Abstract
Our aim was to evaluate the feasibility of using computed tomography (CT) to define the pulmonary artery anatomy in patients with tetralogy of Fallot and pulmonary atresia (TOF-PA). We retrospectively reviewed 110 patients with TOF-PA between 1995 and 2008. Those who received cardiac catheterization and surgery within 3 months of their CT examinations were enrolled. Based on Dr. Somerville’s classification, the pulmonary arterial pattern was determined, including identifiable pulmonary trunk (type I), the presence of both left and right pulmonary arteries without trunk (II), only left or right pulmonary artery present (III), and absent intrapericardial pulmonary arteries (IV). The accuracy of both imaging modalities was evaluated with operation findings as the golden standard. The effective radiation doses and adverse events were also recorded. In the 64 eligible patients (median age, 23 months), CT and catheterization demonstrated accurate pulmonary arterial morphology in 60 (60/64) and 53 (53/64) TOF-PA patients, respectively. Thirty-two of 35 type I patients were correctly identified by CT, whereas 26 were correctly identified by catheterization (p = 0.03). Of the 20 type II TOF-PA patients, 19 were diagnosed by CT, whereas 18 were diagnosed by catheterization. CT and catheterization both successfully defined six type III and three type IV patients. The median calculated radiation doses caused by CT and catheterization were 4.5 and 5.6 mSv, respectively (p > 0.05). Conclusions: For patients with TOF-PA, CT could accurately delineate pulmonary arterial morphology with the same level of accuracy as cardiac catheterization. Therefore, CT can be considered a reasonable diagnostic alternative for such patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetralogy of Fallot with pulmonary atresia (TOF-PA), a specific type of pulmonary atresia with ventricular septal defect, is characterized by a lack of physiologic antegrade pulmonary blood flow and marked morphologic variability in the pulmonary arterial arborization [19]. Treatment of this condition has evolved from palliative shunting or the interruption of collateral arteries in the 1970s to the reconstruction of pulmonary arteries with unifocalization to the right ventricle with or without VSD closure [21]. This progress has been related to the better characterization of native pulmonary arteries together with improvements in the surgical care of small infants. Complete delineation of the anatomy, size, and morphology of the pulmonary circulation is essential and necessary to plan the appropriate surgical management [3, 28, 29]. Ishibashi et al. [17] showed that survival in TOF-PA patients with central pulmonary arteries was significantly higher than the survival of those without. However, such anatomic characteristics have been conventionally assessed by cardiac catheterization [12]. Conventional pediatric catheterization carries risks associated with its invasive nature and with anesthesia [22]. In addition, angiography often provides only indirect information regarding arterial anatomy distal to atresia or high-grade stenosis [27]. Therefore, with the advantage of its noninvasive nature, short scanning time, and high spatial resolution, modern computed tomography (CT) can provide valuable information about intracardiac, extracardiac, arterial, and venous anatomy in patients with congenital heart disease [20, 23]. Several published studies have discussed the application of CT to patients with pulmonary atresia (PA) [26, 33]. Recently, Yin et al. [33] studied 20 patients with pulmonary atresia and ventricular septal defect (age 43 days to 22 years) and demonstrated that measurements of the diameters of native pulmonary and collateral vessels by CT correlated well with the results obtained by catheterization. The depiction rates of CT on the confluence of bilateral pulmonary arteries are better than those of cardiac catheterization (16/16 vs. 10/16, p = 0.024). In the current study, using a relatively large and specific patient cohort of TOF-PA, we plan to validate the feasibility of CT for the detection of pulmonary arterial morphology of such patients, especially the presence of pulmonary trunk and left and right pulmonary arteries. Because previous studies have shown that children suffer from larger radiation dose compared to adults using the same CT protocol [10] and are more susceptible to the effects of radiation [16], we also examined the safety issues of CT and cardiac catheterization in our study cohort.
Material and methods
Study subjects
The study protocol was approved by the local ethics committee and written informed consent was obtained from all patients or their guardians. Between October 1995 and October 2008, 110 consecutive children with TOF-PA visited our hospital, a 2,000-bed tertiary referral general hospital. Medical records were reviewed, and if patients had received CT examinations for evaluation of pulmonary arterial morphology and had undergone cardiac catheterization and a sternotomy approach operation within 3 months of the CT, they were eligible for this study.
Classification of pulmonary arterial morphology
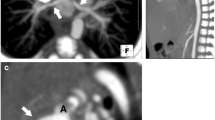
Several classification systems have been proposed for the pulmonary arborization of the TOF-PA patients [4, 29, 30]. Because this study focused on the extent of the atresia and the development of the pulmonary arteries distal to the atresia, we used Dr. Somerville’s classification [29] and four types of pulmonary arterial morphology were identified (Fig. 1). In type I, the pulmonary trunk was present. In type II, the pulmonary trunk was absent, but there were both left and right pulmonary arteries (confluent or nonconfluent). In pattern III, either right or left pulmonary artery only was present. In pattern IV, no intrapericardial pulmonary artery was found.
CT images and schematic drawing of classification (based on Dr. Somerville’s classification [29]) (types N, I, II, III, and IV) of pulmonary arterial morphology. N normal; I the pulmonary trunk was present; II the pulmonary trunk was absent, but there were both left and right pulmonary arteries; III either left or right pulmonary artery was present only; IV no intrapericardial pulmonary artery was found
CT scanning protocol and image analysis
Subjects underwent contrast-enhanced cardiac CT examinations (C150-L [Imatron, South San Francisco, CA, USA] from October 1995 to September 2003 and LightSpeed 16 [General Electric Medical Systems, Milwaukee, WI, USA] from September 2003 to Oct 2008), with electrocardiographic gating, as suggested by the manufacturer. Intracardiac structural anomalies, pulmonary arterial morphology, and mediastinal organs were evaluated at the same time. All images were obtained at the end diastolic phase of the cardiac cycle. Slice thickness was 3 mm using the C150-L scanner and 0.625 mm using the LightSpeed 16 scanner. Computed tomography was performed starting at the level of the lung apex to the cardiac apex without any gaps. The table increment using the C150-L scanner was 3 and 0.625 mm for patients evaluated using the LightSpeed 16 scanner. The temporal resolution ranged from 100 to 250 ms depending on the heart rate of the patients. The matrix of the reconstructed axial image was 512 × 512 pixels. Nonionic iodinated contrast medium (Ultravist 370; Schering, Berlin, Germany) at doses of 2 to 3 mL/kg was delivered using a power injector in all patients. Patients were allowed to breathe freely during the examination. Patients younger than 5 years of age were routinely sedated before imaging with chloral hydrate using a dose of 50 mg/kg in neonates and 50 to 75 mg/kg in children up to age 5.
Two radiologists with 15 (SJ Chen) and 8 (WJ Lee) years of experience in cardiac computed tomography retrospectively assessed the CT images to confirm the anomalies of interest. They were blinded to the results of the cardiac echocardiography, angiography, and operation findings.
Cardiac catheterization
Biplane contrast angiography was used and directed for the evaluation of the pulmonary arteries, aortopulmonary collaterals, and systemic-to-pulmonary shunts. Descending aortograms at the aortic isthmus were routinely conducted to detect the ductus arteriosus (DA) and native pulmonary arteries supplied from the DA. In addition to aortography and ventriculography, selective injections at the DA and certain collaterals or shunts were performed as needed. Two randomly selected investigators (MT Lin, JK Wang, HH Chiu, CA Chen, SN Chiu, ET Wu, CW Lu, and MH Wu) unaware of the CT findings reviewed the angiograms and recorded the pulmonary arterial morphology and the diameters of the left and right pulmonary arteries.
Measurement of diameters of pulmonary arteries
In addition to the direct measurement of vessel diameters, the McGoon index was used to represent the corrected and standardized pulmonary arterial size, as had been done in previous studies [25]. The index is defined as (diameter of left pulmonary artery + diameter of right pulmonary artery) / diameter of the descending aorta at the level of the diaphragm.
Adverse events
An adverse event (AE) was defined as any negative event that occurred during or within the first 24 h after the procedure. The definition of a major AE is the one used by the US Food and Drug Administration [31].
Dosage of radiation
We recorded the tube current time product (milliampere second, mAs), tube voltage (peak kilovoltage), scan length (millimeters), and total collimation in each CT study. The dose length product (milligray centimeter, mGy cm) was calculated and the age-specific effective radiation dose (millisieverts) was thereafter estimated [8]. For cardiac catheterization, we recorded the dose–area product (milligray square centimeter) automatically calculated by the imaging system and multiplied by age-adjusted conversion factors in pediatric interventional cardiology [18].
Statistics
The CT and catheterization findings regarding the pulmonary arterial morphology were recorded on a spreadsheet and analyzed based on the operation golden standard. Analysis was performed using SPSS v15.0. Data are presented as the median with interquartile ranges for the characteristics of patients, whereas the frequencies and percentages (in parentheses) summarize the categorical variables. Differences in accuracy and adverse events between the two imaging modalities were tested with McNemar’s test, based on the concordant and discordant pairs. An exact test was applied if the number of discordant pairs was less than 20 in a 2 × 2 contingency table. The difference was deemed significant at a p value < 0.05.
Results
Between 1995 and 2008, 110 patients (male/female = 68/52) with TOF-PA visited our hospital. Of these 110 patients, 103 ever received CT examinations. Based on the inclusion criteria, 64 patients (male/female = 34/30) were eligible and had concurrent reports from CT, cardiac catheterization, and surgery for analysis. The median age at CT of the 64 patients was 23 months (interquartile range, 4–53 months old). The median body weight at examination was 10.0 kg (interquartile range, 4.5–14.0 kg). According to the operation results recorded by surgeons (YS Chen, SC Huang, IS Chiu, or CI Chang), the distribution of pulmonary arterial patterns for the 64 enrolled patients was as follows (Table 1): type I (identifiable pulmonary trunk)—35 (35/64), type II (left and right pulmonary arteries [LPA and RPA] both exist without pulmonary trunk)—20 (20/64), type III (only LPA or RPA present)—6 (6/64), and type IV (no intrapericardial pulmonary artery)—3 (3/64).
CT findings for pulmonary arteries
CT findings for the pulmonary arterial morphology of the enrolled patients are listed in Table 1. With operation findings as the golden standard, the overall accuracy of CT in the classification of pulmonary arterial morphology was 93.8% (60/64, 95% confidence interval 87.8–99.7%). No significant difference in the accuracy was noted between the two CT machines (C150-L vs. LightSpeed 16, 34/37 vs. 26/27, p > 0.05; Supplementary Table 1). For the specific patterns of pulmonary arterial morphology, 32 of 35 type I and 19 out of 20 type II patients were correctly identified by CT. CT could also define the pulmonary arterial morphology in all type III (six out of six) and type IV (three out of three) patients (Table 1). CT examinations failed to demonstrate the presence of pulmonary trunk in three type I patients. These three patients had a cord-like pulmonary trunk that was too small for the creation of a shunt. Another one examination failed to disclose the small RPA because of motion artifacts.
The diameters of the LPA and RPA in each patient were also measured by CT. The median diameters of the LPA and RPA in 61 patients with intrapericardial pulmonary arteries were 4.83 and 5.19 mm, respectively. For standardization, the McGoon index was calculated and its median was 1.38 (interquartile range, 1.06–1.85). The pulmonary arterial size is significantly larger in the patients with pulmonary trunk (type I, median 1.56), compared to those without (types II, III, and IV; median 1.25, p < 0.01).
Major aortopulmonary collateral arteries (MAPCAs) are another hallmark of TOF-PA. In this study, CT identified a total of 131 MAPCAs in 47 enrolled patients (47/64). One hundred twenty-six MAPCAs arose from the descending aorta, 3 from the left subclavian artery, 1 from the innominate artery, and 1 from the right subclavian artery. Type I patients had fewer MAPCAs (median 1) than the remaining (median 2), but this difference was not significant (p = 0.35).
Comparison between CT and cardiac catheterization
Based on the operation findings, the overall accuracy of cardiac catheterization in the demonstration of pulmonary arterial arborization was 53/64 (82.8%, 95% confidence interval 73.6–92%), relatively lower than 93.8% by CT (95% confidence interval 87.8–99.7%; McNemar’s test, p = 0.05). In each type of pulmonary arterial morphology, cardiac catheterization identified 26 of 35 type I patients, 18 out of 20 type II patients, and all type III and IV patients (Table 1). There was perfect agreement between the two imaging modalities in the detection of LPA and RPA (i.e., types II, III, and IV). Both CT and catheterization failed to detect the pulmonary trunk in three type I cases. However, catheterization misclassified the pulmonary trunk as atretic in six patients for whom a hypoplastic pulmonary trunk was demonstrated by CT and confirmed with surgery. The difference in the detection of pulmonary trunk between CT and catheterization was significant (32/35 vs. 26/35, p = 0.03, Table 1).
The diameters of LPA and RPA were also measured during cardiac catheterization. The median diameters of LPA and RPA of 61 patients with identifiable intrapericardial pulmonary arteries were 4.95 and 5.20 mm, respectively. The median McGoon index was 1.39, which correlated well with the CT measurements (r = 0.86). A paired t test did not show a significant difference in the measurements of pulmonary arterial diameters between catheterization and CT (p > 0.05).
There was complete agreement between the CT and catheterization in the demonstration of 126 MAPCAs from the descending aorta and two MAPCAs from the left subclavian artery [(126 + 2) / 131]. Catheterization did not completely delineate all MAPCAs seen on the CT in three patients. Of the three catheterization-misdiagnosed MAPCAs, two arose from the subclavian arteries and one from the innominate artery.
Of the six patients whose pulmonary trunk could be detected by CT only, five patients were younger than 1 year old. Therefore, we further analyzed the accuracy of these two imaging tools when applied to infants with TOF-PA. Of the 20 enrolled infants (20/64, 31%) with TOF-PA (Table 2), CT could demonstrate correct pulmonary arterial morphology in 13 type I infants (13/14) and in all type II, III, and IV infants (six out of six). Catheterization correctly demonstrated eight pulmonary trunk in 14 type I infants and also did the correct classification for the six type II, III, and IV infants. Thus, in this age group, the McNemar’s test showed that there was a borderline significant difference in the detection of pulmonary trunk between CT and catheterization (p = 0.06).
Dosage of radiation and adverse events
The interquartile range of effective radiation dose equivalent for the enrolled CT studies was 3.3–6.5 mSv (median 4.5 mSv, dose length product 115–218 mGy cm), relatively but not significantly lower than that for the enrolled cardiac catheterizations (5–8.5 mSv, median 5.6 mSv, p > 0.05; dose area product 3.8–6 Gy cm2). Of the 64 instances of cardiac catheterization, five adverse events (5/64, 7.8%, 95% confidence interval 4.5–11.2%) occurred during or immediately after the procedure, including apnea rescued by short-term Ambu-bagging (three cases), absent pulse of the femoral artery with resolution after the infusion of heparin (one case), and apnea necessitating intubation (one case). There was only one episode (1/64) of transient apnea recorded during 64 CT examinations. There was no significant difference in the rate of adverse events between the two imaging modalities (p > 0.05).
Discussion
Our study demonstrates that CT is a practical alternative to cardiac catheterization in the safety and identification of pulmonary arterial morphology for patients with TOF-PA. The major reasons for the excellent detection of pulmonary arterial morphology with CT in this study might be (1) sufficient distribution of contrast medium in the patent part of pulmonary arteries distal to the atretic segment from several cardiac cycles in CT, (2) volumetric data acquisition in CT that prevent an overlapping shadow in projection image like angiography [20, 24, 26], and (3) atretic stump of the pulmonary trunk is sometimes enhanced only by retrograde flow or inadequately filled in ductal angiography or descending aortogram, which has only a limited contrast medium-enhanced vascular shadow [7, 27].
The adequate demonstration of pulmonary arterial aborization should be beneficial to repair TOF-PA [3, 17, 28, 29]. For example, Carotti et al. [3] demonstrated that the absence of confluent intrapericardial pulmonary arteries affected the postoperative right/left ventricular pressure ratio after ventricular septal defect closure, and the right/left ventricular pressure ratio has been shown the most powerful factor related to survival in patients with TOF-PA [19]. Selective angiograms with the injection of contrast medium in the ductus arteriosus are usually suggested to identify the confluence of pulmonary arteries [29]. In the current study, 53 of the 64 enrolled patients had pulmonary confluence. The accuracy of CT in the detection of such anatomic characteristic was 96.2% (95% confidence interval 91.1–100%), similar to that of catheterization (90.6%, 95% confidence interval 82.7–98.4%). Together, the above results indicate that CT could be considered a reliable alternative to imaging studies in patients with TOF-PA. Moreover, CT can help detect several associated extracardiac anomalies like coronary artery crossing the outflow tract of RV [5] and anomalous brachiocephalic vein [6]. These anomalies are not frequent but might change the sites of snaring during cannulation for cardiopulmonary bypass and even compromise the surgical outcomes.
In infants with congenital heart disease, the application of CT angiography to define the anatomy of the great vessels had also been reported [2, 24] and thought of as feasible. Cardiac catheterization in infancy is relatively invasive and carries an inherent risk of vessel damage, bleeding, stroke, and infection [22]. Mehta et al. [22] studied the clinical records of 11,073 children undergoing cardiac catheterization between 1994 and 2006 and found that age (especially younger than 6 months) and the year of the procedure were independent risk factors for major complications like death and cardiac arrest. The adjusted odds ratio for the “age < 6 months old” factor was 3.82 (95% confidence interval 3.0–4.83, p = 0.0003). Furthermore, in infants with TOF-PA, vessel damage should be carefully avoided because of future multistage operations. In the present study, CT did the correct classification on the pulmonary arterial morphology in the 19 enrolled infants (19/20). This implies that CT could serve as a less invasive alternative to conventional angiography in infants with TOF-PA.
Although cardiac catheterization has the aforementioned disadvantages, it can help delineate which regions of the lung have “dual supply” from the native pulmonary arteries as well as from aortopulmonary collaterals [19]. CT is not as good at providing this important information because it is difficult for CT to show the full courses of the lobar or segmental pulmonary arteries based purely on serial sectional images without using three-dimensional reconstruction. In addition, catheterization provides essential hemodynamic data and offers the advantages of intervention using vascular access, such as the dilation of stenotic area and the embolization of unnecessary collateral circulation.
The main drawbacks of CT include patient exposure to ionizing radiation and contrast material, especially for children [9, 14], because children have a longer life expectancy to develop complications such as tumors and are more susceptible to the effects of radiation than adults [10, 16]. Recently, magnetic resonance (MR) imaging had been applied to the examination of congenital heart disease [2, 23] and thought of as a nice alternative to catheterization and CT because it avoids ionizing radiation and uses less nephrotoxic contrast medium [23, 32]. Its utility in patients with TOF-PA has been well studied [11]. High sensitivity and specificity for the diagnosis of pulmonary arterial stenosis have also been demonstrated [11]. Furthermore, Valsangiacomo Buechel et al. [32] demonstrated perfect correlations between the measurements performed on the MR angiography and those from the cardiac catheterization. Therefore, they concluded that MR angiography provided accurate quantitative information of aorta and pulmonary vessels and could be used for planning catheter-guided procedures and reducing the radiation exposure during catheterization [32]. However, in our institution, performing MRI in children with congenital heart disease is difficult because of patient monitoring and accessibility issues. This is why we chose CT examination as an alternative. In addition, the dose of radiation given to patients was significantly reduced by adjusting the diagnostic tube current according to patients’ weight and by doubling the pitch [2, 13]. In this study, we estimated that the effective radiation dose equivalent of CT examination ranged from 3.3 to 6.5 mSv, similar to the results of previous studies [14]. For children aged 1 to 5 years, such CT effective dose is equivalent to 40–80 plain abdomen radiographs (0.08–0.09 mSv/film) or 60–130 chest radiographs (0.06 mSv/film) [15]. Recent studies also revealed that the effective radiation dose would be further reduced to less than 1 mSv with 320-detector CT angiography [1] or prospective ECG-triggered sequential dual-source data acquisition [24]. Therefore, in terms of radiation exposure, CT seems to be a good alternative to cardiac catheterization. Moreover, CT does not expose children to the possible complications associated with invasive angiography, and most importantly, CT can be performed without the need for hospitalization. Therefore, CT is also suitable for sequentially following patients with TOF-PA.
Limitations
First, because the number of MAPCAs and vessel diameters were not always the main focus of the operations (such as the creation of a central shunt), this information was not always available in the operation findings. Therefore, the accuracy of CT and catheterization in these two entities could not be compared with each other. The efficacy of CT in these fields needs to be validated by further studies. Second, this is a retrospective study. Further prospective studies are necessary in the future to compare CT and cardiac catheterization in a direct, contemporaneous fashion for TOF-PA patients.
Conclusions
This retrospective study validates the accuracy of CT for the determination of pulmonary arterial morphology in patients with TOF-PA. CT could identify pulmonary arterial arborization with competent and equivalent accuracy to conventional cardiac catheterization. Therefore, in terms of imaging accuracy and safety, CT can serve as a reasonable alternative to cardiac catheterization for such children.
Abbreviations
- CT:
-
Computed tomography
- TOF:
-
Tetralogy of Fallot
- PA:
-
Pulmonary atresia
- MAPCA:
-
Major aortopulmonary collateral artery
References
Al-Mousily F, Shifrin RY, Fricker FJ, Feranec N, Quinn NS, Chandran A (2011) Use of 320-detector computed tomographic angiography for infants and young children with congenital heart disease. Pediatr Cardiol 32:426–432
Bailliard F, Hughes ML, Taylor AM (2008) Introduction to cardiac imaging in infants and children: techniques, potential, and role in the imaging work-up of various cardiac malformations and other pediatric heart conditions. Eur J Radiol 68:191–198
Carotti A, Albanese SB, Filippelli S, Rava L, Guccione P, Pongiglione G, Di Donato RM (2010) Determinants of outcome after surgical treatment of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg 140:1092–1103
Castaňeda AR, Jonas RA, Mayer JE Jr, Hanley FL (1994) Tetralogy of Fallot. In: Castaňeda AR, Jonas RA, Mayer JE Jr, Hanley FL (eds) Cardiac surgery of the neonate and infant. WB Saunders, Philadelphia, pp 215–234
Chen SJ, Lin MT, Lee WJ, Liu KL, Wang JK, Chang CI, Li YW, Chiu IS (2007) Coronary artery anatomy in children with congenital heart disease by computed tomography. Int J Cardiol 120:363–370
Chen SJ, Liu KL, Chen HY, Chiu IS, Lee WJ, Wu MH, Li YW, Lue HC (2005) Anomalous brachiocephalic vein: CT, embryology, and clinical implications. Am J Roentgenol 184:1235–1240
Davis GD, Fulton RE, Ritter DG, Mair DD, McGoon DC (1978) Congenital pulmonary atresia with ventricular septal defect: angiographic and surgical correlates. Radiology 128:133–144
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
Feng ST, Law MW, Huang B, Ng S, Li ZP, Meng QF, Khong PL (2010) Radiation dose and cancer risk from pediatric CT examinations on 64-slice CT: a phantom study. Eur J Radiol 76:e19–23
Fujii K, Aoyama T, Koyama S, Kawaura C (2007) Comparative evaluation of organ and effective doses for paediatric patients with those for adults in chest and abdominal CT examinations. Br J Radiol 956:657–667
Geva T, Greil GF, Marshall AC, Landzberg M, Powell AJ (2002) Gadolinium-enhanced 3-dimensional magnetic resonance angiography of pulmonary blood supply in patients with complex pulmonary stenosis or atresia: comparison with X-ray angiography. Circulation 106:473–478
Griselli M, McGuirk SP, Winlaw DS, Stümper O, Giovanni JVd, Miller P, Dhillon R, Wright JG, Barron DJ, Brawn WJ (2004) The influence of pulmonary artery morphology on the results of operations for major aortopulmonary collateral arteries and complex congenital heart defects. J Thorac Cardiovasc Surg 127:251–258
Herzog C, Mulvihill DM, Nguyen SA, Savino G, Schmidt B, Costello P, Vogl TJ, Schoepf UJ (2008) Pediatric cardiovascular CT angiography: radiation dose reduction using automatic anatomic tube current modulation. AJR Am J Roentgenol 190:1232–1240
Hollingsworth CL, Yoshizumi TT, Frush DP, Chan FP, Toncheva G, Nguyen G, Lowry CR, Hurwitz LM (2007) Pediatric cardiac-gated CT angiography: assessment of radiation dose. AJR Am J Roentgenol 189:12–18
Huda W (2004) Assessment of the problem: pediatric doses in screen-film and digital radiography. Pediatr Radiol 34(Suppl 3):S173–S182
ICRP (2007) The 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP 2–4:1–332 [ICRP publication 103]
Ishibashi N, Shin'oka T, Ishiyama M, Sakamoto T, Kurosawa H (2007) Clinical results of staged repair with complete unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Eur J Cardiothorac Surg 32:202–208
Karambatsakidou A, Sahlgren B, Hansson B, Lidegran M, Fransson A (2009) Effective dose conversion factors in paediatric interventional cardiology. Br J Radiol 82:748–755
Kouchoukos NT, Blackstone EH, Doty DB, Hanley FL, Karp RB (2003) Ventricular septal defect and pulmonary stenosis or atresia. In: Kirklin JW, Barratt-Boyes BG (eds) Cardiac surgery, 3rd edn. Churchill-Livingstone, Philadelphia, pp 946–1073
Ley S, Zaporozhan J, Arnold R, Eichhorn J, Schenk JP, Ulmer H, Kreitner KF, Kauczor HU (2007) Preoperative assessment and follow-up of congenital abnormalities of the pulmonary arteries using CT and MRI. Eur Radiol 7:151–162
Malhotra SP, Hanley FL (2009) Surgical management of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals: a protocol-based approach. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 12:145–151
Mehta R, Lee KJ, Chaturvedi R, Benson L (2008) Complications of pediatric cardiac catheterization: a review in the current era. Catheter Cardiovasc Interv 72:278–285
Mertens L, Ganame J, Eyskens B (2008) What is new in pediatric cardiac imaging? Eur J Pediatr 167:1–8
Pache G, Grohmann J, Bulla S, Arnold R, Stiller B, Schlensak C, Langer M, Blanke P (2011) Prospective electrocardiography-triggered CT angiography of the great thoracic vessels in infants and toddlers with congenital heart disease: feasibility and image quality. Eur J Radiol PMID: 21310567
Piehler JM, Danielson GK, McGoon DC, Wallace RB, Fulton RE, Mair DD (1980) Management of pulmonary atresia with ventricular septal defect and hypoplastic pulmonary arteries by right ventricular outflow construction. J Thorac Cardiovasc Surg 80:552–567
Rajeshkannan R, Moorthy S, Sreekumar KP, Ramachandran PV, Kumar RK, Remadevi KS (2010) Role of 64-MDCT in evaluation of pulmonary atresia with ventricular septal defect. AJR Am J Roentgenol 194:110–118
Rees RS, Somerville J, Underwood SR, Wright J, Firmin DN, Klipstein RH, Longmore DB (1987) Magnetic resonance imaging of the pulmonary arteries and their systemic connections in pulmonary atresia: comparison with angiographic and surgical findings. Br Heart J 58:621–626
Reddy VM, McElhinney DB, Amin Z, Moore P, Parry AJ, Teitel DF, Hanley FL (2000) Early and intermediate outcomes after repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: experience with 85 patients. Circulation 101:1826–1832
Somerville J (1970) Management of pulmonary atresia. Br Heart J 32:641–651
Tchervenkov CI, Roy N (2000) Congenital Heart Surgery Nomenclature and Database Project: pulmonary atresia—ventricular septal defect. Ann Thorac Surg 69(4 Suppl):S97–105
US Food and Drug Administration website, medwatch section, available from http://www.fda.gov/medwatch/report/DESK/advevnt.htm. Accessed Jan 2011
Valsangiacomo Buechel ER, DiBernardo S, Bauersfeld U, Berger F (2005) Contrast-enhanced magnetic resonance angiography of the great arteries in patients with congenital heart disease: an accurate tool for planning catheter-guided interventions. Int J Cardiovasc Imaging 21:313–322
Yin L, Lu B, Han L, Wu RZ, Johnson L, Xu ZY, Jiang SL, Dai RP (2011) Quantitative analysis of pulmonary artery and pulmonary collaterals in preoperative patients with pulmonary artery atresia using dual-source computed tomography. Eur J Radiol 79:480–485
Acknowledgments
This study was supported by a grant from the Nation Science Council, Taiwan (99-2314-B-002-014-MY3). We also would like to thank Hui-Chi Chen, PhD, post-doc in Genomics Research Center, Academia Sinica, Taipei, Taiwan, for her statistical review and comments.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Accuracy of different CT machines to detect pulmonary arterial morphology compared to the surgical standard (DOC 104 kb)
Rights and permissions
About this article
Cite this article
Lin, MT., Wang, JK., Chen, YS. et al. Detection of pulmonary arterial morphology in tetralogy of Fallot with pulmonary atresia by computed tomography: 12 years of experience. Eur J Pediatr 171, 579–586 (2012). https://doi.org/10.1007/s00431-011-1621-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-011-1621-4