Abstract
Early alterations in glucose homeostasis increase the risk of developing insulin resistance and obesity later in life. The concurrence of altered lipids and insulin sensitivity/resistance markers at birth has been scarcely investigated. The study aimed to ascertain level ranges of homocysteine (tHcyt), arylesterase (AE), lipids/lipoproteins, and insulin resistance/sensitivity markers in full-term neonates and to determine the concurrence effect of dyslipaemia and dysglycaemia on those parameters at birth. Participants were 197 full-term, 2.5 to <4.0 kg, without foetal distress Spanish newborns from the Mérida Study. Parameter percentiles for males and females were stated. The effect of the concurrence high glucose/high triglycerides (high glucose/high TG) or high glucose/low cholesterol transported by HDL (HDL-c) on tHcyt, LDL-c, HDL-c, lipoprotein (a) (Lp(a)), oxidised LDL (oxLDL), AE, glucose, insulin sensitivity (QUICKI) and insulin resistance index (HOMA-IR) was studied. Females had higher total cholesterol (TC), HDL-c, Apo A1, Lp(a) and HDL-c/Apo A1, but lower relative transport of TC (%TC) by the very low lipoprotein fraction than males. No gender differences were found for glucose, HOMA-IR and QUICKI. Neonates at the 2.5- to 2.999-kg range display more adequate HOMA-IR and QUICKI levels that their >3.0 kg counterparts. The concurrence of high glucose/high TG or high glucose/low HDL-c increased TC/HDL-c and HOMA-IR, but decreased, oxLDL, oxLDL/LDL-c and QUICKI with respect to that of low glucose/low TG or glucose/high HDL-c. The concurrence glucose/TG has predictive value for low QUICKI, whilst that of glucose/HDL-c for low QUICKI and high HOMA-IR, suggesting the importance of routine TG, HDL-c and glucose screening at birth as it would identify candidates for insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The perinatal period is very sensitive to several disorders which can increase the risk of metabolic diseases later in life [5, 9, 18]. The detection of high total cholesterol (TC), apolipoprotein (Apo) B, and the low Apo A1 and Apo A1/Apo B ratio in umbilical cord blood from term newborns could identify higher risk for cardiovascular disease (CVD) neonates [1, 10, 30, 34]. Our group has shown that the level of some CVD risk markers (e.g. TC, TC/cholesterol transported by HDL (TC/HDL-c), Apo A1/Apo B) at age 4 can be predicted considering these factors at birth and those of their respective parents [8]. Elevated serum lipoprotein (a) (Lp(a)) [24] and total homocysteine (tHcyt) [32] have been suggested to be independent risk factors for atherosclerosis and CVD. Delivery represents a significant stress to both mother and child. The dramatic decrease of foetal antioxidant capacity at the end of labour may induce lipoprotein oxidation increase. It has been suggested that paraoxonase (PON 1) and the arylesterase (AE)-PON 1 activity protects low- and high-density lipoproteins (LDL and HDL, respectively) from lipid peroxidation [3]. Our group has determined the AE activity in neonates carrying APOA5 S19W gene polymorphisms [14]. However, few studies have defined the normality ranges for oxidised LDL (oxLDL) and AE in full-term neonates.
Early alterations in main hormones controlling glucose homeostasis increase the risk of developing insulin resistance and obesity later in life [17]. Thus, elevated insulin levels during perinatal life may predispose for the development of diabetes mellitus (DM) in children and adults [28]. A relatively high prevalence of increased Homeostatic Model Assessment—Insulin Resistance (HOMA-IR) values has been found in Spanish neonates [12, 13]. Early identification of candidates to develop the metabolic syndrome (MS) and, thus, DMT2 and/or CVD should be demanded as these conditions are becoming more and more frequent in children and adolescents [22, 34].
Taking previous premises into account, the aims of the present study were to ascertain in umbilical cord blood of full-term newborns the influence of gender, gestational age and body weight on (a) tHcyt, lipids, lipoproteins, Apos, as well as the atherogenic indices and (b) glycaemia, insulinaemia, and HOMA-IR indices and the Quantitative Insulin Sensitivity Check Index (QUICKI). In addition, this paper studies the possible relationships between lipoprotein and glucose metabolism markers and the effect of the concurrence of two major components of MS (high glucose/high triglycerides (TG) and high glucose/low HDL-c) on tHcyt, lipoproteins and insulin sensitivity/resistance markers at birth.
Materials and methods
Sample characteristics
This study of 197 neonates from the Hospital of Mérida (Badajoz, Spain), a regional hospital of 400 beds giving medical support to 150,000 people, was performed in accordance with the Helsinki Declaration of 1975 as revised in 1983, following approval by the Management and Ethical Committee of the hospital. All selected neonates were Caucasian, singleton, full-term (between 37 and <42 weeks), normoweight (between 2.5 and <4.0 kg) infants born without foetal distress (Apgar scores ≥7 and ≥9 at the first and fifth minutes, respectively).
Protocol
Data concerning delivery (type, primiparity/multiparity), mothers (age and gestational weight gain) and neonates (birthweight, length, gender, gestational age, and Apgar score at the first and fifth minutes) were obtained from hospital records. Anthropometrical measurements were taken by the Obstetric Department-trained personnel following routine Mérida Hospital protocols. After delivery, the umbilical cord was cut and blood was obtained by arterial puncture and collected in BD Vacutainer® SST II tubes with a separation gel (Becton Dickinson, Plymouth, UK). Blood was centrifuged (3,500 rpm for 5 min) to obtain serum. Aliquots were frozen at −18 °C until processed. Samples for AE and oxLDL determination were frozen at −80 °C.
Assays
Serum glucose was measured using the glucose hexokinase method (Gluco-quant®, Roche Diagnostics) and TC concentrations using the colorimetric enzymatic method (CHOD-PAP, Roche Diagnostics). HDL-c was tested using the HDL-cholesterol plus second-generation homogeneous enzymatic test (Roche Diagnostics). TG were determined using the colorimetric enzymatic method (GPO-PAP, Roche Diagnostics) and LDL-c was calculated using the formula of Friedewald et al. as validated in neonates by Glueck et al. [15]. Apo A1, Apo B and Lp(a) were determined using immunoturbidimetric methods (Tina-quant®, Roche Diagnostics). All these determinations were processed in a Roche/Hitachi Modular P (Roche Diagnostics, Basel, Switzerland) analyser. Insulin concentrations were determined using the electrochemiluminescence immunoassay in a Roche/Hitachi Modular Analytics E 170 analyser (Roche Diagnostics). tHcyt was measured using the fluorescence polarization immunoassay supplied by Abbott in an IMX® System analyser (Abbott Diagnostics, Abbott Park, IL, USA). AE activity was measured according to the method of Nus et al. [25] at 37 °C. One unit of AE was defined as millimoles of phenol formed from phenyl acetate per minute monitored using a thermostated T80+ spectrophotometer (PG Instruments® Ltd. Wibtoft, Leics, UK). OxLDL was determined using an ELISA test kit from Mercodia Laboratories (Uppsala, Sweden). The colorimetric end point was measured at 450 nm using a model ELx808 BioTek® spectrophotometer (BioTek Instruments, Winoosky, VT, USA).
Our laboratory participates in the Spanish Clinical Chemistry Society (SEQC) External Quality Evaluation Program, which follows UNE-EN-ISO 9001:2000 standards and is certified by AENOR. All assays were properly calibrated and performed under internal and external quality controls provided by the manufacturers and SEQC, respectively. The intra- and inter-assay variation coefficients were 1 and 1.7 % for glucose, 1.5 and 4.9 % for insulin, 0.8 and 1.7 % for TC, 1.5 and 1.8 % for TG, 0.9 and 1.85 % for HDL-c, 1 and 2.4 % for Apo A1, 1.5 and 2.5 % for Apo B, 2.3 and 2.8 % for tHcyt, 8 and 8.9 % for AE, and 4.5 and 5.0 % for oxLDL, respectively.
Insulin resistance or sensitivity was tested using the QUICKI [19], calculated as 1/[(logInsulin) (mIU/L) + (logGlucose) (mg/dL)] and HOMA-IR [23] as Glucose (mmol/L) × Insulin (mIU/L)/22.5. In addition, the glucose/insulin ratio was calculated.
Dysglycaemia and dyslipaemia concurrences
The prevalence of high-glucose/high-TG and high-glucose/low-HDL-c levels in the present neonatal population was tested. Data of neonates showing those concurrences were compared with those presenting concurrency of low-glucose/low-TG and low-glucose/high-HDL-c values, respectively.
As neonates were singleton, normoweight, full-term and did not have foetal distress at birth, percentiles 25 and 75 of the current study were selected for defining low-/high-TG and HDL-c cutoff points. As cutoff points 81.3 mg/dL in males and 82.5 mg/dL in females for high serum glucose and 53.5 mg/dL in males and 56.0 mg/dL in females for low glucose were selected as reported in a previous paper performed im control male and female full-term neonates whose mothers were negative at the O’Sullivan test [12].
Statistical studies
The Kolmogorov–Smirnov test was used to analyse the normal distribution of data. TG and Insulin sensitivity/resistance markers were normalised by natural log transformation. Student’s t test was employed to compare male and female neonates. This study was designed to have a power at least of 90 % (nominal α = 0.05) to detect mean differences of 7 mg/dL for TC, 0.25 for TC/HDL-c, 4.0 mUI/L for insulin and 1.5 for HOMA-IR between male and female neonates. One-way ANOVA followed by the Bonferroni post hoc study was used for multiple comparisons. The sample size was also adequate to test absolute differences of 9 mg/dL for TC, 0.30 for TC/HDL-c, 4.5 mUI/L for insulin and 2.5 for HOMA-IR with at least a power of 72 and 60 %, between neonates with high-glucose/high-TG vs. low-glucose/low-TG levels and high-glucose/low-HDL-c vs. low-glucose/high-HDL-c levels, respectively. Spearman correlations between neonatal anthropometrical and clinical data were tested. A contingency table was used to determine the sensitivity and predictive value of the combined use of glucose and TG or glucose and HDL-c as previously indicated on the concentration of insulin sensitivity/resistance markers and lipoprotein levels at different cutoff points. To compare the method predictability of glucose/HDL-c or glucose/TG combinations, a receiver operating characteristic (ROC) curve was constructed in which the sensitivity/resistance markers or the lipoprotein levels and the prognostic probability group were analysed as predictive variables. The area under the curve (AUC) represents the discriminative test power. Values are expected to be between 0.5 (indicating no discriminative ability) and 1.0 (indicating the highest detection accuracy). Statistical significance was set at p ≤ 0.05 using the SPSS (version 15.0) and the SAS (version 9.2) statistical software packages.
Results
Anthropometrical data (mean, SD, 95%CI, percentiles) are shown in Table 1. No significant differences for anthropometric values, except for body mass index (BMI; p = 0.050), were found between male and female neonates.
Data related to lipids, lipoproteins, Apos and tHcyt are shown in Table 2. TC, HDL-c, Apo A1 and Lp(a) were significantly higher (at least p < 0.05) in females. The CVD ratios, the oxLDL/LDL-c and the AE/HDL-c ratios and the relative transport of TC (%TC) by very low-density lipoproteins (VLDL), LDL and HDL are shown in Table 3. The HDL-c/Apo A1 ratio was higher (p = 0.033), but the relative transport of TC (%TC) by the VLDL fraction was significantly lower (p = 0.019) in females. Table 4 shows that significant gender differences were found for insulin (p = 0.026).
Several significant correlations (p < 0.05) were found. However, gestational age did not significantly correlate with any biochemical (tHcyt, lipoprotein and/or insulin resistance/sensitivity) markers. To emphasize relationships between anthropometrical, lipoprotein and insulin sensitivity/resistance markers, Table 5 reports only correlations at the level of p < 0.01. Among them, it can be remarked that bodyweight significantly correlated with insulin and HOMA-IR. Glucose correlated positively with HDL-c and insulin. TG were positively correlated with LDL-c and Apo B and inversely with insulin. Insulin positively correlated significantly with glucose, but negatively with TG. tHcyt was significantly correlated with Apo B. AE correlated negatively with Apo B. oxLDL correlated positively with Apo B and LDL-c. Lp(a) correlated positively with TC.
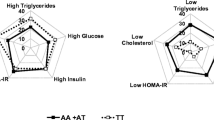
When neonates were classified according to a 500-g scale in 2,500–2,999 g, 3,000–3,499 g and 3,500–3,999 g, oxLDL, HOMA-IR and QUICKI appeared associated with bodyweight (at least p < 0.05). Neonates with 2,500–2,999 g bodyweight showed the lowest oxLDL, AE and HOMA-IR and the highest QUICKI levels (Fig. 1). These 2,500 to 2,999-g neonates also showed reduced BMI, ponderal index and cephalic and thoracic perimeters (data not shown).
Table 6 shows the anthropometrical and analytical data of term neonates in which concurrences of high glucose/high TG and high glucose/low HDL-c were present. The prevalence of neonates with high glucose/high TG was 6.6 %, whilst that of neonates with high glucose/low HDL-c was 2.5 %. Neonates where the concurrence of high glucose/high TG exists present higher TC (p < 0.001), LDL-c (p < 0.05), TC/HDL-c (p < 0.05), %TC by VLDL (p < 0.001), Apo A1 (p < 0.001), glucose/insulin ratio (p < 0.05) and HOMA-IR (p < 0.05), but lower oxLDL (p < 0.05), oxLDL/LDL-c (p < 0.01) and QUICKI (p < 0.05) with respect to their low-glucose/low-TG counterparts. Neonates with the concurrence of high glucose/low HDL-c vs. low glucose/high HDL-c presented higher CT/HDL-c ratio (p < 0.01), insulin (p < 0.01), HOMA-IR (p < 0.001), %TC by LDL (p < 0.05) and %TC by VLDL (p < 0.001), but lower TC (p < 0.001), oxLDL (p < 0.01), Apo A1 (p < 0.001), HDL-c/Apo A1 (p < 0.001), oxLDL/LDL-c (p < 0.05), QUICKI (p < 0.05) and %TC by HDL (p < 0.01).
Table 7 shows data about the sensitivity, specificity and positive predictive values of the combined use of glucose and TG or glucose and HDL-c. The concurrence of glucose/TG displayed significant contingence (p = 0.003) for the distribution of neonates at the lower/higher quartile for QUICKI values. The concurrence of glucose/HDL-c displayed significant values for the distribution of neonates at the lower/higher quartile for insulin (p = 0.016), HOMA-IR (p = 0.005) and QUICKI (p = 0.047).
Figure 2 presents the method predictability by the combination of quartile distributions of glucose and TG or glucose and HDL-c. In the case of QUICKI and HOMA-IR, the combination glucose/TG was associated with large AUC with QUICKI quartile distribution (AUC = 830, 95%CI = 0.621–1.038, p = 0.017) and HOMA-IR quartile distribution (AUC = 813, 95%CI = 0.513–1.032, p = 0.023), whilst the concurrence of glucose/HDL-c was associated with a large AUC with HOMA-IR quartile distribution (AUC = 1,000, 95%CI = 1.000–1.000, p = 0.036).
ROC curve of probabilities obtained from the concurrence of glucose/TG or glucose/HDL-c as dependent variables and quartiles for insulin, HOMA-IR and QUICKI as independent variables. Larger AUC indicates a higher prognostic value. HOMA-IR Homeostatic Model Assessment—Insulin Resistance, QUICKI Quantitative Insulin Sensitivity Check Index. a ROC curve for QUICKI quartiles at the glucose/TG concurrence (AUC = 830, 95%CI = 0.621–1.038, p = 0.017). b ROC curve for HOMA-IR quartiles at the glucose/TG concurrence (AUC = 813, 95%CI = 0.593–1.032, p = 0.023). c ROC curve for HOMA-IR quartiles at the glucose/HDL-c concurrence (AUC = 1,000, 95%CI = 1.000–1.000, p = 0.036
Discussion
To the best of our knowledge, this is the first study relating at birth tHcyt, lipoprotein and Apo values with insulin sensitivity/resistance markers in an ample sample of full-term, 2.5- to <4.0-kg birthweight, and without distress neonates. The results suggest that the concurrence of dyslipaemia and dysglycaemia negatively affected some neonatal CVD risk markers and the insulin resistance/sensitivity indexes.
The anthropometrical characteristics were similar to those of other studies as males tended to have had higher bodyweight and presented higher BMI than females [6, 7, 10, 16, 29]. Our results on lipids, lipoproteins and Apo were consistent with those of different studies [6, 7, 10, 11, 16, 27, 29] which found that neonatal data (e.g. lipids and lipoprotein lipids) are quite similar in newborns from different countries and racial and ethnic origins. However, in previous studies of our group, 5 % of neonates were hypercholesterolaemics [7, 29], whilst in the present study only four neonates (approx. 2 %) showed TC ≥ 100 mg/dL, a marker of neonatal hypercholesterolaemia [16].
The OxLDL levels were lower than those found in control or in intrauterine growth restriction neonates. However, their average gestational age was 35.7 weeks and most of them were delivered by caesarean section [21]. Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of oxidised lipoproteins and apoptotic cells. Among the several suggested immunogens present in the atherosclerotic plaque, oxidation-specific epitopes, as occur in oxLDL, are immunodominant. The oxLDL/LDL-c ratio has been used as a marker of LDL particles being oxidised [31]. This ratio and the AE were also lower (five to seven times) than those found in young Spanish adults [31], suggesting that lipoprotein oxidation significantly increases after birth. The AE is one of the major enzyme activities of PON1 and one of the first enzymes to act in cases of lipid peroxidation [3]. The low AE levels found in neonates suggest that this enzyme was used to maintain oxLDL levels as low as possible due to the high metabolic demands at late pregnancy and delivery. The Lp(a) levels were similar to those of several studies [10, 20, 26], but much lower than those described in a neonatal Iranian population [4]. tHcyt has received interest during the last decades because hyperhomocysteinaemia has been associated with an increased risk of CVD [32]. The present tHcyt data are lower than those found by Couto et al. [11] in Brazilian neonates. Although several factors would be engaged, a high intake of folates during pregnancy (unpublished data) would be involved.
In agreement with other studies, female neonates presented higher levels of TC, HDL-c [4, 27, 29] and Apo A1 [6, 10]. Lp(a) appeared higher in females, suggesting a central role of foetal sexual hormones on lipoprotein metabolism. Other studies did not show any significant gender differences in the mean and median Lp(a) concentrations [4, 10]. However, the number of neonates tested in those studies was lower than that of the current study.
Neonatal glucose, insulin, HOMA-IR and the QUICKI levels concur with others [12, 14, 20, 26]. In addition, the HOMA-IR values were positively correlated with birthweight, and neonates weighing 2.5–2.999 kg showed higher QUICKI values, suggesting that insulin resistance decreases and insulin sensitivity increases in full-term neonates with lower birthweight in order to improve their glucose cell uptake and growth. Increases in insulin sensitivity have been found in undernourished animal models [33] and intrauterine growth restriction neonates [21, 33, 34].
Gestational age did not correlate significantly with any lipoprotein and insulin resistance/sensitivity markers; thus, it did not influence, in general terms, the lipoprotein and insulin resistance/sensitivity markers as only the HDL-c/LDL-c, the Apo B and the glucose/insulin ratio appeared correlated (p < 0.05, but p > 0.01). Our group found in an ample neonatal population that TG [7], Apo B and Apo A1 [6] increased slightly through the term period.
When neonates were classified according to a birthweight scale (2,500–2,999 g, 3,000–3,499 g and 3,500–3999 g), non-significant effects on most lipids and lipoprotein levels, but on AE and oxLDL, were observed due to birthweight, suggesting that in full terms, bodyweight affected more the antioxidant status than the lipoprotein profile. However, HOMA-IR and QUICKI were associated with bodyweight. Hardell [16] reported that birthweight affected TG, but has minimal influence on newborns’ TC. Boyne et al. [9] found that weight was inversely related to HDL-c, but did not significantly correlate with plasma cholesterol, TG and HOMA-IR at birth.
The concurrence of at least three of the following factors is required for MS diagnosis: abdominal obesity, hypertriglyceridaemia, low levels of HDL-c, high blood pressure and hyperglycaemia [2, 35]. One central aspect of MS is the presence of hyperinsulinaemia and/or insulin resistance. Full-term neonates presenting concurrence of high glucose/high TG or high glucose/low HDL-c displayed higher levels of HOMA-IR, but lower QUICKI, oxLDL and oxLDL/LDL-c with respect to their corresponding counterparts. The lower oxLDL levels and oxLDL/LDL-c ratio suggest low lipoprotein oxidation as a consequence of an intra-utero metabolism reduction in those neonates.
The altered HOMA-IR and QUICKI levels suggest insulin resistance and reduced insulin sensitivity, respectively, in those MS-risk neonates. Of the neonates with concurrence of high glucose/low HDL-c, 83 % showed insulin levels >6 mUI/L, which is the considered cutoff point for high insulin levels at birth [13]. In fact, this concurrence showed high sensitivity, specificity and positive predictive values (100 %) for HOMA-IR. Moreover, 33 % of them displayed insulinaemia >15 mUI/L. These results are relevant. As was previously stated, high insulinaemia during perinatal life may cause predispositions for the development of DM in later life [28]. In addition, the relative transport of TC by the VLDL and by the HDL fractions was higher and lower, respectively, suggesting a more atherogenic lipoprotein profile in those neonates. However, other lipoprotein markers were distinctly affected when TG or HDL-c was selected as the MS factor. Thus, the concurrence of high glucose/high TG induced higher TC, LDL-c and Apo A1 levels, whilst the concurrence of high glucose/low HDL-c decreased TC and Apo A1 and increased the TC/HDL-c ratio and %TC by the LDL fraction, suggesting that the last concurrence was most deleterious for the lipoprotein metabolism than that of high glucose/high TG in term neonates. Moreover, the concurrence of high glucose/low HDL-c also showed positive predictive values for high insulin, high HOMA-IR and low QUICKI values whilst that of high glucose/high TG for low QUICKI values. However, the relatively low number of neonates presenting high-glucose/low-HDL-c concurrence demands future studies in more ample newborn populations.
In conclusion, this study has allowed us to establish the ranges for cord serum lipoproteins (LDL-c, HDL-c, Lp(a), oxLDL), AE and tHcyt in an ample sample of Spanish Caucasian, normoweight, full-term non-distressed neonates. The neonatal concurrence of dysglycaemia and dyslipaemia determines increased insulin resistance and negatively modifies the lipoprotein profile metabolism at birth. In addition, the benefit of glucose, triglyceride and HDL-c screening at birth seems evident as it may discover candidates for T2DM and MS, permitting their early follow-up and adequate prevention and/or treatment. However, more studies are needed to assess the importance of these neonatal findings in MS development and diagnosis later in life.
Abbreviations
- AE:
-
Arylesterase
- Apo:
-
Apolipoproteins
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- DMT2:
-
Type 2 diabetes mellitus
- HDL-c:
-
Cholesterol transported by HDL
- HOMA-IR:
-
Homeostatic model assessment—insulin resistance
- tHcyt:
-
Total homocysteine
- LDL-c:
-
Cholesterol transported by LDL
- Lp(a):
-
Lipoprotein (a)
- MS:
-
Metabolic syndrome
- oxLDL:
-
Oxidised LDL
- PON1:
-
Paraoxonase-1
- QUICKI:
-
Quantitative Insulin Sensitivity Check Index
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- VLDL:
-
Very low-density lipoproteins
- %TC:
-
Relative transport of TC
References
Aasvee K, Kurvinen E, Jordania R, Jauhiainen M, Sundvall J (2004) Lipoprotein parameters in relation to other risk factors of atherosclerosis in adults and newborns: Tallinn Young Family Study. Scand J Clin Lab Invest 64:245–253
Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP) (2003) NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 52:1210–1214
Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN (1998) Paraoxonase inhibits high density lipoproteins oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 101:1581–1590
Badiee Z, Kelishadi R (2008) Cord blood lipid profile in a population of Iranian term newborns. Pediatr Cardiol 29:574–579
Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67
Bastida S, Sánchez-Muniz FJ, Cuesta C, Perea S, Ureta A (1996) Serum apolipoproteins A-I and B in male and female full-term newborns of the Toledo-Study (Spain). Acta Paediatr 85:750–752
Bastida S, Sánchez-Muniz FJ, Cuesta C, Perea S, Aragonés A (1997) Male and female cord blood lipoprotein profile differences throughout the term-period. J Perinatal Med 27:184–191
Bastida S, Sánchez-Muniz FJ, Cuena R, Aragonés A, Bravo C (2007) Lipid and lipoprotein concentrations at age 4. Association with neonatal and parental levels. Med Clin (Barc) 128:521–528
Boyne MS, Osmond C, Fraser RA, Reid M, Taylor-Bryan C, Soares-Wynter S, Forrester TE (2010) Developmental origins of cardiovascular risk in Jamaican children: the Vulnerable Windows Cohort Study. Br J Nutr 104(7):1026–1033. doi:10.1017/S0007114510001790
Casanueva V, Cid X, Chiang MT, Molina M, Ferrada MC, Pérez R, Casanueva P (1998) Serum lipids, lipoprotein and apolipoprotein levels in normal newborns. Rev Med Chile 126:1073–1078
Couto FD, Moreira LM, Dos Santos DB, Reis MG, Gonçalves MS (2007) Folate, vitamin B12 and total homocysteine levels in neonates from Brazil. Eur J Clin Nutr 61:382–386
Gesteiro E, Bastida S, Sánchez-Muniz FJ (2009) Insulin resistance markers in term, normoweight neonates. The Mérida cohort. Eur J Pediatr 168:281–288
Gesteiro E, Bastida S, Sánchez-Muniz FJ (2011) Effects of maternal glucose tolerance, pregnancy diet quality and neonatal insulinemia upon insulin resistance/sensitivity biomarkers in normoweight neonates. Nutr Hosp 26:1447–1455
Gesteiro E, Bastida S, Vázquez-Velasco M, Corella D, Guillén M, Ordovas JM, Sánchez-Muniz FJ (2011) Effect of the S19W polymorphism in the APO A5 gene on growth, insulin resistance markers, and lipoprotein levels in normoweight neonates. Eur J Pediatr 170:1551–1558
Glueck CJ, Steiner P, Leuba U (1973) Cord blood low-density lipoprotein-cholesterol estimation versus measurement with the preparative ultracentrifuge. J Lab Clin Med 82:467–472
Hardell LI (1981) Serum lipids and lipoproteins at birth based on a study of 2815 newborn infants. Relation between materno-foetal factors and the concentration of triglycerides and cholesterol. Acta Paediatr Scand 70(Suppl 285):11–20
Hill DJ, Duvillié B (2000) Pancreatic development and adult diabetes. Pediatr Res 48:269–274
Holemans K, Aerts L, Van Assche FA (2003) Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Invest 10:392–399
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Kirimi E, Cesur Y, Gül A (2006) Normal levels of insulin, growth hormone and cortisol levels in venous cord blood of healthy full-term infants: correlation with birthweight and placental weight. Eastern J Med 6:14–17
Leduc L, Delvin E, Ouellet A, Garofalo C, Grenier E, Morin L, Dubé J, Bouity-Voubou M, Moutquin JM, Fouron JC, Klam S, Levy E (2011) Oxidized low-density lipoproteins in cord blood from neonates with intra-uterine growth restriction. Eur J Obstet Gynecol Reprod Biol 156:46–49
Lissau I, Overpeck MD, Ruan WJ, Due P, Holstein BE, Hediger ML (2004) Body mass index and overweight in adolescents in 13 European countries, Israel, and the United States. Arch Pediatr Adolesc Med 158:27–33
Livesey G (2006) A systematic review of the glycaemic response to foods and health. ILSI Europe Workshop. Glycaemic response on health. Life Sciences Institute, Brussels, pp 82–127
Luc G, Bard J-M, Arveiler D, Ferrieres J, Evans A, Amouyel P (2002) Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis 163:377–384
Nus M, Sánchez-Muniz FJ, Sánchez-Montero JM (2006) A new method for the determination of arylesterase activity in human serum using simulated body fluid. Atherosclerosis 188:155–159
Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D (2000) Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab 85:4266–4269
Pardo MCG, Geloneze B, Tambascia MA, Bros-Filho AA (2005) Atherogenic lipid profile of Brazilian near term newborns. Brazil J Med Biol Res 38:755–760
Plagemann A (2005) Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav 86:661–668
Sánchez-Muniz FJ, Cuesta Lorenzo C, Bastida Codina S, Perea Ramos S, Moya Gómez P (1994) Perfil lipoproteico en una muestra seleccionada de neonatos a término del Estudio Toledo. An Esp Pediatr 40:173–180
Srintvasan S, Berenson G (1995) Serum apolipoproteins A1 and B as markers of coronary artery disease risk early in life: the Bogalusa Heart Study. Clin Chem 41:159–164
Vázquez-Velasco M, Esperanza Díaz L, Lucas R, Gómez-Martínez S, Bastida S, Marcos A, Sánchez-Muniz FJ (2011) Effects of hydroxytyrosol-enriched sunflower oil consumption on cardiovascular disease risk factors. Br J Nutr 105:1448–1452, Corrigendum in Br J Nutr 2011;105:1712
Veeranna V, Zalawadiya SK, Niraj A, Pradhan J, Ference B, Burack RC, Jacob S, Afonso L (2011) Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 58:1025–1033
Sánchez-Muniz FJ, Gesteiro, E, Esparrago Rodilla M, Rodriguez Bernal B, Bastida S (2013) Maternal nutrition during pregnancy conditions the fetal pancreas development, hormonal status and Diabetes mellitus and metabolic syndrome biomarkers at birth. Nutr Hosp 28. doi:10.3305/nh.2013.28.2.
Wu G, Imhoff-Kunsch B, Girard AW (2012) Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol 26(Suppl 1):4–26
Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S, IDF Consensus Group (2007) The metabolic syndrome in children and adolescents—an IDF consensus report. Ped Diabetes 8:299–306
Acknowledgments
This study was partially supported by the Spanish AGL-2008 04892-C03. Thanks are due to the Gynecology and Obstetrics Department and Laboratory Services of Mérida Hospital (Badajoz, Spain) and to participant mothers and children. We acknowledge the statistical assistance of Dras Carmen Bravo and Mar Ruperto.
Disclosure statement
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gesteiro, E., Bastida, S. & Sánchez-Muniz, F.J. Cord-blood lipoproteins, homocysteine, insulin sensitivity/resistance marker profile, and concurrence of dysglycaemia and dyslipaemia in full-term neonates of the Mérida Study. Eur J Pediatr 172, 883–894 (2013). https://doi.org/10.1007/s00431-013-1959-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-1959-x