Abstract
Topical therapy is usually of limited benefit in the treatment of severe atopic dermatitis (AD), and the need for a safe and effective systemic treatment may be required in certain cases especially in children. We evaluated the efficacy and safety of methotrexate and cyclosporine in the treatment of 40 children with severe AD. Patients were divided into two groups (each consisting of 20 patients); group A was treated with methotrexate (7.5 mg/week) while group B was treated with cyclosporine (2.5 mg/kg/day). The severity scoring for atopic dermatitis (SCORAD) was used to indicate efficacy of treatment. In group A, the mean SCORAD score at the beginning of the study was 57.90 ± 3.21 that was reduced at the end of the treatment period to reach 29.35 ± 6.32 with a mean absolute reduction of 26.25 ± 7.03. In group B, the mean SCORAD score was 56.54 ± 4.82 at the start of treatment and was 31.35 ± 8.89 at the end of 12 weeks of treatment. The mean absolute reduction was 25.02 ± 8.21. There was no statistically significant difference in the reduction of SCORAD score between both groups (P ± 0.93). Mild and temporary adverse effects were reported in some patients in both groups. Conclusion: Methotrexate or cyclosporine in low doses can be considered as effective, relatively safe, and well-tolerated treatments for severe AD in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
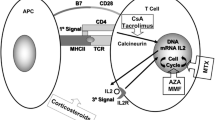
Atopic dermatitis (AD) is a common, chronic, relapsing inflammatory skin disease frequently affecting infants and children [23]. The worldwide prevalence of AD is estimated to be 5–20 % of the pediatric population. However, the prevalence in developed countries may increase up to 30 % which constitutes a public health problem [15]. Severe AD may lead to a major impairment in children's lives, and it may have a significant impact on the morbidity and the quality of life for those children and their parents [20]. Topical therapies are the mainstay in the treatment of AD, and they are effective in the majority of patients with mild or moderate condition. The use of systemic therapy in the treatment of AD is uncommon and usually indicated for only severe and resistant cases. Systemic corticosteroids are recommended to control acute flares, taking into account that new flares are frequent after stopping treatment, and their long-term use in children is not recommended [1]. An effective treatment that can control the variable inflammatory and immunological abnormalities such as elevated serum IgE, depressed cellular immunity, elevated blood eosinophilia, reduced levels of interferon-gamma, increased interleukin 4 production, and high levels of soluble CD30 (sCD30) in severe form of AD is usually required [3]. Cyclosporine was reported to induce some immunological changes in patients with AD. It reduces eosinophilic count and lowers E-selectin levels and soluble CD30 (known as disease markers); in addition, it corrects the imbalance between Th1 and Th2 response [6]. These effects may explain the possibility of an effective role of cyclosporine in the treatment of severe, recalcitrant AD in both adults and children [8, 19]. Methotrexate has been suggested also as a useful immunosuppressant in AD. It has been reported that methotrexate is an effective and well-tolerated therapeutic alternative for late-onset AD or idiopathic eczema in patients unresponsive to either topical or other systemic therapies [2, 9]. The use of methotrexate in both inflammatory and neoplastic diseases in children becomes widely accepted in the last few years, and it is considered an important therapeutic option in many dermatological disorders such as lymphomatoid papulosis [24] and mixed connective tissue disease [16] in addition to common skin diseases such as psoriasis [11] and morphea [22].
In this study, we demonstrated our experience in the management of severe childhood AD with methotrexate or cyclosporine and evaluated the efficacy, safety, and tolerability of both drugs. Both drugs were selected due to their common use in childhood diseases, and many previous reports documented their safety and tolerability in children even with a prolonged period of treatment. Moreover, methotrexate has an advantage of low price so that it can be afforded by most of the patients in developing countries and poor communities.
Materials and methods
The study included a series of 40 children who were diagnosed with severe AD and attended the outpatient dermatology clinics at four different centers: Al-Hussein, Al-Zahraa, Domyate, and Tanta university hospitals. The study was approved by the local ethical committee in Al-Azhar and Tanta universities.
The patients were randomly (computer-generated simple randomization method) divided into two equal groups. The first group (group A) was treated with methotrexate while the other group (group B) was treated with cyclosporine.
The inclusion criteria included children aged 8–14 years who had severe AD and failed to be treated with topical therapy, and who were unfit, uncooperative, or poorly responsive to phototherapy. Children who suffered from chronic or recurrent infections or having a history of severe or uncontrolled systemic diseases were not included in this study. In addition, patients with history of organ transplantation or history of cancer, or those who had herpes zoster infection within 2 months before the study, were excluded. Moreover, patients who are known to have hypersensitivity for methotrexate or cyclosporine were also excluded from the study.
For each patient, the severity scoring of AD (SCORAD) was assessed at start of treatment, 4 and 8 weeks of treatment, as well as at the end of 12 weeks of treatment. The SCORAD score combines both objective items as affected area and intensity of the lesions (erythema, edema/induration, excoriation, oozing/crusting, lichenification, and dryness) and subjective items such as extent of pruritus and sleep loss on a visual analog scale. Scores range from 0 to 108 points [12].
Basic laboratory investigations that were performed for all patients before starting treatment included: complete blood count, erythrocyte sedimentation rate, blood sugar, renal function tests, liver function tests, hepatitis B and C antibodies, serum total IgE, and urine analysis in addition to chest x-ray and Mantoux tuberculin skin test. All patients were requested to stop any topical or systemic treatment except emollients for at least 2 weeks prior to commencement of the study.
Treatment regimens
Before treatment, a written consent was obtained from the parents with complete information about the possible adverse effects of the medications. Group A was treated with methotrexate. The initial dose was 5 mg which represents the test dose; then, a maintenance dose of 7.5 mg as single weekly dose was continued to the end of the treatment period. The drug was administrated orally (2.5 mg/tablet) in three divided doses with 12-h interval. This regimen was more tolerated by the children with a main advantage of less gastric upset than the classical single dose. The patients were also supplemented with folic acid (400 μg once weekly) following the day of methotrexate dose. Group B was treated with cyclosporine 2.5 mg/kg/day (oral solution, 100 mg/ml, diluted in juice and administrated in two divided doses). The treatment period lasts for 12 weeks, and follow-up was continued for 12 weeks after the end of treatment period. The patients were advised not to administrate any other systemic treatment or exposed to phototherapy during the treatment and follow-up period. Sedating antihistamines and topical emollients were only allowed as adjuvant therapy.
Treatment efficacy and safety
Treatment efficacy was assessed by the absolute reduction in SCORAD score at the end of the treatment period. Relapse was considered for each patient when the SCORAD index increased by 50 % or more of the reduction after treatment. Clinical assessment and laboratory investigations were done for each patient at each visit (4, 8, 12 weeks) and at the end of the follow-up period.
Statistical analysis
The data were collected and statistically analyzed using SPSS version 16. For quantitative data, the mean and standard deviation were calculated. The difference between two means was statistically analyzed using Student's t test. A P value ≤0.05 was considered statistically significant.
Results
A total of 40 patients (26 boys and 14 girls) were enrolled in this study. Group A included 12 boys and 8 girls with a mean age of 11.6 ± 1.52 years. The duration of the disease ranged from 4 to 9 years with a mean of 7.2 ± 2.4 years. Group B included 14 boys and 6 girls with a mean age of 10.30 ± 2.82 years. The duration of disease ranged from 4 to 10 years with a mean of 6.80 ± 1.83 years. There was no statistically significant difference between both groups regarding age of patients and duration of the disease.
The lesions were mostly located on the extremities, and the most clinical stage of the disease was subacute/chronic lesions. Cyclosporine showed a rapid onset of action (2–3 weeks) and a rapid relapse (average, 14 weeks), while methotrexate showed a delayed onset of action (3–5 weeks) and a late relapse (average, 20 weeks). Associated allergic disorders included bronchial asthma, hay fever, and allergic rhinitis (Table 1).
In group A, the mean SCORAD score at the beginning of the study was 57.90 ± 3.21 that reduced to 48.45 ± 5.01 at the 4th week and 39.15 ± 7.44 at the 8th week while at the end of the 12th week, the mean SCORAD score was 29.35 ± 6.32. In group B, the mean SCORAD score was 56.54 ± 4.82 before treatment that was reduced to 64.01 ± 5.03, 38.80 ± 6.76, and 31.35 ± 8.89 in the 4th, 8th, and 12th weeks, respectively. After the follow-up period (24 weeks), the mean SCORAD score was 31.35 ± 9.52 in group A compared with 35.5 ± 14.54 in group B. The mean absolute reduction in SCORAD score in group A at the 4th, 8th, and 12th weeks were 9.95 ± 3.15, 19.75 ± 5.20, and 26.25 ± 7.03, respectively, while in group B, it was 10.55 ± 3.43 at the 4th week, 16.90 ± 6.15 at the 8th week, and 25.02 ± 8.21 at the end of the treatment period (12 weeks). The mean absolute reduction at the end of the follow-up period (24 weeks) was 24.90 ± 10.88 in group A and 21.01 ± 10.91 in group B (Fig. 1). There were no statistically significant differences between both groups in the reduction of SCORAD score either at the end of the treatment period (P = 0.93) or at the end of the follow-up period (P = 0.29) as shown in Table 2. Common adverse effects in group A included anemia (30 %), fatigue (30 %), abnormal liver functions (25 %), nausea and vomiting (20 %), and glossitis with oral ulceration (20 %). In group B, common complications included fatigue (45 %), leukopenia (35 %), headache (25 %), anemia (20 %), and flu-like symptoms (20 %). Adverse effects in both groups are listed in Table 3. None of the reported adverse reactions necessitated discontinuing or decreasing the dose of the drug, and all adverse effects disappeared at the end of the follow-up period.
Discussion
In this study, we present our experience in the treatment of severe AD in a small group of children with either methotrexate or cyclosporine. We proposed a therapeutic regimen with the lowest effective dose; hence, serious complications or adverse effects can be avoided. In both groups, there was a significant reduction in SCORAD score after treatment. These results may suggest the efficacy of both drugs in the treatment of severe childhood AD. In both groups, there were no serious side effects or complications that required cessation of treatment, and the safety profile of both drugs was nearly similar. Previous reports that were concerned with the use of cyclosporine in the treatment of severe AD in children showed variable results and different regimens. Leonardi et al. used cyclosporine (5 mg/kg/day) in the treatment of three children with severe AD, and they suggested that therapeutic courses of 8 weeks seem to be effective and safe as well as longer time courses in producing early remission with the advantage of a low cumulative exposure to the drug [14]. In another study that compared multiple short courses (each is 12 weeks) of cyclosporine (5 mg/kg/day) with continuous therapy (for 1 year), it was found that a continuous treatment is more effective in controlling the disease, although short-course therapy showed prolonged remission in some patients [10].
Similar to our low dose of cyclosporine (2.5 mg/kg/day), Bunikowski et al., in a prospective controlled study that included ten children, reported a SCORAD reduction of at least 35 % in four patients. In addition, there was a significant reduction in interferon-gamma (IFN-gamma), IL-2, IL-4, IL-13, and HLA-DR-positive CD3(+) cells proving that the low dose (2.5 mg/kg/day) of cyclosporine not only improves the clinical measures of disease, but also reduces T lymphocyte cytokine production and regulates T cell activation [5]. With a slightly higher daily dose of 2.8 mg/kg, Lee et al. observed a rapid improvement within the first 2 weeks, a maximum benefit at a mean of 10 weeks, and significant improvement in 73 % of patients at the end of the treatment period. However, all patients relapsed within 3 months after cessation of cyclosporine [13]. From these results, cyclosporine can be considered as an effective treatment for severe childhood AD. Although the improvement usually appears rapidly within a few weeks, relapse seems to occur shortly thereafter. A good safety profile of the drug was demonstrated with the different doses (2.5–5 mg/kg/day), and the drug was generally well tolerated without reports of any serious complications. This was concomitant with that of Zonneveld et al., who showed no differences in efficacy or adverse events between two different dosage schedules (3 and 5 mg/kg/day) in long-term treatment with cyclosporine [27].
There were many other open studies that suggested cyclosporine as an effective and safe short-term treatment for severe AD in children. Zaki et al. achieved a good to excellent response in 88.9 % of patients with a daily dose of 5 or 6 mg/kg, but most were relapsed within a few weeks [25].
Berth et al. also found a marked improvement or total clearance in 81.5 % of patients with significant improvement of the quality of life for the children and their families [4]. In another study which assesses the effectiveness of cyclosporine in patients with severe AD in addition to meta-analysis of the controlled and uncontrolled trials, it was found that in 15 studies which met the eligibility criteria, cyclosporine consistently decreased the severity of AD. After 2 weeks of treatment, it was found to be a dose-related response with a pooled mean decrease in disease severity of 22 % under low dose (3 mg/kg) and 40 % at dosages ≥4 mg/kg, while after 6–8 weeks, the relative effectiveness was 55 %. Moreover, the effectiveness of cyclosporine was found similar in both adults and children, but tolerability might be better in children [18].
In contrast to cyclosporine, there were few reports that demonstrated the efficacy of methotrexate in severe AD. Weatherhead et al. evaluated the efficacy of oral methotrexate in 12 adult patients with severe AD; they reported significant improvements in the quality of life, BSA affected, and itch scores, and the majority of improvement in disease activity was seen by week 12. The drug was well tolerated by 91.7 % of patients with a median dose of 15 mg weekly, and 66.7 % of patients had a persistent improvement 12 weeks after stopping methotrexate [21]. Zoller et al. also reported a significant improvement in six patients with late-onset AD after treatment with methotrexate; the initial response was noted after 3–7 weeks, and complete remission was achieved after 3 months. The condition worsened in one patient after complete withdrawal of the drug, but no serious adverse events were noted during treatment [26]. These results, in addition to our results, suggested that methotrexate is an effective treatment in severe AD. The low dose has a higher therapeutic safety in spite of the delayed response comparing with cyclosporine. However, the efficacy of the drug lasts for a longer time, and this can be more noticed in children than adults. The prolonged action of methotrexate in our study (up to 20 weeks) was also observed in another study that showed an average clearance of eczema for 9 months after withdrawal of the drug [17]. Moreover, methotrexate showed a prolonged efficacy in other inflammatory diseases affecting children, specifically psoriasis [7]. The low cost, easy administration, and high safety of methotrexate may play a significant role in increasing the usage of the drug as an alternative method in the treatment of severe childhood AD.
Conclusion
Methotrexate or cyclosporine in low doses can be considered as relatively safe and well-tolerated treatments for severe AD in children. Cyclosporine has the advantage of inducing a rapid response while methotrexate has the benefit of longer-lasting effect.
References
Akdis CA, Akdis M, Biber T, Bindslev-Jensen C, Boquniewicz M, Eigenmann P, European Academy of Allergology, Clinical Immunology/American Academy of Allergy, Asthma And Immunology/PRACTALL Consensus Group (2006) Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol 118:152–169
Balasubramaniam P, Ilchyshyn A (2005) Successful treatment of severe atopic dermatitis with methotrexate. Clin Exp Dermatol 30:436–440
Bateman EA, Ardern-Jones M, Ogg GS (2007) Dose-related reduction in allergen-specific T cells associates with clinical response of atopic dermatitis to methotrexate. Br J Dermatol 156:1376–1377
Berth-Jones J, Finlay AY, Zaki I, Tan B, Goodyear H, Lewis-Jones S, Cork MJ, Bleehen SS, Salek MS, Allen BR, Smith S, Graham-Brown RA (1996) Cyclosporin in severe childhood atopic dermatitis: a multicenter study. J Am Acad Dermatol 34:1016–1021
Bunikowski R, Staab D, Kussebi F, Bräutigam M, Weidinger G, Renz H, Wahn U (2001) Low-dose cyclosporin a microemulsion in children with severe atopic dermatitis: clinical and immunological effects. Pediatr Allergy Immunol 12:216–223
Caproni M, Salvatore E, Cardinali C, Brazzini B, Fabbri P (2000) Soluble CD30 and cyclosporin in severe atopic dermatitis. Int Arch Allergy Immunol 121:324–328
Collin B, Vani A, Ogboli M, Moss C (2009) Methotrexate treatment in 13 children with severe plaque psoriasis. Clin Exp Dermatol 34:295–298
Cordero Miranda MA, Casas Becerra B, Reyes Ruiz NI, Avila Castañón L, del Río Navarro BE, Sienra Monge JJ (2002) Cyclosporin A in atopic dermatitis. Rev Alerg Mex 49:129–134
Goujon C, Bérard F, Dahel K, Guillot I, Hennino A, Nosbaum A, Saad N, Nicolas JF (2006) Methotrexate for the treatment of adult atopic dermatitis. Eur J Dermatol 16:155–158
Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, Finlay AY, Wilson NJ, Graham-Brown RA, Sowden JM, Beard AL, Sumner MJ, Berth-Jones J (2000) Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol 142:52–58
Kaur I, Dogra S, De D, Kanwar AJ (2008) Systemic methotrexate treatment in childhood psoriasis: further experience in 24 children from India. Pediatr Dermatol 25:184–188
Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A (1997) Clinical validation and guidelines for the SCORAD index: consensus report of the European task force on atopic dermatitis. Dermatology 195:10–19
Lee SS, Tan AW, Giam YC (2004) Cyclosporin in the treatment of severe atopic dermatitis: a retrospective study. Ann Acad Med Singapore 33:311–313
Leonardi S, Marchese G, Rotolo N, Miraglia Del Giudice M, La Rosa M (2004) Cyclosporin is safe and effective in severe atopic dermatitis of childhood. Report of three cases. Minerva Pediatr 56:231–237
Leung DY, Bieber T (2003) Atopic dermatitis. Lancet 361:151–160
Nakata S, Uematsu K, Mori T, Mitsushita N, Kinoshita T, Ishioka C, Mutou K, Yokota S, Hirose Y, Komiyama A (1997) Effective treatment with low-dose methotrexate pulses of a child of mixed connective tissue disease with severe myositis refractory to corticosteroid. Nihon Rinsho Meneki Gakkai Kaishi 20:178–183
Roberts H, Orchard D (2010) Methotrexate is a safe and effective treatment for paediatric discoid (nummular) eczema: a case series of 25 children. Australas J Dermatol 51:128–130
Schmitt J, Schmitt N, Meurer M (2007) Cyclosporin in the treatment of patients with atopic eczema—a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 21:606–619
Sowden JM, Berth-Jones J, Ross JS, Motley RJ, Marks R, Finlay AY, Salek MS, Graham-Brown RA, Allen BR, Camp RD (1991) Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet 338:137–140
Su JC, Kemp AS, Varigos GA, Nolan TM (1997) Atopic eczema: its impact on the family and financial cost. Arch Dis Child 76:159–162
Weatherhead SC, Wahie S, Reynolds NJ, Meggitt SJ (2007) An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol 156:346–351
Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI (2006) Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol 155:1013–1020
Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R (1999) Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J Allergy Clin Immunol 103:125–138
Yip L, Darling S, Orchard D (2011) Lymphomatoid papulosis in children: experience of five cases and the treatment efficacy of methotrexate. Australas J Dermatol 52:279–283
Zaki I, Emerson R, Allen BR (1996) Treatment of severe atopic dermatitis in childhood with cyclosporin. Br J Dermatol 135:21–24
Zoller L, Ramon M, Bergman R (2008) Low dose methotrexate therapy is effective in late-onset atopic dermatitis and idiopathic eczema. Isr Med Assoc J 10:413–414
Zonneveld IM, De Rie MA, Beljaards RC, Van Der Rhee HJ, Wuite J, Zeegelaar J, Bos JD (1996) The long-term safety and efficacy of cyclosporin in severe refractory atopic dermatitis: a comparison of two dosage regimens. Br J Dermatol 135:15–20
Conflict of interest
The authors declare that they have no conflict of interest, and no financial support was received to perform this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Khalawany, M.A., Hassan, H., Shaaban, D. et al. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr 172, 351–356 (2013). https://doi.org/10.1007/s00431-012-1893-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-012-1893-3