Abstract
Posterior reversible encephalopathy syndrome (PRES) is characterized clinically by headaches, seizures, vomiting, nausea, visual abnormalities, and altered mental function and is often (but not invariably) accompanied by parieto-occipital imaging features. The aim of this study is to describe the clinical and radiological features and outcome following PRES in a paediatric cohort. From a retrospectively identified cohort, case records were studied to confirm a diagnosis of PRES. Neuroimaging was reviewed again to assign to recently described radiological subtypes parieto-occipital pattern, holohemispheric watershed pattern, dominant superior frontal sulcus pattern, and asymmetrical or partial expression of the three primary patterns (A/P). Patient outcome was measured by the modified Rankin scale (mRS) scores. Nine boys and three girls with mean age of 12 were identified. Hypertensive episodes (n = 11), tacrolimus toxicity (n = 4), and autoimmunity (n = 1) were identified as potential risk factors/etiologies. Their median mRS at the peak of illness was 2 (range 2–5); three children required intensive care support. After mean follow-up of 35 months (median 37 months; range 3–60 months), all patients improved significantly with mean mRS of 1 (median 1; range 0–1). Conclusion: PRES is easily recognizable by the clinical and radiological features. Although severe at presentation, the outcome from this condition is favorable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological entity characterized by a variable combination of consciousness impairment, seizure activity, headaches, visual abnormalities, nausea/vomiting, and focal neurological signs. Hinchey and colleagues described patients with headache, altered sensorium, seizures, vomiting, and abnormalities in visual perception, with edema involving the white matter in the posterior portion of the cerebral hemispheres, especially bilaterally in the parieto-occipital regions, and these patients were reported as having a “reversible posterior leukoencephalopathy syndrome” [11]. In recognition of cortical (frontal lobe) and subcortical (basal ganglia) involvement, this distinct clinical radiological syndrome has now been revised to PRES [14].
Among potential triggers, malignant or rapid onset of hypertension, immunosuppressive therapy, and transplantation are commonly identified [4, 9, 17]. PRES may occur as a result of either breakdown of cerebral autoregulation and disruption of blood brain barrier, causing vasogenic edema [8, 11] or endothelial dysfunction resulting from circulating toxic mediators (CNI), sepsis, autoimmune conditions, or eclampsia [4, 15, 16].
Overall, the reported prognosis is favorable; timely recognition is essential with removal of potential triggering factors although there have been isolated case reports of permanent sequelae and associations with mesial temporal sclerosis bit with no medium- to long-term follow-up [1, 2].
Here, we report the clinical and radiological features of 12 children with PRES who improved significantly after medium- to long-term follow-up.
Material and methods
Twelve patients with clinical and radiological findings consistent with PRES were identified at two tertiary pediatric hospitals in South London, over an 8-year period 2006–2013. In this cohort, we included all children with clinico-radiological findings in keeping with PRES based on the most contemporary criteria.
We collected data including demography, underlying primary disease and associated co-morbidities, relevant clinical features, and details regarding medications and management (NO, RS). Hypertension was defined as blood pressure above the 95th percentile using the Task Force report (group (revised [10])). Tacrolimus was suspected to be a trigger factor in patients, who were on tacrolimus, in absence of any identifiable trigger factors, like hypertension, autoimmune, or infective triggers.
Modified Rankin scale (mRS) scoring adapted for children is the most widely used outcome measure of the degree of disability or dependence, in patients with stroke [6]. We used mRS scores for our children during acute illness and at the time of their last clinic follow-up (RS, ML, and MDS).
Imaging studies were reviewed by two neuroradiologists (AS, JU) independently, and discrepancies were reviewed (AS, JU, RS, ML) to reach a consensus. As a minimum, all patients had MRI imaging with T2 weighted, T2 FLAIR, T1-weighted, and diffusion-weighted imaging (DWI) sequences and these were reviewed in all children. Additional sequences like contrast enhanced T1 weighted (n = 2) and time-of-flight intracranial MR angiography (n = 3) were reviewed. A diagnosis of PRES confirmed if typical imaging findings were found as described by Bartynski and Boardmann: dominant parieto-occipital (PO) pattern, holohemispheric (HH) watershed pattern, dominant superior frontal sulcus (SFS) pattern, and asymmetrical or partial expression (A/P) of the three primary patterns [5]. The imaging findings were also described according to their location, i.e., frontal, parietal, occipital, temporal, deep white matter, basal ganglia/thalami, brain stem, and cerebellum.
As the data collection was retrospective with no additional data being collected beyond what was required for the standard medical care of the patients, a full ethics review was not deemed necessary by the authors.
Results
Twelve children (nine boys) with a median age of 10 years, range 14 months to 14 years, were identified with PRES based on contemporary criteria. Four children had a recent renal transplant, and two had recent liver transplant. The non-transplant cohort included one child each with acute renal failure, chronic kidney disease on hemodialysis, and acute Henoch-Scholein purpura (HSP) nephritis, and two with lupus nephritis of whom one also had sickle cell anemia (HbSS).
Children presented with multiple neurological symptoms, and the most common was seizures (11 of 12 children). Three children were admitted to the pediatric intensive care unit with prolonged seizure and reduced level of consciousness. Seizure pattern was varied with predominantly generalized (8/12), and one child had prolonged post-ictal period and was found to have non-convulsive status epilepticus on EEG. Other symptoms included confusion (3/12), irritability, and vomiting (3/12) visual symptoms (3/12). None of the children had any evidence of septicemia.
The suspected etiology at the time of the presentation is displayed in Table 1 and commonly included multiple factors including hypertension in 11 patients, tacrolimus toxicity in four, and autoimmunity induced in one patient. Six post-transplant patients were on tacrolimus and one on cyclosporine, and the median onset of PRES was 17.6 days (range, 6–41 days) following transplantation.
PRES was managed with a combination of anti-hypertensive medications, reduction of steroid dose (one child), and reduction of calcineurin inhibitor (CNI) dose (three children) or change of CNI agent to alternative immunosuppressant (one child).
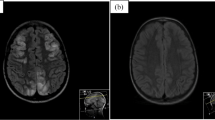
Eight children had MRI changes, predominantly parietal and occipital regions (PO pattern) (Table 2), two had holohemispheric involvement (HH), and two had predominantly superior frontal sulcus (SFS) involvement (Fig. 1). On diffusion-weighted imaging, the lesions showed predominantly free diffusion characteristics in all cases, with the observation of minimal foci of diffusion restriction in the cortex and splenium of corpus callosum in four children. In three children who had post-gadolinium imaging, mild smooth sulcal enhancement (Fig. 2d, f) was noticed corresponding to the regions showing parenchymal T2/FLAIR signal change (Fig. 2c, e). MR angiography was done in five children, and three of them showed subtle multifocal narrowing in the posterior circulation (Fig. 2b). Follow-up MRI performed in four children showed resolution in all cases except one child (case 8) had two episodes of PRES with serial imaging and clinical findings confirming resolution of initial episode but with subsequent development 10 days later of PRES with typical clinical and radiological findings.
Axial T2 FLAIR images at various levels in three patients with PRES showing the different imaging patterns. Top row (case 11) shows the dominant parieto-occipital (PO) pattern in a 6-year-old boy with acute renal failure with past history of meningococcal septicemia. Middle row (case 8) shows the dominant holohemispheric (HH) watershed pattern with changes extensively in both hemispheres but predominantly in the watershed distribution with involvement of the frontal poles (dashed arrows) in a 10-year-old girl with ESRF secondary to pANCA positive vasculitis who presented with a seizure. Bottom row shows MRI of an 11-year-old boy presenting with a seizure 9 days post renal transplant (case 5) showing the dominant superior frontal sulcus (SFS) pattern with linear changes mainly along the superior frontal sulci (solid arrows)
a Time-of-flight MR angiography images in case 8, demonstrating an initial normal study. b Subsequent MR angiography on day 10 after onset of PRES shows multifocal narrowing of the intracranial arteries (solid arrows), particularly in the posterior circulation, mirroring the distribution of parenchymal changes (not shown). c–f Axial FLAIR (c, e) and post-gadolinium T1-weighted imaging (d, f) in two patients (case 2 (c–d) and case 6 (e–f)) showing smooth sulcal enhancement (arrows) on the post-contrast images, adjacent to areas of parenchymal signal changes on FLAIR. g, h Axial FLAIR images in a patient (case 3) showing typical parieto-occipital changes (g) which later resolved completely on follow-up imaging (h)
In both instances, hypertension was identified as triggering etiology and all our children made a complete recovery on formal neurological and visual assessment at a mean follow up of 35 months..
Discussion
In 12 children with radiologically and clinically confirmed PRES, hypertension was the most common but not invariable causative factor, consistent with previous reports. The threshold whereby hypertension would attribute to PRES was lower, if co-occurring associated factors, such as tacrolimus, were present as reported [11, 13]. This finding was also observed in our patients on tacrolimus (in case 3 with high tacrolimus level 27 ug/L and normal blood pressure, also in case 5 with tacrolimus level of 10 ug/L and patient having malignant hypertension). Trough tacrolimus levels of above 12 ug/L was considered toxic, as per our local protocol; however, even lower trough levels were considered to be a trigger in relevant clinical context (case 5). In our children, the level of tacrolimus did not correlate with the severity of PRES, but all patients improved once CNI was reduced or withdrawn.
Most of our children had parieto-occipital lobe involvement as reported in literature. Interestingly, four of our children had involvement of brainstem, which is an uncommon finding. Three of our children who required PICU admissions did not show any specific pattern of involvement or increased lesion load.
Our findings on diffusion imaging were consistent with previously described changes, with predominantly free diffusion indicating vasogenic edema and suggesting reversibility of changes. Additional novel findings in our case series include the long-term outcome confirming reversibility of acute findings in PRES (Fig. 2g, h). Vascular/angiographic imaging is not routinely performed or required in these patients. In the two of five cases wherein MR angiography was performed in our series (e.g., for underlying sickle cell disease in one case), vasculopathy was identified with multifocal areas of dilatation and narrowing matching the posterior distribution of the changes, similar to previously described findings in literature [3].
Median follow-up for our patients was 37 months (3–60 months). The median mRS score at the peak of their illness was 2 suggesting slight disability and unable to carry out all previous activities, but were able to look after their own affairs without assistance. Three patients had scores of 4–5 at their peak illness, and they required PICU admission. The median mRS score at their last clinic visit was 1, which reflected no significant disability despite of their underlying chronic disease or other conditions except one child (case 7) with Henoch-Scholein purpura. mRS Score of 1 in 11/12 children reflects preceding co-morbidities (Fig. 3). All children had pre-PRES level mRS scores reflecting reversibility of this clinical entity irrespective of the lesion load and extent on MRI studies and hence favorable outcome of this condition. This is likely to reflect the underlying pathophysiology of PRES that is disruption of blood brain barrier and endothelial dysfunction, triggered either by rapid surges in blood pressure, toxic, or autoimmune mediated inflammation, leading to vasogenic edema. Importantly, persisting vasogenic edema may massively compromise local microcirculation leading to cytotoxic injury or irreversible neuronal injury, as reported [7, 12].
Our study, being a retrospective one, is subject to selection and recall bias. Nevertheless, our study with 12 children shows good neurological outcome, without any long-term sequelae. Importantly, prompt diagnosis and the empirical modification of all identifiable risk factors are ultimately the determinants of outcome in these children.
Abbreviations
- AI:
-
Autoimmune
- A/P:
-
Atypical or partial expression
- CNI:
-
Calcineurin Inhibitors
- CKD:
-
Chronic kidney disease
- DWI:
-
Diffusion-weighted imaging
- EEG:
-
Electroencephalogram
- ESRF:
-
End stage renal failure
- FLAIR:
-
Fluid-attenuated inversion recovery
- HSP:
-
Henoch-Scholein purpura
- HbSS:
-
Sickle cell anemia
- HH:
-
Holohemispheric
- HT:
-
Hypertension
- MRI:
-
Magnetic resonance imaging
- mRS:
-
Modified Rankin scale
- Non T:
-
Non-transplant
- PO:
-
Parieto-occipital
- PRES:
-
Posterior reversible encephalopathy syndrome
- SFS:
-
Superior frontal sulcus
- SLE:
-
Systemic lupus erythematosus
- TT:
-
Tacrolimus toxicity
References
Aboian MS, Junna MR, Krecke KN, Wirrell EC (2009) Mesial temporal sclerosis after posterior reversible encephalopathy syndrome. Pediatr Neurol 41(3):226–228
Auntunes NL, Small TN, George D (1999) Posterior leukoencephalopathy syndrome may not be reversible. Pediatric Neurol 20(3):241–243
Bartynski W (2008) Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical feature. Am J Neuroradiol 29:1036–1042
Bartynski WS, Boardman JF et al (2006) Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. Ajnr: Am J Neuroradiol 27(10):2179–2190
Bartynski WS, Tan HP et al (2008) Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol 29(5):924–930
Bigi S, Fischer U et al (2011) Acute ischemic stroke in children versus young adults. Ann Neurol 70(2):245–254
Covarrubias DJ, Luetmer PH et al (2002) Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. Am J Neuroradiol 23(6):1038–1048
Dinsdale HB, Robertson DM, Chiang TY, Mukherjee SK (1971) Hypertensive cerebral microinfarction and cerebrovascular reactivity. Eur Neurol 6:29–33
Fugate JE, Claassen DO et al (2010) Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 85(5):427–432
Group, N. H. B. P. E. P. W. (2005) National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin M, Lamy C, Mas J, Caplan L (1996) A reversible leukoencephalopathy syndrome. N Engl J Med 334:494–500
Onder AM, Lopez R et al (2007) Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr Nephrol 22(11):1921–1929
Port JD, Beauchamp N (1998) Reversible intracerebral pathologic entities mediated by vascular autoregulatory dysfunction. Radiographics 18(2):353–367
Pula JH, Eggenberger E (2008) Posterior reversible encephalopathy syndrome. Curr Opin Ophthalmol 19(6):479–484
Rodgers GM, Taylor RN, Roberts JM (1988) Pre-eclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol 159:908–914
Schwartz RB, Bravo SM, Kulfas RA, Hsu L, Branes PD (1995) Calcineurin neurotoxicity and its relationship to hypertensive encephalopathy: CT and MRI finding in 16 cases. AjNR Am J Roentgenol 165:627–631
Zeppa P, Fonio P et al (2012) Posterior reversible encephalopathy syndrome: description of a case in the setting of severe infection. Recenti Prog Med 103(11):526–530
Acknowledgments
The authors MDS and ML acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Disclosure
ML receives research grants from Action Medical Research and MS Society, receives research support grants from the London Clinical Research Network and Evelina Appeal, has received consultation fees from CSL Behring, received travel grants from Merck Serono, and awarded educational grants to organize meetings by Novartis, Biogen Idec, Merck Serono, and Bayer.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Peter de Winter
PRES in children
NO MS helped collecting data and preparing initial draft. NO, SW, and MS reviewed the data and helped in preparing the initial draft, and MS helped in subsequent reviews. AS and JU reviewed all the images independently. AS, JU, RS, and ML reviewed all the images to reach to a consensus. ML, MS, and RS assigned mRS scoring and in preparing the final draft and submission.
What is known about this condition and what is new?
PRES is a reversible condition, and there has been no report of quantitative measurement of outcome in this group of children at the time of illness and on complete recovery. We report that irrespective of the lesion load and distribution of lesion, the outcome on modified Rankin scale (mRS) is good.
Rights and permissions
About this article
Cite this article
Singh, R.R., Ozyilmaz, N., Waller, S. et al. A study on clinical and radiological features and outcome in patients with posterior reversible encephalopathy syndrome (PRES). Eur J Pediatr 173, 1225–1231 (2014). https://doi.org/10.1007/s00431-014-2301-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-014-2301-y