Abstract
Based on previous studies, we had made a try to administer sodium pyruvate to newborn Wistar rats suffering repetitive and profound hypoglycemia, which can induce brain injury. Fluoro-Jade B was used to marked degenerative neurons 1 day after the third hypoglycemic insult, and Morris water navigation task was performed to assess cognitive function when the rats were 6 weeks old. We found that administration of sodium pyruvate to those rats whose hypoglycemia was terminated by dextrose can reduce neurodegeneration induced by hypoglycemia and improve the cognitive function. Supplementing sodium pyruvate with glucose to terminate severe neonatal hypoglycemia is an effective intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuron death induced by hypoglycemia is the result of a series of events triggered by decreasing glucose levels. Once the events are started, restoring blood glucose levels cannot prevent or reverse cell death. Repetitive and profound hypoglycemia can result in cellular energy failure, mitochondrial dysfunction, production of reactive oxygen species, and DNA damage. DNA strand breaks can increase the activity of poly (ADP-ribose) polymerase 1 (PARP-1) up to 500 times of normal [1]. PARP-1, an enzyme that promotes ADP ribosylation with nicotinamide-adenine dinucleotide (NAD+) to form branched ADP-ribose polymers, promotes DNA repair, and prevents chromatid exchange. NAD+ is necessary for the last step in glycolysis involving glyceraldehyde-3-phosphate dehydrogenase. Excessive activation of PARP-1 depletes cytoplasmic NAD+, which prevents cells from using glucose, even if the glucose supply is restored. In turn, this results in cellular energy depletion [5, 14, 17]. Repetitive and profound neonatal hypoglycemia can often cause serious brain damage with irreversible neurological sequelae. Treating hypoglycemia in the clinic usually includes providing intravenous glucose infusions to raise blood sugar levels as soon as possible. However, because depleted intracellular NAD+ prevents cells from using glucose, glucose infusions for repetitive and profound hypoglycemia cannot repair cell damage caused by low blood sugar. Recent studies have also shown that high blood glucose levels after severe hypoglycemia can increase zinc levels and activate neuronal nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, both of which can induce neuronal death. Neuronal cell injury caused by glucose reperfusion is similar to hypoxia reperfusion-induced brain injury [11]. However, infusing pyruvate, glutamine, α ketoglutarate, or other compounds involved in oxidative metabolism can reduce cell death induced by PARP-1 activation because their metabolism do not need cytoplasmic NAD+ [14].
Our recent studies have been focused on successfully establishing a newborn Wistar rat model of brain injury induced by hypoglycemia through repetitive subcutaneous injection of insulin (15 U/kg). We examined and confirmed brain damage in this model by Fluoro-Jade B (FJB) staining, a specific fluorescent marker that stains and localizes degenerative neurons. We confirmed that repetitive and profound neonatal hypoglycemia can result in extensive neurodegeneration, and it appeared that neurons of the cortex, dentate gyrus of the hippocampus, the thalamus, and the hypothalamus are more vulnerable to hypoglycemic insult in newborn rats [18]. Based on the findings of Suh et al. [10] and Lee et al. [6], in this study, we supplied sodium pyruvate to newborn rats with brain injury induced by repetitive and profound insulin-induced hypoglycemia to study if pyruvate can protect the rats against brain injury.
Materials and methods
Thirty-six newborn Wistar rats (purchased from Animal House Center, Medical School of Shandong University, Jinan, China) were used in the experiments. Experimental animals were randomly divided into three groups: insulin-treated rats with prolonged hypoglycemia terminated by dextrose (INS-P; n = 12), insulin-treated rats with prolonged hypoglycemia terminated by dextrose + pyruvate (INS-PP; n = 12), and control rats (CON; n = 12). All the newborn animals were cared for by their own mother rats, one litter per cage. All animals had free access to water and standard animal food, with a 12-h light/dark cycle (lights on between 7:00 and 19:00) and a thermoregulated environment (20°C). The animal care and experimentation were approved by Shandong University School of Medicine Animal Studies Committee. All efforts were made to minimize animal suffering and reduce the number of animals used.
Treatment
INS-P
On postnatal day 2 (P2), P4, and P6, 12 newborn rats received 15 U/kg of insulin lispro (Eli Lilly, IN, USA; hypodermic injection). Blood glucose was measured with a One Touch II blood glucose meter (Lifescan, Inc., AW, USA) before insulin injection, 0.5, 1.5, and 2.5 h after insulin injection. To terminate hypoglycemia after the fourth blood glucose measurement, 0.01 ml of 50% dextrose was given by hypodermic injection and blood glucose was measured again 0.5 h later, and then the animals were returned to their mother rats.
INS-PP
On P2, P4, and P6, 12 newborn rats received 15 U/kg of insulin lispro. Blood glucose was measured before insulin injection, 0.5, 1.5, and 2.5 h after insulin injection. To terminate hypoglycemia after the fourth blood glucose measurement, 0.01 ml of 50% dextrose was given by hypodermic injection, and 30% sodium pyruvate (500 mg/kg, Ganghua, Inc., Beijing, China) was given by peritoneal injection. Blood glucose was measured again 0.5 h later, and then the animals were returned to their mother rats.
CON
On P2, P4, and P6, 12 newborn rats received 0.9% sodium chloride solution instead of insulin. Blood glucose was measured before injection. Then they were returned to their mother rats.
Histology
In INS-P, INS-PP, and CON, six rats of each group were sacrificed respectively to quantify cell death 1 day after the third treatment. These rats were anesthetized with 10% chloral hydrate and intracardiac perfused with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were postfixed (4°C) overnight then cryoprotected in PBS with 30% sucrose over 48 h. Twenty-five-micron coronal sections were prepared with a freezing sliding microtome (LEICA CM1900). The sections were typically mounted on 2% gelatin-coated slides and then air dried on a warmer slide at 50°C for at least half an hour.
Fluoro-Jade B staining
Fluoro-Jade B (FJB, HistoChem, AR, USA) staining was performed as described by Schmued and Hopkins [8]. The slides were first immersed in 100% alcohol for 5 min. This was followed by 2 min in 70% alcohol and 2 min in distilled water. The slides were then transferred to a solution of 0.06% potassium permanganate for 10 min, shaking gently. The slides were then rinsed in distilled water for 2 min, and stained with FJB (from this step on, performed in darkroom). The concentration of staining solution was 0.001%. After 20 min in the staining solution, the slides were rinsed for 1 min in each of three distilled water washes. Excess water was removed by briefly (about 15 s) draining the slides vertically on a paper towel. The slides were then placed on a warmer slide, set at approximately 50°C, until they were fully dry (e.g., 5–10 min). The dry slides were cleared by immersion in xylene for at least a minute before cover slipping with DPX (Sigma Chem. Co., St. Louis MO, USA).
Analysis
FJB-stained sections were then observed and photographed with an Olympus BX51 fluorescence microscope equipped with an Olympus DP71 camera.
Quantify degenerating neurons
We selected one representative section of each brain referring to a rat brain atlas [7]. The fluorescent cells were counted by an observer in a blind manner. FJB positive neurons were visually identified and manually counted in a random 100 × 100 μm square in ×400 campus visuals within rectangular regions (rectangular regions in Fig. 1) of parasagittal cortex and piriform cortex. The dentate gyrus (DG) of hippocampus, thalamus, and hypothalamus within each section were also surveyed for FJB positive neurons. The summation of each encephalic region of six sections of each group was recorded as the number of FJB+ cell of the region.
Morris water maze test
The Morris water navigation task was to detect spatial learning and memory in rats. When the rats were 6 weeks old, six rats in each of the three groups were subjected to the Morris water maze navigation procedure to test animals’ spatial learning and memory. Three days before testing, all rats were kept in the water maze test facility to acclimate them. Rats were kept separately in three groups to reduce possible influence on behavior between rats. Tank diameter was 1.5 m, tank height was 0.5 m, and water depth was 0.3 m. Water temperature was maintained at 24 ± 2°C. The experimental water tank was placed in the same location for all tests to keep surrounding visual clues identical in all tests. To reduce researchers’ influence on experimental animals and to record the trajectory of animals in the experimental tank, a camera was installed approximately 2 m above the experimental tank to monitor animal behavior. All tests were conducted in silence to avoid noise interference. The experimental tank was divided into four quadrants. An 8 × 8-cm platform was placed in the center of the first quadrant at 2 cm depth. During experiments, water surface was covered with a layer of white plastic foam particles to avoid visual disturbance. The study lasted five consecutive days. Video observation was recorded twice every day (in the morning and the afternoon) with a minimum of 3 h between observations. Rats were gently placed into the water in each of the four quadrants maze back-end first to avoid stress and facing the pool-side to avoid bias. We recorded latency—the time from when the animal was placed in the water in each quadrant to the time for each of them to reach the platform. Once rats found the platform, they were allowed to stay on it for 10 s. If rats could not find the platform in 120 s, they would be guided to find it and remain there for 30 s for them to observe the surrounding visual clues to increase their learning. In this case, the time was recorded as 120 s. In 5 days of testing, the first 3 days were used as training time and the results of the fourth day were compared between rat groups. The explorative test was conducted on day 5. In this test, we removed the platform and recorded times for rats to swim to where the original platform had been located, and the time for them to stay in the quadrant where the original platform was in within 1 min.
Data analysis
Data of blood glucose levels, the latency to find the platform, and swimming distance in the Morris water navigation task are presented as mean ± SD. T test and one-factor analysis of variance analyses were performed to compare the difference. A value of p < 0.05 was considered statistically significant.
The numbers of FJB+ cells, the percentage of time that rats remained in the quadrant with the platform, and the times for rats to cross to the original platform are expressed as median and interquartile range. Differences between two groups were assessed by Mann–Whitney test. A value of p < 0.05 was considered statistically significant. Differences between three groups were assessed by Kruskal–Wallis test; Pairwise comparisons were assessed by Mann–Whitney test and Bonferroni correction was used to adjust p values for multiple comparisons.
Results
Blood glucose
Blood glucose levels of INS-P and INS-PP were shown in Table 1. There is no difference between them. The mean blood glucose of 12 measurements from 12 CON rats were 2.86 ± 0.26 mmol/L on P2, 4.09 ± 0.65 mmol/L on P4, and 4.88 ± 0.56 mmol/L on P6. Glucose levels of three groups showed obvious differences (P2: F 2,33 = 345.81; P4: F 2,33 = 284.74; P6: F 2,33 = 569.92; p < 0.01).
Histology
FJB staining of brains of CON did not result in any detectable fluorescence, while FJB+ cells were seen in all brains of INS-P and INS-PP. FJB+ cells were numerous in all sections of brains of INS-P and dispersedly distributed in the sections of brains of INS-PP (Fig. 2). The numbers of each region of the two groups of rats were shown in Table 2. The numbers of FJB+ cells of two groups showed differences.
Fluoro-Jade B staining of brain coronal sections adjacent to the sagittal plane, dentate gyrus of the hippocampus, thalamus, and hypothalamus of rats in the INS-P group and the INS-PP group. A large number of FJB positive cells in all brain regions of rats in the INS-P group were observed. A small number of scattered FJB positive cells were found in rats in the INS-PP group. Par Parasagittal cortex, DG dentate gyrus, Tha thalamus, Hth hypothalamus, Pir piriform cortex (the scale is applicable to all fluorescence photos)
Morris water maze test
Rats in the three groups had good mobility during the water maze test and no significant movement disorders were observed.
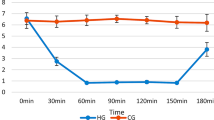
-
1.
Changes of the latency for rats of three groups to find the platform. During the water navigation task, the operating capacity of rats in the three groups was significantly enhanced. We observed that there was a trend toward reduced latency—the time for rats to find the platform. On the third day of the test, the latency was stable (Fig. 3). On the fourth day of the test, we found significant differences in latency between the three groups (F 2,141 = 34.82, p < 0.01). There was a significant difference in latency between the INS-P group and the INS-PP group (t = 5.72, p < 0.01) or CON group (t = 6.98, p < 0.01). There was no significant difference in latency between the INS-PP group and the CON group (t = 1.59, p = 0.06).
-
2.
Comparison of rats’ swimming distance in the Morris water navigation task. There were significant differences between the rat groups in swimming distances from the point where they entered water to the platform (F 2,141 = 37.53, p < 0.01). Swimming distances of rats in the INS-P group were significantly longer than those of rats in the INS-PP group (t = 7.44, p < 0.01) and the CON group (t = 6.55, p < 0.01). We observed no significant differences in swimming distances between the INS-PP group and the CON group (t = 0.87, p = 0.19; Fig. 4).
-
3.
Comparison of rat behavior in Morris water navigation task. Swimming mobility of rats in the three groups was good once they were in the water, and rats’ search and exploration behaviors were observed. However, the performances of rats in three groups were significantly different. First, the time rats remained in the quadrant with the platform was different in the groups (Table 3). For rats in the INS-P group, the time remaining in the quadrant was significantly shorter than that of rats in the INS-PP group (Mann–Whitney U = 3.50, p = 0.015) or the CON group (Mann–Whitney U = 3.63, p = 0.016). However, there was no significant difference between the INS-PP group and the CON group for this variable (Mann–Whitney U = 16.50, p = 0.82). Bonferroni correction was used to adjust p values for multiple comparisons (p = 0.05/3 = 0.017. A value of p < 0.017 was considered statistically significant).
Second, there were significant differences in the times for rats to cross to the original platform (Table 4). Times for rats in the INS-P group was significantly less than times for rats in the INS-PP group (Mann–Whitney U = 3.50, p = 0.015) or the CON group (Mann–Whitney U = 3.27, p = 0.013). However, there was no significant differences between the INS-PP group and the CON group for this variable (Mann–Whitney U = 17.00, p = 0.973). Bonferroni correction was used to adjust p values for multiple comparisons (p = 0.05/3 = 0.017. A value of p < 0.017 was considered statistically significant).
Discussion
Glucose is the main energy substrate of brain. After glucose enters brain tissues, it can be directly used by neurons and glial cells. Alternatively, glucose can also be metabolized to lactic acid by glial cells, and neurons can then oxidatively metabolize the produced lactic acid [2, 3, 12]. Although the major metabolic substrate of brain is glucose, fetal and newborn brains can also use ketone, lactic acid, and other metabolic substrates for energy. These substrates can be metabolized into pyruvate in the cytoplasm with NAD+, and then enter the Krebs or citric acid cycle to provide energy. However, when PARP-1 is overactivated as in animals with profound hypoglycemia, these substrates are unable to provide energy.
Previous studies have shown that neuronal death is not directly or immediately caused by low levels of blood glucose, but rather from a cascade of reactions due to insufficient energy supplies. Among these reactions, glutamate release [4], zinc translocation [9], and PARP-1 activation [13] has been identified as the key event of cell death. Activated PARP-1 consumes cytosolic NAD+, and the depletion of intracellular NAD+ can inhibit glucose metabolism. In cell culture, after PARP-1 activation, supplementing the medium with NAD+ can prevent cell death [15, 16]. Similarly, supplementing the media with substrates that do not need NAD+ for energy metabolism, can also provide energy for cells and avoid cell death [14]. Pyruvate can be transferred across the blood–brain barrier in a facilitated way and increase blood and brain pyruvate concentrations. In this study, we used sodium pyruvate (500 mg/kg) as a substrate, which could increase the concentration of blood pyruvate to 5 mmol/L, which is 100 times of the normal blood pyruvate concentration [11]. In one study, investigators infused a similar dose of pyruvate as we used here, which significantly reduced damage of hippocampal neurons caused by hypoxia in rats [6].
In this study, FJB staining of coronal brain tissue sections showed that the number of degenerated neurons of different brain areas in rats of the INS-PP group was less than that of the INS-P group. It was confirmed that providing pyruvate at the same time that glucose is given to terminate low blood sugar levels promoted neuron survival. However, when glucose alone was given, neuronal death could not be stopped. This indicates that pyruvate can protect neurons with brain injury caused by severe hypoglycemia. This neuroprotective effect indicates that low blood sugar levels can cause sustained disruption of glucose metabolism. Even if the supply of glucose is restored, neurons cannot effectively use glucose.
Of all the brain areas that are readily injured by repeated and severe neonatal hypoglycemia, the dentate gyrus, part of the hippocampal formation, is central to learning and memory. Therefore, evaluating cognitive function is an important addition for studies of animal models with brain injury, and the Morris water navigation task is a standard method of assessing learning and spatial memory capabilities. In our experiments, we found that rats of the INS-P group had significantly longer swim distances and therefore latency to locate the platform than rats in other groups. No significant differences in latency was observed between the INS-PP group and the CON group, indicating that rats in the INS-P group had a significantly decreased learning ability, and that rats in the INS-PP group and the CON groups had similar normal learning abilities. In the pre-test period of the Morris task, compared with CON and INS-PP group rats, those in the INS-P group had significantly shorter staying time in the quadrant with the platform and less times crossing the location of the original platform. This suggested that INS-P rats had decreased spatial memory ability. In contrast, the learning and memory abilities of rats in the INS-PP showed no apparent reduction.
In our study, newborn rats with brain injury caused by repetitive and profound insulin-induced hypoglycemia had reduced cognitive abilities and a poor performance in the Morris navigation task. However, rats provided with sodium pyruvate showed comparable cognitive abilities to the normal controls. Fluoro-Jade B dye staining of brains damaged by low blood sugar levels confirmed that supplementing pyruvate at the same time that glucose is given can decrease degenerative neurons. The cognitive abilities of 6 weeks old rats with neonatal hypoglycemia can also be significantly improved. This indicates sodium pyruvate can protect neuron structure and function in newborn rats with brain injury induced by repetitive and profound insulin-induced hypoglycemia. In addition, sodium pyruvate is inexpensive and readily available and has no toxic side effects. In conclusion, in our Wistar rat model, we have demonstrated that supplementing sodium pyruvate with glucose to newborn animals with severe hypoglycemia is an effective and safe intervention. Such an intervention may be useful to improve outcomes in newborn patients with severe hypoglycemia and may have a good prospect in clinic.
Abbreviations
- CON:
-
Control rats
- FJB:
-
Fluoro-Jade B
- INS-P:
-
Insulin-treated rats with prolonged hypoglycemia terminated by dextrose
- INS-PP:
-
Insulin-treated rats with prolonged hypoglycemia terminated by dextrose + pyruvate
- NAD+ :
-
Oxidized form of nicotinamide-adenine dinucleotide
- P:
-
Postnatal day
- PARP-1:
-
Poly (ADP-ribose) polymerase-1
- PBS:
-
Phosphate-buffered saline
References
D’Amours D, Desnoyers S, D’Silva I, Poirier GG (1999) Poly (ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J 342:249–268
Dienel GA, Cruz NF (2004) Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int 45:321–351
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Engelsen B, Westerberg E, Fonnum F, Wieloch T (1986) Effect of insulin-induced hypoglycemia on the concentrations of glutamate and related amino acids and energy metabolites in the intact and decorticated rat neostriatum. J Neurochem 47:1634–1641
Ha HC, Snyder SH (1999) Poly (ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A 96:13978–13982
Lee JY, Kim YH, Koh JY (2001) Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci 21(20):RC171
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic, San Diego
Schmued LC, Hopkins KJ (2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874:123–130
Suh SW, Garnier P, Aoyama K, Chen Y, Swanson RA (2004) Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol Dis 16:538–545
Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA (2005) Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes 54:1452–1458
Suh SW, Gum ET, Hamby AM (2007) Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 117(4):910–918
Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR (2000) Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci 20:6804–6810
Wieloch T (1985) Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science 230:681–683
Ying W, Chen Y, Alano CC, Swanson RA (2002) Tricarboxylic acid cycle substrates prevent PARP-mediated death of neurons and astrocytes. J Cereb Blood Flow Metab 22:774–779
Ying W, Garnier P, Swanson RA (2003) NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun 308:809–813
Ying W, Alano CC, Garnier P, Swanson RA (2005) NAD+ as a metabolic link between DNA damage and cell death. J Neurosci Res 79:216–223
Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL (2002) Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297:259–263
Zhou D, Qian J, Liu CX, Chang H, Sun RP (2008) Repetitive and profound insulin-induced hypoglycemia results in brain damage in newborn rats: an approach to establish an animal model of brain injury induced by neonatal hypoglycemia. Eur J Pediatr 167(10):1169–1174
Acknowledgments
The authors gratefully acknowledge Dr. Li Fuhai, Dr. Xu Guangrun, and Dr. Chen Teng for their scientific expertise and Dr. Liu Chun-xi for laboratory investigations and technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
No organization sponsored our research.
In this experiment the animal care and experimental protocol were approved by The Chinese Academy of Sciences. All efforts were made to minimize animal suffering and reduce the number of animals used. There has been no duplicate publication or submission elsewhere of this work. All authors have read and approved the manuscript, are aware of the submission for publication, and agree to be listed as co-authors.
Rights and permissions
About this article
Cite this article
Zhou, D., Qian, J., Chang, H. et al. Pyruvate administered to newborn rats with insulin-induced hypoglycemic brain injury reduces neuronal death and cognitive impairment. Eur J Pediatr 171, 103–109 (2012). https://doi.org/10.1007/s00431-011-1489-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-011-1489-3