Abstract
We aimed to assess the efficacy and safety of low-dose propranolol for treatment of infantile hemangiomas (IHs) in China. Our prospective study included data from 89 patients with IH, aged 1–12 months. Plasma renin activity, angiotensin II, and aldosterone were measured before initiation of propranolol therapy. Patients were administered propranolol (0.75–1 mg/kg/day) under close observation. The volume, texture, and color of lesions were used to evaluate efficacy. Safety endpoints included heart rate, systolic and diastolic blood pressures, alanine transaminase, aspartate transaminase, thyroid function tests, and fasting blood glucose. Adverse effects were recorded. Mean plasma angiotensin II concentration in patients with IH was higher than that in age-matched healthy children, whereas mean plasma renin activity was lower. Mean aldosterone level was higher at 1–3 months but lower at 4–12 months, than values reported previously. After propranolol therapy for 6 months, IH regression was classed as grade IV in 44 patients (49.4 %), grade III in 21 patients (23.6 %), and grade II in 24 patients (27.0 %); none were grade I. Mild adverse effects, including diarrhea, restless sleep, nausea, cold extremities, and hypoglycemia, occurred in 12 patients (13.5 %). Slight decreases in heart rate and blood pressure occurred in all patients (p < 0.05). The IHs of four patients (4.5 %) relapsed after treatment cessation at 4–5 months. Conclusion: Low-dose propranolol is effective and safe for Chinese children with IH, and larger-scale studies are merited. Mechanisms underlying IH pathogenesis, and possible involvement of the renin–angiotensin–aldosterone system, deserve study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infantile hemangiomas (IHs) are the most common vascular tumors of infancy, with an estimated worldwide prevalence of 1–10 % in newborns and infants [5]. IHs are benign tumors, and the majority are small in size and self-limiting; however, in some cases, severe or life-threatening problems can arise [3]. In 2008, Léauté-Labrèze et al. [11] published their initial report of propranolol as an effective treatment for IHs. Since then, numerous studies have confirmed that propranolol is an effective and highly promising therapy for IH, and many clinicians now recommend propranolol as the first-line treatment for problematic IHs. Compared with people of White race, Chinese individuals have a greater sensitivity and lower tolerance to beta-blockers, due to decreased plasma protein binding [17, 18]. Thus, a lower dose of propranolol should be used for the treatment of IH in Chinese patients. However, a systematic study of the use of low-dose propranolol for management of IH in Chinese patients has not yet been described.
High levels of renin have been found in infants, Caucasians, females, and premature infants. Itinteang et al. [9] have recently proposed a crucial role for the renin–angiotensin system (RAS) in IH, supported by the clinical observation of a higher incidence of this tumor in Caucasians, females, and premature infants [15]. However, to date, no experimental evidence has been provided to confirm this hypothesis. The aims of the present study were to analyze the RAS level in patients with IH, before the use of propranolol treatment, and to evaluate the efficacy, safety, and adverse effects of low-dose propranolol for the management of IH. Here, we present our preliminary experience on the treatment of IH with low-dose propranolol in Chinese children.
Materials and methods
Patients
The ethics committee of Xinhua Hospital, affiliated to Shanghai Jiaotong University School of Medicine, approved this study. All parents whose infants participated in this study were informed of the research purpose and provided written informed consent. This prospective, interventional case series was conducted between May 2009 and December 2011. The inclusion criteria were as follows: infants less than 1 year of age, newly diagnosed with IH, and who had not been previously treated with corticosteroids or other medications. The contraindications for use of propranolol included: a history or risk of asthma, reactive airway disease, impaired renal or liver function, heart defects or arrhythmia, hypotension, central nervous system disorders, neonates under the age of 1 month, preterm babies not reaching a gestational age of 40 weeks, and allergy to propranolol. Infants who withdrew from follow-up within 6 months were also excluded from the study.
Clinical assessment prior to treatment with propranolol
The patients were admitted to hospital for 7 days. Before the initiation of propranolol treatment, a careful physical examination was carried out to rule out respiratory and cardiologic diseases. Baseline heart rate, systolic and diastolic blood pressures, and blood oxygen saturation were monitored and recorded by an ECG monitor (Philips SureSigns VM6 Monitor). A standard set of laboratory blood tests were undertaken, including complete blood count, alanine transaminase (ALT), aspartate transaminase (AST), thyroid function tests (thyroid-stimulating hormone, total thyroxine [total T4], free thyroxine [free T4], total triiodothyronine [total T3], and free triiodothyronine [free T3]), fasting blood glucose, plasma renin activity, angiotensin II (ATII), and aldosterone. Color Doppler echocardiography was performed to exclude any patients with heart defects.
Propranolol treatment protocol
On the second day of hospitalization, the patients were treated with propranolol, given as two separate doses (given at 8 am and 8 pm). The total dosage was determined by the age of the patient (1–3 months, 0.75 mg/kg/day; 4–12 months, 1 mg/kg/day). It was ensured that the patients were adequately fed every 3 h, in order to avoid drug-induced hypoglycemia. The patients were kept under close observation and received ECG monitoring during the first 3 days. Heart rate and systolic and diastolic blood pressures were recorded 1 h after each dose of propranolol, which corresponded with the time of peak absorption. Propranolol was omitted if blood pressure was measured to be less than the 5th percentile, according to age [10], and resumed once the blood pressure had normalized. If the patient tolerated the total dosage well, we arranged for discharge from the hospital after 1 week, with follow-up at an outpatient clinic every 3 months. ALT, AST, thyroid function tests, and fasting blood glucose were examined at each visit to the outpatient clinic. Propranolol treatment was maintained for at least 6 months, and in some patients was continued until the age of 18 months. If the tumor did not respond to low-dose propranolol, an additional dose of 0.5 mg/kg/day was given. Cessation of propranolol treatment was achieved with dose de-escalation, over a period of 1–2 weeks.
Clinical evaluation
The evaluation of efficacy was determined on the basis of three parameters: (a) the reduction in tumor volume. Estimates of tumor volume were made using ultrasonography or MRI, prior to medication and 3 and 6 months after medication. Superficial lesions were measured manually at each visit; (b) improvement in color; (c) improvement in texture. The results were classified into four grades, based on a previously used rating system: grade I, poor (0–25 % regression); grade II, fair (26–50 % regression); grade III, good (51–75 % regression); and grade IV, excellent (76–100 % regression) [1].
Statistical analysis
Analysis was performed using the SAS statistical software package (SAS 9.1.3; SAS Institute Inc., Cary, USA). All data are expressed as numbers, percentages, or means ± standard deviations. Student’s t test was used for comparisons between groups. A statistical threshold of p < 0.05 was used as the criterion for statistical significance between groups.
Results
Patient characteristics
A total of 125 patients were initially enrolled in our study, and 89 of these completed the full follow-up period. The characteristics of these 89 patients are summarized in Table 1. Among these, 52 patients were female and 37 were male, corresponding to a female-to-male ratio of 1.4:1. The average age at which medication was initiated was 3.56 months (1–3 months, 47 patients; 4–6 months, 31 patients; 7–9 months, 8 patients; and 10–12 months, 3 patients). The locations of the IHs were: the scalp, face (including orbital, periorbital and perioral regions, and the nose), neck, trunk, extremities, and perineal region.
Levels of plasma renin activity, ATII, and aldosterone in patients with IH
The mean plasma renin activity was 0.91 ng/ml/h (range, 0.1–7.92 ng/ml/h; Fig.1a). Since the test kit used for determination of plasma ATII could only detect concentrations in the range 0–800 pg/ml, values more than 800 pg/ml were recorded as >800 pg/ml. Of the 89 patients, 52 (58.4 %) had high plasma ATII (>800 pg/ml). In the remaining 37 patients (41.6 %), the mean plasma ATII level was 371.17 pg/ml (range, 36.8–779.33 pg/ml; Fig. 1b). The mean plasma aldosterone level was 462.35 pg/ml (range, 185.36–1,634.45 pg/ml; Fig.1c, Table 2).
Effect of low-dose propranolol treatment
In 86 of the 89 patients, the IHs responded to low-dose propranolol treatment and decreased in size. The other three patients were given an additional 0.5 mg/kg/day propranolol during the first week. The parents of the patients were satisfied by the obvious improvement in the color (changing from intense-red to red or purple) of the superficial lesions, during the first week following the initiation of therapy. In addition, subcutaneous tumors had changed in texture from hard to medium by the end of the first week.
After treatment with propranolol for 3 months, most of the IHs regressed. MRI scanning showed that the volumes of deep lesions in the orbital and parotid areas had regressed by more than 30 %. Furthermore, noticeable improvements in color were observed in superficial and cutaneous tumors, with lesions turning purple with areas of gray. The texture also changed to that of soft in most cases. Three patients who started therapy at the age of 1–3 months, and four patients who started therapy at the age of 4–6 months, displayed only mild regression at the time of the first follow-up visit. For this reason, the dose of propranolol was increased by an additional 0.5 mg/kg/day for subsequent therapy.
After treatment with propranolol for 6 months, even better results were observed: parts of the IHs had completely regressed, and the patients had stopped taking medication. The results were rated as follows: 44 patients (49.4 %) showed a grade IV response, 21 patients (23.6 %) a grade III response, and 24 patients (27.0 %) a grade II response; no patients had a grade I response (0.0 %) (Fig. 2). At the beginning of the study, it had been arranged that the patients should continue to receive propranolol until the tumor had completely regressed, up to an age limit of 18 months. The mean medication duration was found to be 13.6 months (range, 5–16 months).
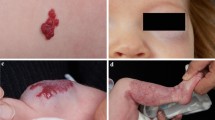
a, e, i Patients presenting with proliferating IH, involving the orbital and periorbital region. b, f, j MRI of the IHs. c, g, k After treatment with propranolol for 3 months, obvious regression could be observed. d, h, l Follow-up at 6 months showed further regression, and no rebound growth occurred
Safety of low-dose propranolol treatment
We observed that slight decreases in heart rate and blood pressure occurred in all patients during hospitalization (Table 3). Although blood pressure was seen to decrease, the average blood pressure value in all patients remained above the 5th percentile value for patients of that age [10]. In 5 of the 89 patients (5.6 %), ALT and AST were slightly elevated after medication with propranolol for 3 months. The dosage in these patients was adjusted to 0.75 mg/kg/day, and the patients were seen in the Department of Pediatrics to receive further advice. No obvious suppression of thyroid function was observed after treatment for 6 months with low-dose propranolol.
Adverse effects of low-dose propranolol treatment
Adverse effects of propranolol, besides those mentioned above, were suspected in 12 patients (13.5 %). Three patients developed mild diarrhea on the second day of propranolol treatment, but this had almost completely resolved spontaneously by the end of the first week, without any intervention. One patient had restless sleep during the course of treatment, and another two experienced mild nausea during the first week, but these symptoms disappeared during the second week. One patient, receiving 0.75 mg/kg/day propranolol, developed cold lower extremities on the third day of treatment. We adjusted the dosage to 0.5 mg/kg/day, and the symptoms disappeared. We increased the dosage to 0.75 mg/kg/day in the second week of treatment, and no recurrence of the symptom of cold extremities occurred. Slight hypoglycemia was detected in four patients (4.5 %) after treatment with propranolol for 3 months. We asked the parents of these patients to administer the drug around mealtimes and to provide more regular feeding. No patient was found to develop bronchospasm or leukocytosis.
Relapse of IHs after cessation of propranolol treatment
Obvious rebound growth of IHs was observed in 4 of the 89 patients (4.5 %); these patients had discontinued propranolol treatment after 4–5 months. As a result, propranolol treatment was restarted in these patients, according to our dosage regimen.
Discussion
IHs affects 1–10 % of newborns and infants worldwide. Although IHs are generally benign and self-limiting in nature, a significant minority involve complications. In a prospective cohort study at seven US pediatric dermatology clinics, with a consecutive sample of 1,058 children, 24 % of patients were found to experience complications related to their IHs [6]. In patients with complications, a wait-and-see policy should be abandoned and proper treatment instigated.
Propranolol is usually used for treating cardiac arrhythmias and hypertension. However, in the past 4 years, numerous groups worldwide have demonstrated the efficacy of propranolol for treating IHs, used at an empirical dosage of 2–3 mg/kg/day [11] Although many questions remain unanswered, propranolol is recommended as a first-line treatment for all rapidly proliferating IHs with functional deficit and disfigurement.
There are three main mechanisms by which propranolol has been suggested to hasten the involution of rapidly proliferating IHs. The early effects have been attributed to vasoconstriction, due to decreased release of nitric oxide. Intermediate effects are thought to be due to block of pro-angiogenic signals (vascular endothelial growth factor [VEGF], basic fibroblast growth factor, and matrix metalloproteinases 2 and 9) that result in growth arrest. Long-term effects are believed to be due to induction of apoptosis in proliferating endothelial cells.
There may also be alternative mechanisms by which propranolol exerts its effects on IHs. In the recent study of Itinteang et al. [9], the authors hypothesized that VEGF and ATII could drive both proliferation and differentiation of endothelial progenitor cells into mitotically active endothelial cells that characterize IH. Propranolol, acting primarily at the kidneys, reduces renin activity, and thereby decreases the conversion of angiotensinogen to angiotensin-I (ATI), and ultimately the conversion of ATI to ATII by angiotensin converting enzyme. The authors proposed a crucial role for the RAS in the biology of IHs, since clinical observations had reported a higher incidence of this tumor in White, female, and premature infants whose renin level was higher than their counterparts. Despite the higher level of renin and greater incidence of IHs in White, female, and premature infants, no direct evidence has confirmed the relationship between renin level and the onset of IH. Thus, we analyzed the RAS level in patients with IH to verify this hypothesis. Compared with that of age-matched healthy children [4], we found that patients with IH demonstrated an elevated level of plasma ATII, but decreased plasma renin activity. The aldosterone level was higher at 1–3 months, but lower at 4–12 months, than values reported in a previous study by Fiselier [4] (Table 2). Hence, our laboratory findings did not support the hypothesis of Itinteang et al. [9] but instead revealed a higher plasma ATII level in patients with IH. Therefore, it remains unclear whether the RAS contributes to the onset or development of IH, and if so, what the underlying mechanism is. Further studies should focus on revealing the relationship between the RAS and IH.
Propranolol has been widely used at an empiric dosage of 2–3 mg/kg/day in Western countries. Some authors have suggested that a good treatment response may be related to the dose, such that the maximum safe dose (3 mg/kg/day) should be used [7]. In the last 2 years, more and more authors have noticed that a propranolol dosage less than 2–3 mg/kg/day may also achieve satisfactory outcomes. Tan and colleagues reported that propranolol at 1.5–2.0 mg/kg/day was effective and safe for the treatment of problematic proliferating IHs [15, 16]. In China, more and more clinicians have started using propranolol for the treatment of IH [2, 8, 12], but the dosages chosen have been similar to those used in children in Western countries.
Zhou revealed that Chinese individuals had at least a twofold greater sensitivity to the beta-blocking effects of propranolol than White subjects. In Chinese people, less propranolol is bound to plasma protein, resulting in a greater proportion of pharmacologically active, unbound drug in the plasma that may contribute to the increased sensitivity to this agent [17]. The concentration of propranolol required to produce 50 % suppression of the exercise-induced increase in plasma renin activity was sevenfold lower in Chinese individuals than in White subjects [19]. In addition, infants may be more susceptible to the adverse effects of propranolol because hepatic first-pass metabolism is immature and bioavailability therefore greater. Thus, we considered it appropriate to treat our patients with IHs with a lower dosage of propranolol than that used for White individuals. In spite of the considerable amount of clinical studies published in China, only one research article has considered low-dose propranolol treatment [14], and hence there is a lack of information concerning the efficacy and safety of low-dose propranolol treatment.
In the present study, we found that the color and texture of the IHs were clearly improved during the first week. After treatment with low-dose propranolol for 6 months, the results were similar to those reported for use of 2–3 mg/kg/day propranolol in children from Western countries. We believe that the IHs of patients continuing to take propranolol would further involute. Thus, the final efficacy of our low-dose propranolol treatment would be expected to be even more encouraging. In the review of Menezes [13], adverse events occurred in 18.1 % of 154 patients treated with 2–3 mg/kg/day propranolol. Compared with patients treated with a higher dose, the incidence of adverse events was lower in our study. Taken together, these data suggest that a propranolol dose of 0.75–1 mg/kg/day is appropriate for Chinese children. Larger-scale studies should be undertaken to further investigate the safety and efficacy of low-dose propranolol in Chinese children.
References
Achauer BM, Chang CJ, Vander Kam VM (1997) Management of hemangioma of infancy: review of 245 patients. Plast Reconstr Surg 99:1301–1308
Chik KK, Luk CK, Chan HB, Tan HY (2010) Use of propranolol in infantile haemangioma among Chinese children. Hong Kong Med J 16:341–346
Denoyelle F, Leboulanger N, Enjolras O, Harris R, Roger G, Garabedian EN (2009) Role of propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma. Int J Pediatr Otorhinolaryngol 73:1168–1172
Fiselier TJ, Lijnen P, Monnens L, van Munster P, Jansen M, Peer P (1983) Levels of renin, angiotensin I and II, angiotensin-converting enzyme and aldosterone in infancy and childhood. Eur J Pediatr 141:3–7
Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA et al (2007) Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr 150:291–294
Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA et al (2006) Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics 118:882–887
Holmes WJ, Mishra A, Gorst C, Liew SH (2011) Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg 64:445–451
Hsu TC, Wang JD, Chen CH, Chang TK, Wang TM, Chou CM et al (2012) Treatment with propranolol for infantile hemangioma in 13 Taiwanese newborns and young infants. Pediatr Neonatol 53:125–132
Itinteang T, Brasch HD, Tan ST, Day DJ (2011) Expression of components of the renin-angiotensin system in proliferating infantile haemangioma may account for the propranolol-induced accelerated involution. J Plast Reconstr Aesthet Surg 64:759–765
Kent AL, Kecskes Z, Shadbolt B, Falk MC (2007) Blood pressure in the first year of life in healthy infants born at term. Pediatr Nephrol 22:1743–1749
Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A (2008) Propranolol for severe hemangiomas of infancy. N Engl J Med 358:2649–2651
Lv MM, Fan XD, Su LX (2012) Propranolol for problematic head and neck hemangiomas: an analysis of 37 consecutive patients. Int J Pediatr Otorhinolaryngol 76:574–578
Menezes MD, McCarter R, Greene EA, Bauman NM (2011) Status of propranolol for treatment of infantile hemangioma and description of a randomized clinical trial. Ann Otol Rhinol Laryngol 120:686–695
Qin ZP, Liu XJ, Li KL, Zhou Q, Yang XJ, Zheng JW (2009) Treatment of infantile hemangiomas with low-dose propranolol: evaluation of short-term efficacy and safety. Zhonghua Yi Xue Za Zhi 89:3130–3134
Tan ST, Itinteang T, Leadbitter P (2011) Low-dose propranolol for infantile haemangioma. J Plast Reconstr Aesthet Surg 64:292–299
Tan ST, Itinteang T, Leadbitter P (2011) Low-dose propranolol for multiple hepatic and cutaneous hemangiomas with deranged liver function. Pediatrics 127:e772–776
Zhou HH, Koshakji RP, Silberstein DJ, Wilkinson GR, Wood AJ (1989) Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American Whites. N Engl J Med 320:565–570
Zhou HH, Shay SD, Wood AJ (1993) Contribution of differences in plasma binding of propranolol to ethnic differences in sensitivity. Comparison between Chinese and Caucasians. Chin Med J (Engl) 106:898–902
Zhou HH, Wood AJ (1991) Increased suppression of exercise-induced increase in renin by propranolol in Chinese subjects. Clin Pharmacol Ther 50:150–156
Acknowledgments
This research was supported by the Bureau Level Research Project of Shanghai Municipal Health Bureau (20114020).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, X., Zhao, T., Xiao, Y. et al. Preliminary experience on treatment of infantile hemangioma with low-dose propranolol in China. Eur J Pediatr 172, 653–659 (2013). https://doi.org/10.1007/s00431-012-1928-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-012-1928-9