Abstract
Infant cereals are often the elected foodstuff for beginning complementary feeding and provide carbohydrates which are different to those found in maternal milk. The objective of this preliminary study was to ascertain the colonic effects of two infant cereals, with different carbohydrate profiles, in a randomised and double-blind trial in healthy infants. Nineteen term infants between 6.3 and 9.8 months of age were enrolled, after written informed consent was obtained from parents. Ten subjects were allocated to take infant cereal A and nine, infant cereal B. An intervention period was 2 months, with five visits every 15 days, to take anthropometric measurements and faeces samples for the analysis of microbiota, short-chain fatty acids concentration (SCFA), pH value and secretory immunoglobulin A (sIgA). An adequate growth and stool frequency was registered in both intervention groups. Faecal counts of Bifidobacterium, Lactobacillus, Enterobacteriaceae, Enterococcus, Clostridium and Bacteroides did not show any statistical differences. However, a significantly (P < 0.05) higher butyric acid and sIgA, and lower faecal pH were observed in infants who had ingested infant cereal A, with a higher ratio complex/simple carbohydrates. In conclusion, small changes in the carbohydrate profile of infant cereals could lead to significant differences in parameters related to fermentative activity of intestinal microbiota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cereals are the elected foodstuff for beginning complementary feeding because they provide high energy value and are well accepted by infants in terms of sensory and digestive perspectives. Starch absorption and utilisation is limited by pancreatic α-amylase activity. At birth, pancreatic amylase activity is negligible, and this enzyme does not reach the adult level till 6–12 months of age, coinciding with pancreatic maturity [10, 20]. For this reason, infant cereals are hydrolysed during processing with the aim to improve the starch digestibility, because complex carbohydrate digestibility may be compromised, due to the low efficiency of pancreatic amylase and poor chewing physiology [6]. During weaning, infant cereals provide digestible carbohydrates (maltose, dextrin and starch), different to those presented in human milk (lactose and oligosaccharides), and also indigestible carbohydrates, resistant starch (RS) and dietary fiber (DF). RS and soluble fraction of DF withstand gastrointestinal enzyme hydrolysis and reach the large intestine intact, where they serve as a fermentation substrate for colonic microbiota [1, 32]. The nutritional guidelines recommended that carbohydrates must provide 45–60 % of dietary energy intake, with up to 10 % in sugar form, for the general population and children over 12 years old [8, 33]. Related to DF, there are recommendations for young children from 1 to 3 years old, in order to achieve a normal laxation [8, 18] but not for children under one-year old.
In nutritional studies, the role of complex and indigestible carbohydrates in infants and young children has been underestimated, because its ingestion could cause excessive energy losses and fermentation in the large intestine, consequently producing large amounts of gas, diarrhea and general discomfort. Intestinal microbiota salvage energy through fermentation of substrates not digested in the upper gut [5]. The main substrates are dietary carbohydrates that escape digestion or absorption in the upper gastro-intestinal tract. These include RS, DF compounds, non-digestible oligosaccharides and sugar alcohols, which can be fermented by microbiota with the generation of SCFAs, mainly acetic, propionic and butyric acids [4]. For these reasons, indigestible carbohydrates provided through complementary feeding are responsible for the microbiota intestinal transitional changes which occur from the neonatal stage until the adult stage, mainly increasing Bacteroides population. The establishment of intestinal microbiota is a progressive process, and the main functions attributed to microbiota present in the gut begin to manifest before the second year of life and comprise: nutrient absorption and food fermentation, stimulation of the host immune system and barrier effects against pathogens [21]. During the complementary feeding, several authors have reported an increase of Bacteroides and Clostridium and a decrease of Bifidobacterium and Enterococcus populations [9, 11, 12, 26, 30]. Moreover, these changes in microbiota are related to SCFAs, increasing butyric acid and decreasing acetic acid production [17, 23, 27], which can also carry out beneficial effects at colonic and systemic levels in young children’s physiology. In addition, modulating faecal pH is an important regulating factor for different bacterial populations, especially pathogenic ones [4].
The objective of the current preliminary assay was to ascertain the colonic effects of two infant cereals with different carbohydrate profiles in a randomised and double-blind trial in infants aged from 6 to 12 months. To achieve this objective, different parameters were analysed in the faeces such as microbiota, SCFAs concentration, pH value and secretory immunoglobulin A (sIgA).
Materials and methods
Subjects and study design
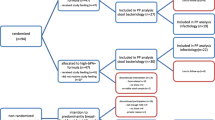
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were evaluated and approved by the Ethics Committee of “Hospital Universitario Virgen de la Arrixaca” (Murcia, Spain). Parents were approached regarding participation in the study if their infant met the following eligibility criteria: full-term, birth weight of 2,500 to 4,200 g, cow milk and lactose tolerance, exclusive infant formula feeding at least since 4 months of age, no infant formula intake with pre/probiotics, no gastrointestinal anomalies or congenital diseases at birth, and no acute disease prior to recruitment.
Nineteen full-term infants were enrolled after written informed consent was obtained from parents. At recruitment, ages ranged from 6.3–9.8 months. Subsequently, the infants were randomised to each intervention group, according to the type of infant cereal. Ten subjects were allocated to take infant cereal A, and nine subjects were allocated to take infant cereal B. The carbohydrate profiles of both infant cereals are shown in Table 1. To ensure homogenous feeding and preparation of infant cereals, both intervention groups were supplied the same follow-on formula without probiotics or prebiotics. The intervention period was 2 months. During this period, parents completed a daily information intake of food intake and stool frequency. Infants were monitored and overseen by four primary attention paediatricians every 15 days. At all visits, the infants underwent clinical examination in terms of anthropometric measurements (weight, length and head circumference); faeces samples were collected and were given the infant cereals and formula specific to their intervention group. Before the first intervention (baseline) (visit 1) and every 15 days during the 2-month period (visits 2, 3, 4 and 5), two stool samples (fresh and frozen) were taken in sterile conditions by the parents on the same day of clinical revision. Parents had prior training in the collection of stool samples, and they delivered them to the laboratory within 2 h of collection in refrigerated containers. The fresh sample was taken in anaerobic conditions (sterile vessel and sterile spoon, anaerobic plastic pouches, AnaeroGen sachet OX AN0035 and anaerobic indicator BR55), and the frozen ones were stored at −80 °C. Fresh stools were used to determine the microbiota and pH, whereas SCFAs and sIgA were quantified in frozen stools.
Analytical methodology
For microbiological assays, immediately after fresh stool collection, an aliquot of 1 g was homogenised in 9 ml in phosphate buffered saline with 100 μl of cystein (4.48 % w/v). The suspension was homogenised with a stomacher lab blender (IUL Instruments, Spain) for 1 min. Tenfold serial dilution was made in a pre-reduced salt medium. The specific agar plates used were as follows: Beerens agar [3] for Bifidobacterium spp., Rogosa agar (Merck, 5413) for Lactobacillus spp., MacConkey agar (Merck, 5465) for Enterobacteriaceae, KAA agar (Merck, 5222) for Enterococcus spp., reinforced clostridia agar (Oxoid, CM0151) for Clostridium spp.; colony counts were obtained and expressed as log10 of the colony-forming units (CFU) per gram of fresh faeces.
The quantification of the Bacteroides populations in faeces by real time-polymerase chain reaction (PCR) was performed by modification of the methodology described by Layton et al. [19]. Before quantification, genomic DNA in faecal samples (200 mg) and DNA from bacterial cultures used for calibration curves were extracted by using the QIAamp DNA stool mini kit (Qiagen, Hilden, Germany) [16]. All reactions were performed in a thermocycler Mastercycler ep Realplex (Eppendorf, Hamburg, Germany). The strain used was Bacteroides fragilis DSM 2151 obtained by The Deutsche Sammlung von Mikroorganismen und ZellKulturem GmbH (DSMZ) (Braunschweig, Germany), and the primers were GAGAGGAAGGTCCCCCAC (forward) CGCTACTTGGCTGGTTCAG (reverse). For PCR experiments, a standard curve was prepared, in duplicate, using DNA extracted from culture at concentrations ranging from 105 to 108 colony-forming units per millilitre. The correlation coefficient for the standard curve was above 0.95. Bacterial count was expressed as log10 CFU per gram of faecal samples.
To measure faecal pH, an aliquot of 1 g of faeces was diluted tenfold in Milli-Q water. After the dilution was homogenised, the faecal pH was measured with a pH meter GLP21 (Crison, Barcelona, Spain).
The detection and quantification of SCFAs was detected and quantified using gas chromatography the by methodology described Girard-Pipau et al. [15].
The faecal sIgA concentration was quantified using an enzyme-linked immunosorbent assay (Immunodiagnostik K8870, Bensheim, Germany), reading the absorbance at 450 nm in a microplate reader (Biotek μQuant, Vermont, USA).
Statistical analysis
Data were checked for normality using the Shapiro-Wilk test. Normal variables were expressed as mean ± standard deviation, and not normally distributed dates were expressed as median and ranges. A difference of P < 0.05 was considered statistically significant. Data microbiological and pH were considered as not normally distributed, and a Mann–Whitney U test was performed to assess differences between both intervention groups. The Friedman test was used to detect differences among visits in case of non-parametric variables. SCFAs and normalised faecal sIgA data were compared by analysis of variance (ANOVA) in repeated measures to analyse time effects and feeding groups. Pearson’s correlation coefficient was calculated between all parameters analysed. Statistical analyses of data were performed with the Statistical Package for the Social Sciences (SPSS version 18.0; Inc., Chicago, IL, USA).
Results
As has been mentioned above, the carbohydrate profile of both infant cereals is shown in Table 1. Infant cereal A showed a significantly higher content of digestible starch (DS) and RS than infant cereal B. On the contrary, infant cereal B provided a significantly higher content of digestible carbohydrates as dextrins and total free sugars (TFS). However, both infant cereals showed a similar content of (DF) due to the cereal ingredients used in the formulation.
During the intervention period, all girls and boys had a normal growth pattern, since mean values of weight, length and head circumference for both intervention groups registered in each visit were not significantly different to the standard curves reported by WHO [34] (data not shown). Moreover, there were no differences for all anthropometric measurements between groups according to the infant cereal intake (P > 0.05). The daily stool frequency ranged from 1 to 3 (data not shown), and there was no difference between intervention groups or among visits.
The evolution of microbiota of faeces according to the intervention group and visit number is shown in Fig. 1. In general, there were no significant differences between both intervention groups, since microbiota were kept constant during the 2 months of intervention period, except to Enterococcus, which underwent a decrease from the baseline visit until the last visit in both groups, decreasing significantly (P = 0.030) around 1 log CFU/g. Although nothing statistically significant was observed, the faeces of infants fed with infant cereal A tended to have a higher Bifidobacterium count in all visits than infants fed with infant cereal B. In log CFU per gram, the values were: 10.06 versus 9.74 in visit 1, 9.73 versus 9.29 in visit 2, 9.75 versus 9.48 in visit 3, 9.64 versus 9.52 in visit 4 and 9.79 versus 9.24 in visit 5 (Fig. 1). In the case of Bacteroides, the population shows a contrary tendency, being higher in the count for infants in group B than group A for the five visits (Fig. 1). No significant differences were observed in the mean values, mainly due to the high individual variability of faecal counts, whose individual values ranged as follows—6.20–12.08 log CFU/g for Bifidobacterium, 4.00–11.30 log CFU/g for Lactobacillus, 5.92–11.15 log CFU/g for Enterobacteriaceae, 9.54-4.65 log CFU/g for Enterococcus, 7.23–12.00 log CFU/g for Clostridium and 4.49–10.99 log CFU/g for Bacteroides.
Faecal counts of Bifidobacterium, Lactobacillus, Enterobacteriaceae, Enterococcus, Clostridium and Bacteroides populations obtained in infants belonging to both intervention groups during each visit. Expressed results as log CFU per gram of fresh faeces (mean ± SD). *Statistical differences during the study P < 0.05
Table 2 shows the total SCFAs and the individual content of the main compounds (acetic, propionic and butyric acids) and the faecal sIgA content in the samples collected from the five visits throughout the period of the intervention study. No significant differences in total faecal SCFAs were observed between groups A and B during the intervention period, with the mean values ranging from around 80 to 110 mmol/kg of fresh stool (Table 2). Acetic, propionic and butyric acids comprise around 90 % of the total SCFAs, with acetic acid being the main SCFA comprising more than 60 % of the total amount. In the first visit, the mean values of SCFAs, expressed as a percentage of the total amount for both groups, were 68.7 %, 18.5 % and 7.1 % for acetic acid, propionic acid and butyric acid, respectively (Table 2). There were no significant differences between visits and feeding groups for absolute values of acetic and propionic acids, due to individual variability. However, the content of faecal butyric acid showed significant differences relating both to feeding group and intervention time (P = 0.026), because the butyric acid content in the faeces of infants fed infant cereal A were significantly higher than the faecal content of infants fed with infant cereal B, besides showing an increase over time (Table 2).
Related to the faecal sIgA concentration, the ANOVA showed that the results obtained were statistically influenced by visit and feeding intervention (P = 0.030), achieving higher values in infants who received infant cereal A compared with those who received infant cereal B (Table 2). In addition, the concentration of sIgA was significant and positively correlated with the Bifidobacterium count (r = 0.213, P = 0.040).
At baseline, the pH mean values were 6.97 and 6.74 in infants fed with infant cereals A and B, respectively, without significant differences (Fig. 2). Faecal pH values remained constant during the four first weeks of the intervention period, but in the last visit the pH changed, decreasing significantly in infants who ingested infant cereal A (P = 0.038) (Fig. 2). The Pearson correlation showed that changes in faecal pH were related to the fermentative activity of microbiota and to SCFA concentration, exhibiting a significant negative correlation with total SCFA (r = −0.358, P = 0.001), specifically with acetic acid (r = −0.369, P < 0.001) and butyric acid (r = −0.325, P = 0.002). In addition, we also obtained a negative significant correlation between pH and Enterococcus (r = −0.375, P < 0.001), Lactobacillus (r = −0.215, P = 0.037) and Bifidobacterium (r = −0.208, P = 0.044).
Discussion
In the early stages, intestinal microbiota is highly variable, and it is influenced by several factors, such as the geographical area, feeding type at pre-weaning period (formula or breastfed) and delivery type (vaginal or caesarean) [11]. During the first year of life, the gastrointestinal tract progresses from sterility to a dense colonisation, ending with a mixture similar to the microbiota in an adult [26]. And the beginning of complementary feeding, up to 2 years of age, is a period of important transition for the dietary pattern which coincides with intestinal microbiota change until reaching the adult pattern. In fact, differences in microbiota between pre-weaning (6 weeks of age) and post-weaning periods (at 4 weeks after beginning complementary feeding) have been described, with a significant decrease in Bifidobacterium, Enterobacteriaceae and Clostridium difficile + Clostridium perfringens and an increase in Clostridium coccoides and Clostridium leptum populations, whereas Atopobium, Bacteroides, Lactobacillus and Streptococcus populations remained constant [11].
During weaning period, intestinal microbiota show a high variability and the introduction of complex carbohydrates in the diet is the main cause behind these changes, since they are the principal substrates of bacterial fermentation [11]. Stark and Lee [30] reported the followings range values for Bifidobacterium (9.8–10.2 log CFU/g), Enterobacteriaceae (8.1–8.5 log CFU /g), Enterococcus (8.5–9.5 log CFU/g) and Bacteroides (8.7–9.3 log CFU/g) in breast-fed and formula-fed infants in early weaning. Other authors have found similar values for Bifidobacterium, Enterobacteriaceae, Enterococcus and Bacteroides, in the faeces of infants from 10–18 months of age [17] and in infants during weaning with follow-on formulas supplemented with galacto-oligossacharides [12]. The mean values obtained in our study for Bifidobacterium, Enterobacteriaceae, Enterococcus and Bacteroides are in agreement with the data reported in the scientific literature [12, 17, 30]. However, for other microorganisms, we found different values than those reported previously. So, Clostridium counts were higher than those described by Stark and Lee [30] and Fanaro et al. [12] whereas for Lactobacillus, our counts were higher than those reported previously [12, 17].
During this period of life, a progressive colonisation by Bacteroides spp., Clostridium and anaerobe Streptococcus take place, hence, higher Enterobacteriaceae and Enterococcus are counted during weaning than in adulthood, decreasing gradually these populations until reaches the adult pattern [30]. An increase in Bacteroides and Clostridium populations has been described in faeces after weaning, possibly due to the introduction of complex carbohydrates, being favourite substrates of these bacterial populations [9, 14]. However, Bifidobacterium populations remain constant, showing similar counts during weaning and after introducing complementary feeding [9, 14]. In our intervention study, the Bifidobacterium, Clostridium and Bacteroides populations were predominant in infants between 6.3 and 12.4 months. We did not find differences in microbial populations according to the carbohydrate profile of infant cereals. Only the decrease of Enterococcus was observed in both groups, which was related to the age of the children as have been described by other authors [9, 30] but not to the infant cereal ingested. We did not find differences in the microbiota between the two intervention groups, which might be due to individual variability and also to the fact that children eat foods during this period other than infant cereals (such as fruits and vegetables) that can also provide a fermentable colonic substrate.
There are few studies in the scientific literature related to the effects of complex carbohydrates in young children, but a recent study is of interest in order to consider the beneficial effects of complex carbohydrates in human development and physiology. De Filippo et al. [7] studied the gut microbiota of children aged from 1–6 years with different dietary patterns, hygiene habits and geographic areas (children from European and rural African). The scientists reported significant differences in gut microbiota between the two groups. The African children showed a higher enrichment in Bacteroidetes and depletion in Firmicutes, whereas the Enterobacteriaceae were higher in European children. Moreover, African children had a higher SCFA concentration than European ones, mainly in terms of propionic and butyric acid. These changes will be related to the major intake of dietary fibre, resistant starch and oligosaccharides, as well as carbohydrates that escape digestion in the small intestine, which are eaten by the African children, whilst European children consume high amounts of energy, fat and protein [7].
In fact, there is a relationship between substrate fermentation and bacterial population, since each microbiota group has a specific substrate for their fermentative activity. In general, it is known that Bifidobacterium spp. are the main fermenters of lactose and human milk oligosaccharides; Lactobacillus spp. and Enterococcus spp. also ferment the lactose, and Bacteroides population, the most frequent bacterial genus in adult subjects, are the result of complex carbohydrates fermentation which reach the colon intact. SCFAs represent the main bacterial fermentation product in the human intestine, and it is responsible for the beneficial effects attributed to bacterial flora and to different carbohydrates as fermentation substrate. Returning to this study, the mean SCFA values obtained in the infants’ stools after the intake of different infant cereals are in agreement with those reported previously in the scientific literature [17, 23]. In the present study, the total SCFAs content remained more or less constant in both groups throughout the intervention study without significant differences. Midtvedt and Midtvedt [23] reported for total SCFAs mean values of 83.8 mmol/kg stools in infants of 6 months of age and 120.1 mmol/kg stools in infants of 2 years of age. Moreover, they observed that, in the weaning stage, acetic acid is the main SCFA such as that in formula-fed as well as breast-fed infants; however, after the introduction of solid food, acetic acid undergoes a detriment increasing propionic and butyric acids [27]. In our study, we found higher butyric acid content in those infants whose intake were the infant cereals with higher complex carbohydrates, indicating higher variety in the intestinal microbial population. Therefore, SCFAs in faeces are associated with the diet, and during this complementary feeding, a change can be observed in the percentage of individual acids according to foods introduced.
The SCFA formation decreases the pH in intestinal lumen, which appears to have quite beneficial effects for the host. Among these effects is an emphasis on the prevention of pathogen microorganism over-growth sensitive to acid pH [31, 32]. In our study, there were no significant differences detected in the pH of stools during the 4 weeks of intervention, as have been described by other authors for young children [24, 27]. However, in the last visit, a significant decrease was observed, which might be related to the changes of acetic and butyric acids. In addition to SCFAs and pH changes, intestinal microbiota plays an important role in acquired immunity, especially in the intestinal immunological system and sIgA production [28]. Several studies have proved that the supplementation of infant formulas with prebiotics contributed to a higher faecal sIgA concentration than those found in infants feeding with formulas without prebiotics [2, 28]. According to this previous knowledge, we hypothesised changes in the sIgA content related to the different content of complex and non-digestible carbohydrates in infant cereals. Our results of faecal sIgA are in according with the data published by Maruyama et al. [22] and higher than those shown by Scholtens et al. [28]. The correlation results among pH, Bifidobacterium population and sIgA obtained in the current study could confirm that Bifidobacterium presence can produce a higher sIgA secretion and a lower pH in the intestinal lumen. These results are in agreement with these shown by other researchers who stated that Bifidobacterium and Lactobacillus groups can stimulate the intestinal immune system, increasing the sIgA production in vitro [36]. Fukushima et al. [13], in an in vivo study, suggested that the intake of formula supplemented with Bifidobacterium spp. stimulates sIgA production in the infant gut. Moreover, it showed that Bifidobacterium influences sIgA production against virus enteropathogens; so, food products that favour Bifidobacterium population proliferation could be instrumental for endogen stimulation of sIgA and to improve the immune system in infants [25]. Although the beneficial effects of Bifidobacterium on sIgA secretion are known, the mechanism of immune stimulation by Bifidobacterium on sIgA is unknown [2]. In infant feeding exclusively with human milk, during the first 3 months of life, there is a significant correlation between Bifidobacterium count at 2 months and salivary sIgA secretion at 6 and 12 months of age [29]. Despite the fact that Yanagibashi et al. [35] described in an in vitro study that Bacteroides stimulate sIgA production by Peyer’s patches lymphocytes, we observed a negative correlation between sIgA and Bacteroides.
This study is a preliminary assay that shows that higher proportion of complex carbohydrates, which escape to digestion and reach the colon intact, in infant cereals could lead to a higher fermentative activity of microbiota, producing in faeces high butyric acid and sIgA concentrations and low pH values. However, taking into account the high variability intra- and inter-individual analysed parameters, the increase of subject numbers would weaken this variability. Sample size apart, another important limitation of the present study is the variable diet of infants during this period, since other sources of complex carbohydrates (vegetables and fruit) could mask the effects of carbohydrates from the infant cereals; however, we are certain of the beneficial effect of complex carbohydrates during this stage of life. Further studies are necessary to determine the impact of carbohydrate types in the health of infants, particularly during the complementary feeding period.
References
Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL (2009) Health benefits of dietary fiber. Nutr Rev 67:188–205
Bakker-Zierikzee AM, van Tol EAF, Kroes H, Alles MS, Kok FJ, Bindels JG (2006) Faecal SIgA secretion in infants fed on pre- or probiotics infant formula. Pediatr Allergy Immunol 17:134–140
Beerens H (1991) Detection of bifidobacteria by using propionic acid as a selective agent. Appl Environ Microbiol 57:2418–2419
Brownawell AM, Caers W, Gibson GR, Kendall CWC, Lewis KD, Ringel Y, Slavin JL (2012) Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr 142:962–974
Christian MT, Edwards CA, Preston T, Johnston L, Varley R, Weaver LT (2003) Starch fermentation by faecal bacteria of infants, toddlers and adults: importance for energy salvage. Eur J Clin Nutr 57:1486–1491
Christian MT, Edwards CA, Weaver LT (1999) Starch digestion in infancy. J Pediatr Gastroenterol Nutr 29:116–124
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS 107:14691–14696
EFSA panel on Dietetic Products, Nutrition, and Allergies (NDA) (2010) Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J 8:1462
Enck P, Zimmerman K, Rusch K, Schwiertz A, Klosterhalfen S, Frick JS (2009) The effects of maturation on the colonic microflora in infancy and childhood. Gastroenterol Res Pract. doi:10.1155/2009/752401
ESPGHAN (European Society for Pediatric Gastroenterology, Hepatology, and Nutrition) (2008) Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 46:99–111
Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Dore J, Edwards C, Team TI (2011) Determinants of the human infant intestinal microbiota after introduction of first complementary foods in five European centres. Microbiology 157:1385–1392
Fanaro S, Marten B, Bagna R, Vigi V, Fabris C, Peña-Quintana L, Argüelles F, Scholz-Ahrens KE, Sawatzki G, Zelenka R, Schrezenmeir J, de Vrese M, Bertino E (2009) Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr 48:82–88
Fukushima Y, Kawata Y, Harac H, Teradac A, Mitsuoka T (1998) Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 42:39–44
George M, Nord KE, Ronquist G, Hedenstierna G, Wiklund L (1996) Faecal microflora and urease activity during the first six months of infancy. Ups J Med Sci 101:233–250
Girard-Pipau A, Pompei A, Schneider S, Nano JL, Hebuterne X, Boquet P, Rampal P (2002) Intestinal microflora, short chain and cellular fatty acids, influence of a probiotic Saccharomyces boulardii. Microb Ecol Health Dis 14:220–227
Gueimonde M, Tölkko S, Korpimäki T, Salminen S (2004) New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl Environ Microbiol 70:4165–4169
Guérin-Danan C, Andrieux C, Popot F, Charpilienne A, Vaissade P, Gaudichon C, Pedone C, Bouley C, Szylit O (1997) Pattern of metabolism and composition of the fecal microflora in infants 10 to 18 months old from day care centers. J Pediatr Gastroenterol Nutr 25:281–289
Institute of Medicine (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids (macronutrients). The National Academies Press, Washington, DC
Layton A, McKay L, Williams D, Garret V, Gentry R, Sayler G (2006) Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224
Maldonado J, Gil-Campos M, Lara F (2010) Nutrición del lactante. In: Gil A (ed) Tratado de Nutrición. Tomo III Nutrición humana en estado de salud, 2nd edn. Medica Paramericana, España, pp 207–226
Mariat D, Firmesse O, Levenez F, Guimaraes VD, Sokol H, Doré J, Gorthier G, Furet JP (2009) The Firmicutes/Bacteroidetes ratio on the human microbiota changes with age. BMC Microbiol. doi:10.1186/1471-2180-9-123
Maruyama K, Hida M, Kohgo T, Fukunaga Y (2009) Changes in salivary and fecal secretory IgA in infants under different feeding regimens. Pediatr Int 51:342–345
Midtvedt AC, Midtvedt T (1992) Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr 15:395–403
Moore N, Chao C, Yang LP, Storm H, Oliva-Hemker M, Saavedra JM (2003) Effects of fructo-olisaccharide-supplemeted infant cereals: a double-blind randomized trial. Br J Nutr 90:581–587
Moreau MC, Baforiau-Routhiau V (2002) Influence of resident intestinal microflora on the development and functions of the interstinal-associated lymphoid tissue. In: Fuller R, Perdigon G (eds) Probiotics 3. Kluwer Academic Publishers, Dordrecht, pp 69–114
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO (2007) Development of the human infant intestinal microbiota. PLOS Biol 5:1556–1573
Scholtens PAMJ, Alles MS, van der Linde EGM, Knol J (2004) Disturbance of the intestinal microflora of fully breast fed infants that are introduced to solid weaning foods. J Pediatr Gastroenterol Nutr 39:S488–S489
Scholtens PAMJ, Alliet P, Raes M, Alles MS, Kroes H, Boehm G, Knippels LM, Knol J, Vandenplas Y (2008) Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J Nutr 138:1141–1147
Sjögren YM, Tomicic S, Lundberg A, Böttcher MF, Björkstén B, Sverremark-Ekström E, Jenmalm MC (2009) Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy 39:1842–1851
Stark PL, Lee A (1982) The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol 15:89–203
Topping DL (2007) Cereal complex carbohydrates and their contribution to human health. J Cereal Sci 46:220–229
Topping DL, Cliftton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and non-starch polysaccharides. Physiol Rev 81:1031–1064
WHO (World Health Organisation) (2003) Diet, nutrition and the prevention of chronic diseases. Report of a Joint WHO/FAO Expert Consultation. World Health Organisation, Geneva
WHO Multicentre Growth Reference Study Group (2006) WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. WHO, Geneva
Yanagibashi T, Hosono A, Oyama A, Tsuda M, Hachimura S, Takahashi Y, Itoh K, Hirayama K, Takahashi K, Kaminogawa S (2009) Bacteroides induce higher IgA production than Lactobacillus by increasing activation-induced cytidine deaminase expression in B cells in murine Peyer’s patches. Biosci Biotechnol Biochem 73:372–377
Yasui H, Nagaoka N, Mike A, Hayakawa K, Ohwaki M (1992) Detection of Bifidobacterium strains that induce large quantities of IgA. Microb Ecol Health Dis 5:155–162
Acknowledgements
We would like to thank our volunteers and co-workers, especially the five paediatricians for the recruitment and follow-up of subjects: Rosario Hurtado, Begoña Almenzar, Begoña Pelegrín, Josefina Cámara and Carlos Morte. The study was supported by Hero Group.
Conflict of interest
María-José Bernal, Rosario Martínez, Inmaculada Ortuño, Fernando Romero and Pedro Abellán are members of the Research & Development Department, Global Technology Center, Hero Group. No other authors declare any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernal, M.J., Periago, M.J., Martínez, R. et al. Effects of infant cereals with different carbohydrate profiles on colonic function—randomised and double-blind clinical trial in infants aged between 6 and 12 months—pilot study. Eur J Pediatr 172, 1535–1542 (2013). https://doi.org/10.1007/s00431-013-2079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-2079-3