Abstract
Recombinant soluble human thrombomodulin (TM-α) has been shown to be useful in the treatment of disseminated intravascular coagulation (DIC) in a heparin-controlled study and has been available for clinical use in Japan since 2008. However, data on its use for neonatal DIC have not been reported from any clinical studies, so efficacy and safety were analyzed in 60 neonatal DIC patients identified in post-marketing surveillance. The DIC resolution rate as of the day after last administration of TM-α was 47.1 %, and the survival rate at 28 days after last administration was 76.7 %. Hemostatic test result profiles revealed decreased levels of fibrin/fibrinogen degradation products and increased platelet counts and antithrombin activity. Incidences of adverse drug reactions, bleeding-related adverse drug reactions, and bleeding-related adverse events were 6.7, 6.7, and 16.7 %, respectively, with no significant differences between neonatal, pediatric (excluding neonates), and adult DIC patients. Conclusion: This surveillance provided real-world data on the safety and effectiveness of TM-alpha in the treatment of neonatal DIC in general practice settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disseminated intravascular coagulation (DIC) is a disorder characterized by a systemic thrombohemorrhagic event seen in association with well-defined clinical situations and laboratory evidence of procoagulant activation, fibrinolytic activation, inhibitor consumption, and biochemical and clinical evidence of multiple organ failure [4]. In newborns, especially those born prematurely, reserves of pro- and anticoagulant coagulation factors are physiologically very limited; for this reason, neonates are susceptible to thrombus formation and hemorrhage due to consumption of procoagulant factors when exposed to the stress of hypercoagulability [1, 2, 21, 28]. In addition, neonates are at high risk of developing DIC as a complication of a variety of underlying characteristics, such as: (1) susceptibility to hypoxic ischemia, (2) labile blood pressure and cardiac output, (3) intravascular volume contraction after birth, (4) immature mucosal and skin barrier functions, and (5) vulnerability to multiple invasive procedures and devices. Reversal of the condition inducing hypercoagulability is paramount in achieving treatment success in newborns with DIC. However, neonatal DIC progresses rapidly, often necessitating replacement therapy and anticoagulant therapy. To that end, several drugs, such as heparin, antithrombin concentrates, and protein C concentrates, have been used to date, but little evidence has been accumulated to confirm the utility of any of these treatments in neonatal DIC patients [3, 10, 11, 13, 22, 23, 25, 26, 29].
Recombinant soluble human thrombomodulin (TM-α) is the first anticoagulant drug in the world shown to be superior to heparin in a phase III randomized clinical trial of adult DIC patients with infection or hematological malignancy as the underlying disease [24]. In post-marketing surveillance (PMS) of TM-α, more than 4,000 patients including newborn patients were enrolled after approval [20]. Since the phase III study did not collect any data on neonatal and pediatric patients with DIC, we decided to analyze and compare the data from 60 neonatal patients with the data for adult and pediatric (excluding newborns) patients with DIC obtained from PMS.
Materials and methods
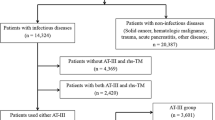
This PMS was conducted in consecutive subjects administered TM-α for approximately 2 years between May 2008 and April 2010. The study was performed in accordance with the Japanese Society on Thrombosis and Hemostasis Postmarketing Surveillance Committee for Thrombomodulin Alfa. The primary analysis set comprised 60 neonatal DIC patients under 4 weeks old from among a total of 4,062 subjects registered during the surveillance. Pediatric patients excluding newborn (n = 210) and adult (n = 3,786) patients were also compared. Six subjects of unknown age were excluded from comparisons by age group.
The standard dose of TM-α was 380 U/kg/day given by continuous drip infusion for 30 min once daily. In patients with severe renal impairment, however, the dose was reduced to 130 U/kg/day as needed.
Patients were consecutively registered at the initiation of TM-α treatment and prospectively monitored during the observation periods. At the start of TM-α administration, information about the following patient characteristics was collected: age, sex, severity of underlying disease (as assessed by the attending physician), duration of DIC, DIC treatment at baseline, type of complications (renal impairment, hepatic impairment, or others), bleeding symptoms, and organ damage or failure symptoms. Underlying diseases were coded using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA/J) version 13.1.
Efficacy assessment
Efficacy was assessed on the basis of the DIC improvement rate, DIC resolution rate, and survival rate at 28 days after the final treatment with TM-α. DIC improvement rate was assessed by the attending physician as “improved,” “unchanged,” or “worsened” based on clinical symptoms and laboratory test results on the day after final treatment with TM-α. The DIC resolution rate was evaluated by calculating DIC scores using the diagnostic criteria of the Japanese Ministry of Health and Welfare (JMHW) before and after TM-α treatment, as described previously [24]. The following cases were excluded from analysis of the DIC resolution rate: cases in which DIC score did not apply to diagnosis according to the former JMHW DIC diagnostic criteria at baseline and cases in which the DIC score could not be calculated because of missing data such as laboratory test results at baseline and/or from the day after final treatment.
Safety assessment
Data on adverse drug reactions (ADRs) and cause of death were collected up to 28 days after final treatment with TM-α. Data on other adverse events (AEs) occurring from baseline until the day after final treatment were also collected. Safety data were coded using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA/J) version 13.1. The definitions of AEs and ADRs were based on International Conference on Harmonization guidelines.
Statistical analysis
The Wilcoxon signed-rank test was used to compare baseline test results to the results obtained during treatment or on the day after final treatment. The χ 2 test was used in both comparisons of safety by age group and of efficacy by age group. Values of p < 0.05 for test results were considered to indicate a significant difference. Statistical tests were two-sided and were performed using SAS version 8.0 software (SAS Institute, Cary, NC).
Results
Age at baseline ranged from the day of birth to 3 weeks old. The underlying disease causing DIC was neonatal asphyxia in 13 cases, sepsis in 12 cases, intrauterine infection in 3 cases, peritonitis in 3 cases, hypoxic–ischemic encephalopathy in 3 cases, infection (location unknown) in 2 cases, acute lymphocytic leukemia in 2 cases, myeloproliferative disorder in 2 cases, small for gestational age in 2 cases, and “other” in 18 cases. Other patient characteristics are shown in Table 1. The dose of TM-α was 330–429 U/kg (nearly the same as the standard adult dose of 380 U/kg) in 46 cases (76.7 %), representing a large number of cases. The second most common dose category was 110–149 U/kg (encompassing the reduced dose of 130 U/kg used in patients with severely decreased renal function), which was administered in seven cases (11.7 %, Fig. 1), three of which were judged by the physician to have renal impairment.
On the other hand, treatment lasted longer than the target duration of 6 days in 31.7 % of subjects. Treatment with TM-α was discontinued prematurely because of improvement in DIC in 59.4 % of subjects. Treatment was completed as scheduled in 15.8 % of subjects, and no change in DIC was apparent in the same percentage of subjects.
Anticoagulant therapy consisted of TM-α monotherapy in 31.7 % of all subjects, but the remaining 68.3 % made concomitant use of another anticoagulant (Table 2). The most commonly used concomitant medications were antithrombin concentrates (60.0 %), followed by nafamostat mesilate (16.7 %), unfractionated heparin (13.3 %), and gabexate mesilate (5.0 %). No subject made concomitant use of low molecular weight heparin or heparan sulfate. Concomitantly, used blood components products included platelet concentrate (48.3 %), fresh frozen plasma (70.0 %), and red blood cell concentrate (36.7 %) (Table 2). No subject made concomitant use of any fibrinogen concentrates. Concomitant use was made of some type of blood components products in 86.7 % of subjects overall. In 61.7 % of cases, anticoagulants other than TM-α were used together with various blood products.
The DIC resolution rate (as determined in accordance with the former JMHW DIC diagnostic criteria; Table 3), DIC improvement rate determined by the attending physician, and day 28 survival rate are shown for neonates (<28 days old), children (≥28 days old), and adults in Table 4. DIC improvement rate and survival rate were greater in neonatal patients than in children and adults, but the DIC resolution rate was lower.
Figures 2, 3, and 4 show profiles for platelet counts, fibrin/fibrinogen degradation products (FDP) levels, and levels of antithrombin activity at baseline, during treatment, and the day after final treatment. Median platelet counts were 7.9 × 104/μL at baseline, 8.7 × 104/μL during treatment, and 12.0 × 104/μL on the day after final treatment, respectively, indicating a gradually return toward the normal range. Antithrombin activity increased significantly on the day after final treatment (p = 0.0014).
Four ADRs associated with TM-α, all bleeding-related, occurred in four subjects (6.7 %) in this surveillance. These comprised intracranial hemorrhage in three patients and pulmonary hemorrhage in one patient. The incidence of ADRs was 5.2 % in pediatric subjects and 7.0 % in adult subjects, revealing no apparent differences from neonatal subjects (Table 5).
Discussion
In cases of neonatal DIC, outcomes of patients are generally poor because of a rapidly deteriorating condition. Management for overt DIC with prominent hemorrhagic symptom is extremely difficult; anticoagulant treatment in the pre-DIC state (the state in which bleeding and organ symptoms are absent) should thus be recommended in Japan. To achieve this aim, medications such as heparin, gabexate mesilate [23], nafamostat mesilate [13], and antithrombin concentrates [26] have been used in Japan, but little evidence is available to confirm whether these medications are particularly useful in the treatment of DIC, including in adults and older children. In a controlled study on the administration of antithrombin concentrates in neonatal respiratory distress syndrome, which is often associated with the development of DIC, infusion of antithrombin concentrates was reported to significantly prolong the period of time for which mechanical ventilation and supplemental oxygen were needed and resulted in a greater number of deaths [25]. Although activated protein C concentrates are not approved for DIC in Japan, a controlled study on the administration of activated protein C concentrates in children with sepsis-induced cardiovascular and respiratory failure revealed a high incidence of intracranial hemorrhage in children under 60 days old in the treatment group [22]. This background has tended to limit anticoagulant therapy for neonatal DIC in Europe and the USA [30].
Thrombomodulin is a glycoprotein expressed on vascular endothelial cells and was identified in 1982 as an anticoagulant substance that plays an important role in regulating blood coagulation [9]. Specifically, thrombomodulin binds to thrombin that has been produced as a result of intravascular hypercoagulation and stimulates the activation of protein C by thrombin. The resulting activated protein C uses protein S as a cofactor to inactivate factors Va and VIIIa [18]. Thrombomodulin is also known to directly suppress inflammatory reactions by inhibiting the production of inflammatory cytokines such as interleukin-1β [8]. Recently, however, TM-α was developed in Japan, where it was genetically engineered in animal cells using only the extracellular domain containing the active site of human thrombomodulin to achieve solubility [12].
Based on the results of a randomized clinical study of TM-α in adult patients with DIC associated with infection or hematological malignancy [24], approval for the indication of DIC was obtained in Japan before anywhere else in the world and case report forms for 60 cases of neonatal DIC have been collected in approximately 2 years of this PMS since administration to neonatal DIC patients also became available. As previously noted, the results showed an extremely high DIC improvement rate of 80.4 %, as judged by physicians. However, these results can only be properly interpreted after considering the following: (1) results were ultimately physician-based, (2) many cases involved DIC caused by the underlying disease of neonatal asphyxia which has a relatively favorable prognosis, (3) many cases involved pre-DIC preceding onset of bleeding symptoms or thrombotic symptoms, and (4) other anticoagulants and blood-derived products were frequently used concomitantly. On the other hand, the DIC resolution rate of 47.1 % was lower than the DIC improvement rate and was lower than the rate in children other than neonates and in adults. One reason for this is that the results were significantly affected by use of the DIC criteria of the JMHW for calculating the resolution rate, as those criteria were prepared for adults [15]. Reference values for neonates differ from those for adults, potentially representing one reason for the results obtained. The results for neonatal FDP in particular may be considerably higher than in adults, depending on the test method used [14, 16, 27]. The wide range of results for FDP may also presumably have resulted from the use of varying methods of measurement, an unavoidable feature of PMS.
With regard to the changes in platelet count and antithrombin activity seen following TM-α treatment, those groups concomitantly administered platelet concentrate or antithrombin medication showed more pronounced increases in platelet count and antithrombin activity than the groups that did not receive these medications concomitantly (data not shown). The significant increase in antithrombin activity was therefore attributed to concomitant use of antithrombin medications.
ADRs, all of which were bleeding-related, occurred in 6.7 % of subjects. Intracranial hemorrhage occurred in three subjects. However, hemorrhage and intracranial hemorrhage in particular, is well known to occur at a frequency of 13–38 % in neonatal patients with DIC complications [5, 7, 17, 19] and the incidence of hemorrhage in this surveillance certainly cannot be considered all that high. Analysis of the data for all 4,062 patients in the TM-α PMS identified concurrent hepatic impairment and bleeding symptoms as risk factors for hemorrhage in DIC patients, including both children and adults [20]. Similar analysis is needed with the acquisition of additional neonatal DIC cases.
Various limitations apply to the results of PMS, and prudent interpretation is thus essential, but TM-α appears to offer promise as a safer and more effective option for neonatal patients compared to other anticoagulant drugs available to date. Protein C levels are physiologically lower in neonates than in adults [2, 6] and are pathologically lower in septic neonates than in normal neonates [7]. Therefore, as more cases continue to be collected, further efforts will be needed to clarify appropriate dosages and methods of administration for neonatal DIC and the criteria for using concomitant medications, such as replacement therapy with fresh frozen plasma to replenish protein C.
Abbreviations
- ADRs:
-
Adverse drug reactions
- AEs:
-
Adverse events
- DIC:
-
Disseminated intravascular coagulation
- FDP:
-
Fibrinogen/fibrin degradation products
- JMHW:
-
Japanese Ministry of Health and Welfare
- PMS:
-
Post-marketing surveillance
- TM:
-
Thrombomodulin
References
Andrew M, Paes B, Johnston M (1990) Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol 12(1):95–104
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Castle V, Powers P (1988) Development of the human coagulation system in the healthy premature infant. Blood 72(5):1651–1657
Bhat R, Monagle P (2012) The preterm infant with thrombosis. Arch Dis Child Fetal Neonatal Ed 97(6):F423–F428
Bick RL (2002) Disseminated intravascular coagulation: a review of etiology, pathophysiology, diagnosis, and management: guidelines for care. Clin Appl Thromb Hemost 8(1):1–31
Dairaku M, Sueishi K, Tanaka K (1982) Disseminated intravascular coagulation in newborn infants. Prevalence in autopsies and significance as a cause of death. Pathol Res Pract 174(1–2):106–115
De Carolis MP (2010) Use of protein C concentrate in neonatal period. Minerva Pediatr 62(3 Suppl 1):29–30
El Beshlawy A, Alaraby I, Abou Hussein H, Abou-Elew HH, Mohamed Abdel Kader MS (2010) Study of protein C, protein S, and antithrombin III in newborns with sepsis. Pediatr Crit Care Med 11(1):52–59
Esmon CT (2002) Protein C pathway in sepsis. Ann Med 34(7–8):598–605
Esmon NL, Owen WG, Esmon CT (1982) Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem 257(2):859–864
Fischer D, Schloesser RL, Nold-Petry CA, Nold MF, Veldman A (2009) Protein C concentrate in preterm neonates with sepsis. Acta Paediatr 98(9):1526–1529
Gobel U, von Voss H, Jurgens H, Petrich C, Pothmann R, Sprock I, Lemburg P (1980) Efficiency of heparin in the treatment of newborn infants with respiratory distress syndrome and disseminated intravascular coagulation. Eur J Pediatr 133(1):47–49
Gomi K, Zushi M, Honda G, Kawahara S, Matsuzaki O, Kanabayashi T, Yamamoto S, Maruyama I, Suzuki K (1990) Antithrombotic effect of recombinant human thrombomodulin on thrombin-induced thromboembolism in mice. Blood 75(7):1396–1399
Hanesaka Y, Takahashi Y, Kawaguchi C, Morikawa H, Yasuhara H, Yoshida K, Yoshioka A (2004) Clinical effect of nafamostat mesilate for disseminated intravascular coagulation of neonate the comparison with gabexate mesilate. Jpn J Pediatr Hematol 18:23–28
Hudson IR, Gibson BE, Brownlie J, Holland BM, Turner TL, Webber RG (1990) Increased concentrations of D-dimers in newborn infants. Arch Dis Child 65(4 Spec No):383–384
Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H (1983) Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol 49(49):265–275
Koh SC, Cheong YC, Arulkumaran S, Ratnam SS (1997) Coagulation activation, fibrinolysis and inhibitors in neonates. Ann Acad Med Singap 26(6):767–771
Kusuda S, Fujimura M, Sakuma I, Aotani H, Kabe K, Itani Y, Ichiba H, Matsunami K, Nishida H, Neonatal Research Network J (2006) Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics 118(4):e1130–e1138
Marlar RA, Kleiss AJ, Griffin JH (1982) Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood 59(5):1067–1072
McDonald MM, Johnson ML, Rumack CM, Koops BL, Guggenheim MA, Babb C, Hathaway WE (1984) Role of coagulopathy in newborn intracranial hemorrhage. Pediatrics 74(1):26–31
Mimuro J, Takahashi H, Kitajima I, Tsuji H, Eguchi Y, Matsushita T, Kuroda T, Sakata Y (2013) Impact of recombinant soluble thrombomodulin (thrombomodulin alfa) on disseminated intravascular coagulation. Thromb Res 131(5):436–443
Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, De Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S (2006) Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost 95(2):362–372
Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B, REsearching severe Sepsis and Organ dysfunction in children: A gLobal perspective (RESOLVE) study group (2007) Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 369:836–843
Nakamura T, Ariyoshi N, Asakura A, Shirahata A (1987) Effects of gabexate mesilate on DIC of infancy and childhood. Jpn J Pediatr Hematol 1:75–83
Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M, Aoki N (2007) Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 5(1):31–41
Schmidt B, Gillie P, Mitchell L, Andrew M, Caco C, Roberts R (1998) A placebo-controlled randomized trial of antithrombin therapy in neonatal respiratory distress syndrome. Am J Respir Crit Care Med 158(2):470–476
Shirahata A, Shirakawa Y, Yoshida Y, Ichikawa K, Takahashi Y, Kajino Y, lguchi Y, Sato A, Ariyoshi N, Miyakawa T (1993) The usefulness of antithrombin III concentrate in disseminated intravascular coagulation of newborn infants. J Jpn Soc Perinat Neonatal Med 29:98–105
Tay SP, Cheong SK, Boo NY (2003) Circulating tissue factor, tissue factor pathway inhibitor and D-dimer in umbilical cord blood of normal term neonates and adult plasma. Blood Coagul Fibrinolysis 14(2):125–129
Veldman A, Fischer D, Nold MF, Wong FY (2010) Disseminated intravascular coagulation in term and preterm neonates. Semin Thromb Hemost 36(4):419–428
von Kries R, Stannigel H, Gobel U (1985) Anticoagulant therapy by continuous heparin-antithrombin III infusion in newborns with disseminated intravascular coagulation. Eur J Pediatr 144(2):191–194
Williams MD, Chalmers EA, Gibson BE, Haemostasis, Thrombosis Task Force BCfSiH (2002) The investigation and management of neonatal haemostasis and thrombosis. Br J Haematol 119(2):295–309
Acknowledgments
This study used the TM-α PMS database. We wish to thank all the participating physicians and registered patients who took part in this surveillance. We are also grateful to Katsuhito Nihashi for his support with statistical analyses. This surveillance was supported in part by the Japanese Society on Thrombosis and Hemostasis.
Conflict of interest
Masahiro Kajiki and Goichi Honda are employees of Asahi Kasei Pharma Corporation. The other authors have no competing financial interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirahata, A., Mimuro, J., Takahashi, H. et al. Recombinant soluble human thrombomodulin (thrombomodulin alfa) in the treatment of neonatal disseminated intravascular coagulation. Eur J Pediatr 173, 303–311 (2014). https://doi.org/10.1007/s00431-013-2155-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-2155-8