencies were compared among patient and control groups, no significant differences were observed. For FV Leiden and FII G20210A, we did not find any homozygote carriers either in patients or in control group.
Inflammation factors IL-3 and TNF-α
A perfect linkage disequilibrium was observed between the C-16T and C132T polymorphisms in IL-3 gene (D' = 1.0, r 2 = 1.0). Therefore, IL-3 C-16T and C132T genotypes and alleles occurred with identical frequencies. The comparison of genotype and allele frequencies among patients and controls did not reveal significant differences neither for TNF-α nor for IL-3 analyzed gene variants (Table 2).
Expression of apoptotic factors in Perthes patients
Stratification of patients for expression analyses
The thirty-one patients used for expression analyses (further designated as total patient group) were sampled in different phases of the disease: 6 patients in avascular, 14 in fragmentation, 8 in reossification and 3 in residual phase. All patients were sampled only once within an indicated phase of the disease. As mentioned before, one of the most important factors in the pathophysiology of Perthes disease is the imbalance between resorption and bone anabolism, which takes place during the fragmentation phase of the disease. Our assumption was that an impaired apoptotic process, in particular during the fragmentation phase as well as during the avascular phase which precedes it, could contribute to the lack of new bone formation. Therefore, in order to test that hypothesis, a subgroup of patients that were sampled in the avascular and fragmentation phases (designated as A + F patient group) was formed and used in analyses alongside the total patient group. The A + F patient subgroup consisted of 15 males and 5 females with a mean age of 6.10 (SD—1.69).
Expression of inner apoptotic pathway genes Bax, Bcl-2 and Bcl2L12 in Perthes patients
To determine the expression pattern of inner apoptotic pathway-related genes in Perthes disease, we detected the expression levels of Bax, Bcl-2 and Bcl2L12 genes in PBMNC of patient and control groups. There was no significant difference in the Bax expression level between the total patient and control groups (Fig. 1a), but the Bax expression level was significantly increased in the A + F patient subgroup in comparison to the control group (P = 0.023, Fig. 1a). No significant difference in the expression level of Bcl-2 or Bcl2L12 genes was detected (Fig. 1b, c). Since it has been suggested that the ratio of Bax and Bcl-2, rather than the level of either gene, is predictive of cell fate [27], we also determined the Bax/Bcl-2 ratio. Our results showed that the Bax/Bcl-2 ratio was significantly higher in the total patient group compared to the control group (P = 0.047, Fig. 1d). In addition, the Bax/Bcl-2 ratio was significantly higher in A + F patient subgroup in comparison to the control group (P = 0.032, Fig. 1d).
Relative expression of genes of intrinsic and extrinsic apoptotic pathways in Perthes patients and controls. a Bax, b Bcl-2, c Bcl2L12, d Bax/Bcl2 ratio, e Fas, f FasL. Total represents total patient group, A + F the subgroup of patients sampled in avascular and fragmentation phases, and IQR represents the interquartile range. The line bars represent the median value (50th percentile) for each cohort. P values were calculated by Mann–Whitney U test
Expression of extrinsic apoptotic pathway genes Fas and FasL in Perthes patients
Next, we evaluated the expression level of genes involved in extrinsic apoptotic pathway, Fas and FasL, in the PBMNC of patient and control groups. Comparison of the total patient group and A + F subgroup to the control group showed no significant differences in the expression of Fas or FasL genes (Fig. 1e, f).
Discussion
Many studies have been performed with the aim of explaining the nature of Perthes disease, but it still remains one of the most controversial conditions in pediatric orthopedics [17, 30]. The focus of our study was to examine genetic factors, such as gene variants in coagulation- and inflammation-related genes, as well as the gene expression profiles of the main regulators of intrinsic and extrinsic apoptotic pathways, as possible causative factors involved in the pathogenesis of Perthes disease.
The role of genetic factors involved in the coagulation process that could contribute to impaired local circulation has been extensively studied. Recent meta-analysis has summarized conflicting results from previous studies on gene variants in coagulation factors FV, FII, and MTHFR and Perthes disease [37]. It showed that FV might increase the odds of developing Perthes disease about threefold. In our cohort of patients, we did not find significant association of gene variants in coagulation factors with Perthes disease.
Although studies on inflammatory cytokines in different bone and cartilage diseases have been conducted [9, 20, 36], this is the first report regarding the gene variants of inflammatory mediators TNF-α and IL-3 in Perthes patients. We found no significant difference between the frequencies of analyzed gene variants in TNF-α or IL-3 genes between the patient and control groups. In our study group, the C132T variant in the IL-3 gene was in perfect linkage disequilibrium with the promoter polymorphism IL-3 C-16T. It is interesting to note that the frequency of −16C/132C allele of the IL-3 gene was rather high in our patient and control group, pointing to the population specificity of frequency of this allele. This allele frequency in our population was similar with other Caucasian subjects [13], while the studies from Asia reported much lower frequency in their populations [5, 29, 39].
This is the first report on the status of the main apoptotic factors in Perthes patients. Research on osteonecrosis of the femoral head has shown that the process leading to cell death in the femoral head of such patients includes an increased rate of apoptosis rather than purely the necrosis of bone cells [3]. Additionally, apoptotic bodies and DNA fragmentations have been observed in the osteocytes and marrow cells of the ischemically necrotic femoral heads of rats [2].
Skeletal tissue integrity is a result of the delicate balance between bone formation by osteoblasts and bone resorption by osteoclasts [23]. In addition to osteoclasts, a distinct population of resident macrophage cells, OsteoMacs, has been shown to be an integral component of bone tissue and to play an important role in bone homeostasis [32]. These cells constitute approximately one sixth of the total cells within osteal tissues [4]; they are intercalated with bone lining cells and promote osteoblast matrix production and/or mineral deposition by secreting a wide range of osteo-active factors [32]. Additionally, it has been shown that the systemic or local depletion of macrophages delayed bone healing and impaired bone formation, thus showing that OsteoMacs have a role in bone modeling and remodeling [4].
Our study points to the relevance of the inner apoptotic pathway in the pathophysiology of Perthes disease. More precisely, we found a significantly increased expression level of the pro-apoptotic factor Bax in the PBMNC of the subgroup of patients that were sampled in the avascular and fragmentation phases of the disease. In addition, the Bax/Bcl-2 ratio was significantly higher in both the total patient group and in a subgroup of patients that were sampled in the avascular and fragmentation phases of the disease, in comparison to the control group. We hypothesized that this distorted apoptotic process may contribute to the pathological remodeling of the affected femoral head through the significant decrease in the number of resident osteal macrophages, OsteoMacs.
Our study has a number of strengths and some limitations. This is the first report showing increased expression level of a pro-apoptotic factor, implicating the role of apoptotic process in the pathogenesis of Perthes disease. The limitation of the present work is the relatively small sample size and control group design. Since Perthes disease is a rare disease with a limited number of patients diagnosed in Serbia, a relatively small group of patients was studied. This resulted in the low power of the test for associative study (<0.47), but the number of patients included in the expression analysis is quite relevant. Also, different control groups were used in this study. For coagulation and inflammation factors, we used the same control groups as in previous studies of these factors in Serbia [8, 34]. A new control group was designed for the expression study of apoptotic factors.
In conclusion, our study brings new insights into the role of genetic factors in the pathophysiology of Perthes disease. If further studies on larger patient cohorts confirm that apoptosis is disturbed in Perthes patients, apoptotic factors may become reliable biomarkers for the evaluation of the status or the prognosis of the disease, as well as a potential target for therapy.
Abbreviations
- BAX:
-
Bcl-2-associated X protein
- BCL-2:
-
B cell lymphoma 2
- BCL2L12:
-
BCL2-like 12
- FAS:
-
Fas receptor
- FASL:
-
Fas ligand
- FV:
-
Factor V Leiden
- FII:
-
Factor II
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- IL-3:
-
Interleukin-3
- LCPD:
-
Legg–Calve–Perthes’ disease
- MTHFR:
-
Methylenetetrahydrofolate reductase
- PAI-1:
-
Plasminogen activator inhibitor-1
- PBMNC:
-
Peripheral blood mononuclear cells
- TNF-α:
-
Tumor necrosis factor alpha
References
Baluchamy S, Sankar N, Navaraj A, Moran E, Thimmapaya B (2007) Relationship between E1A binding to cellular proteins, c-myc activation and S-phase induction. Oncogene 26:781–787
Boss JH, Misselevich I (2003) Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol 40:345–354
Calder JD, Buttery L, Revell PA, Pearse M, Polak JM (2004) Apoptosis—a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg Br 86:1209–1213
Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR (2008) Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 181:1232–1244
Chu X, Dong C, Lei R, Sun L, Wang Z, Dong Y, Shen M, Wang Y, Wang B, Zhang K, Yang L, Li Y, Yuan W, Wang Y, Song H, Jin L, Xiong M, Huang W (2009) Polymorphisms in the interleukin 3 gene show strong association with susceptibility to Graves' disease in Chinese population. Genes Immun 10:260–266
Clark JP, Munson KW, Gu JW, Lamparska-Kupsik K, Chan KG, Yoshida JS, Kawachi MH, Crocitto LE, Wilson TG, Feng Z, Smith SS (2008) Performance of a single assay for both type III and type VI TMPRSS2:ERG fusions in noninvasive prediction of prostate biopsy outcome. Clin Chem 54:2007–2017
Dillman JR RJH (2009) MRI of Legg-Calve-Perthes disease. Am J Roentgenol 193:1394–1407
Djordjevic V, Stankovic M, Brankovic-Sreckovic V, Rakicevic L, Damnjanovic T, Antonijevic N, Radojkovic D (2012) Prothrombotic genetic risk factors in stroke: a possible different role in pediatric and adult patients. Clin Appl Thromb Hemost 18:658–661
Edwards CJ, Williams E (2010) The role of interleukin-6 in rheumatoid arthritis-associated osteoporosis. Osteoporos Int 21:1287–1293
Feldmann M, Brennan FM, Maini RN (1996) Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440
Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P (1998) The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 102:1369–1376
Floros KV, Thomadaki H, Florou D, Talieri M, Scorilas A (2006) Alterations in mRNA expression of apoptosis-related genes BCL2, BAX, FAS, caspase-3, and the novel member BCL2L12 after treatment of human leukemic cell line HL60 with the antineoplastic agent etoposide. Ann N Y Acad Sci 1090:89–97
Jeong MC, Navani A, Oksenberg JR (1998) Limited allelic polymorphism in the human interleukin-3 gene. Mol Cell Probes 12:49–53
Joseph B, Willoughby R (2010) Perthes' disease: a review of contributions from the Asia-pacific region. Malays Orthop J 4:1–8
Kim H, Cho C, Cho Y, Cho S, Yoon K, Kim K (2011) Significant associations of PAI-1 genetic polymorphisms with osteonecrosis of the femoral head. BMC Musculoskelet Disord 12:160
Kim HK (2010) Legg-Calve-Perthes disease. J Am Acad Orthop Surg 18:676–686
Kim HK (2012) Pathophysiology and new strategies for the treatment of Legg-Calve-Perthes disease. J Bone Joint Surg Am 94:659–669
Kim HK, Herring JA (2011) Pathophysiology, classifications, and natural history of Perthes disease. Orthop Clin North Am 42:285–295, v
Kim HK, Morgan-Bagley S, Kostenuik P (2006) RANKL inhibition: a novel strategy to decrease femoral head deformity after ischemic osteonecrosis. J Bone Miner Res 21:1946–1954
Lacey DC, Simmons PJ, Graves SE, Hamilton JA (2009) Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage 17:735–742
Loder RT, Skopelja EN (2011) The epidemiology and demographics of Legg-Calve-Perthes' disease. ISRN Orthop 2011:504393
Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL (1999) Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol 154:203–210
Nagase Y, Iwasawa M, Akiyama T, Kadono Y, Nakamura M, Oshima Y, Yasui T, Matsumoto T, Hirose J, Nakamura H, Miyamoto T, Bouillet P, Nakamura K, Tanaka S (2009) Anti-apoptotic molecule Bcl-2 regulates the differentiation, activation, and survival of both osteoblasts and osteoclasts. J Biol Chem 284:36659–36669
Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B (1996) Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun 64:2371–2380
Nanes MS (2003) Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene 321:1–15
Nelitz M, Lippacher S, Krauspe R, Reichel H (2009) Perthes disease: current principles of diagnosis and treatment. Dtsch Arztebl Int 106:517–523
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Papageorgiou SG, Kontos CK, Pappa V, Thomadaki H, Kontsioti F, Dervenoulas J, Papageorgiou E, Economopoulos T, Scorilas A (2011) The novel member of the BCL2 gene family, BCL2L12, is substantially elevated in chronic lymphocytic leukemia patients, supporting its value as a significant biomarker. Oncologist 16:1280–1291
Park BL, Kim LH, Choi YH, Lee JH, Rhim T, Lee YM, Uh ST, Park HS, Choi BW, Hong SJ, Park CS, Shin HD (2004) Interleukin 3 (IL3) polymorphisms associated with decreased risk of asthma and atopy. J Hum Genet 49:517–527
Perry DC (2013) Unravelling the enigma of Perthes disease. Ann R Coll Surg Engl 95:311–316
Perry DC, Machin DM, Pope D, Bruce CE, Dangerfield P, Platt MJ, Hall AJ (2012) Racial and geographic factors in the incidence of Legg-Calve-Perthes' disease: a systematic review. Am J Epidemiol 175:159–166
Pettit AR, Chang MK, Hume DA, Raggatt LJ (2008) Osteal macrophages: a new twist on coupling during bone dynamics. Bone 43:976–982
Scorilas A, Kyriakopoulou L, Yousef GM, Ashworth LK, Kwamie A, Diamandis EP (2001) Molecular cloning, physical mapping, and expression analysis of a novel gene, BCL2L12, encoding a proline-rich protein with a highly conserved BH2 domain of the Bcl-2 family. Genomics 72:217–221
Srzentic S, Spasovski V, Spasovski D, Zivkovic Z, Matanovic D, Bascarevic Z, Terzic-Supic Z, Stojiljkovic M, Karan-Djurasevic T, Stankovic B, Pavlovic S, Nikcevic G, Vukasinovic Z (2014) Association of gene variants in TLR4 and IL-6 genes with Perthes disease. Srp Arh Celok Lek 142:450–456
Vandermeer JS, Kamiya N, Aya-ay J, Garces A, Browne R, Kim HK (2011) Local administration of ibandronate and bone morphogenetic protein-2 after ischemic osteonecrosis of the immature femoral head: a combined therapy that stimulates bone formation and decreases femoral head deformity. J Bone Joint Surg Am 93:905–913
Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, Burr DB, Gravallese EM (2009) Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res 24:1572–1585
Woratanarat P, Thaveeratitharm C, Woratanarat T, Angsanuntsukh C, Attia J, Thakkinstian A (2014) Meta-analysis of hypercoagulability genetic polymorphisms in Perthes disease. J Orthop Res 32:1–7
Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM (2003) Osteoclast apoptosis: the role of Fas in vivo and in vitro. Endocrinology 144:5545–5555
Yamada R, Tanaka T, Unoki M, Nagai T, Sawada T, Ohnishi Y, Tsunoda T, Yukioka M, Maeda A, Suzuki K, Tateishi H, Ochi T, Nakamura Y, Yamamoto K (2001) Association between a single-nucleotide polymorphism in the promoter of the human interleukin-3 gene and rheumatoid arthritis in Japanese patients, and maximum-likelihood estimation of combinatorial effect that two genetic loci have on susceptibility to the disease. Am J Hum Genet 68:674–685
Yogesha SD, Khapli SM, Wani MR (2005) Interleukin-3 and granulocyte-macrophage colony-stimulating factor inhibits tumor necrosis factor (TNF)-alpha-induced osteoclast differentiation by down-regulation of expression of TNF receptors 1 and 2. J Biol Chem 280:11759–11769
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (Grant No. III41004). This study is in memoriam of Prof. Zoran Vukasinovic, who was the research coordinator for the subproject: “Orthopedic rare diseases: molecular basis of Perthes disease.” His enthusiasm and willpower contributed most to the opening of this new research topic in Serbia.
Ethical statements
The study was approved by the Ethics Committee of the Institute for Orthopedic Surgery “Banjica,” Belgrade, Serbia (I-86/34; 7-2, 12. 12. 2012.). Written informed consent was obtained for all patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Author’s Contributions
DS, ZB and ZŽ performed the case and control sample collection and clinical management of patients. ZTŠ and DM participated in clinical management of patients and participated in studz design. VDJ provided control sample for coagulation study and participated in data analyzes. SS and VS performed the laboratory work and statistical analysis. SS and GN analyzed data and wrote first draft of the paper. VS and SP designed the study and wrote the final version of the paper. VS take the primary responsibility for the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Beat Steinmann
Sanja Srzentić and Gordana Nikčević contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Srzentić, S., Nikčević, G., Spasovski, D. et al. Predictive genetic markers of coagulation, inflammation and apoptosis in Perthes disease—Serbian experience. Eur J Pediatr 174, 1085–1092 (2015). https://doi.org/10.1007/s00431-015-2510-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2510-z