Abstract
Background
Besides profound hypoglycemia with hyperlacticemia, glycogen storage disease type Ia (GSD Ia) presents hypertriglyceridemia that is often resistant to dietary treatment with cornstarch. The present study aimed to evaluate the effects of medium-chain triglycerides (MCT)—which are absorbed via the portal vein without being incorporated into chylomicrons—on hypertriglyceridemia and to explore otherwise metabolic changes in children with GSD Ia.
Patients and methods
A 13-year-old boy with GSD Ia who received a dietary treatment with MCT milk after cornstarch administration and two infants also with GSD Ia, ages 6 and 7 months, who received MCT milk after carbohydrate-rich, lipid-poor milk were enrolled. In addition to serum glucose and lactate levels, serum levels of total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol were serially determined. Simultaneously, serum levels of total carnitine, free carnitine, acylcarnitine, and ketone bodies were determined to evaluate fatty acid beta-oxidation.
Results
Mean glucose level (mmol/l) of patient 1 remained stable, the value being around 4.5, while those of patients 2 and 3 increased to this level from 4.00 and 3.72, respectively. Lactate levels were significantly decreased in all patients. Mean triglyceride levels (mM) of patient 1 decreased from 3.00 to 2.05. Also, triglyceride levels of patients 2 and 3 decreased from 2.74 and 3.15 to 2.13 and 2.70, respectively. HDL cholesterol, acylcarnitine, and ketone body levels increased in all patients after MCT administration, while total and free carnitine levels decreased.
Conclusion
We describe here the beneficial effects on lipid and carbohydrate metabolisms in three Japanese children with GSD Ia. In light of the unfavorable influence of lipid restriction on growth and development in infancy, dietary treatment with MCT milk may be a better treatment for infants with GSD Ia. Further investigation should be required to confirm the efficacy of MCT milk in GSD Ia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycogen storage disease type Ia (GSD Ia), also referred to as von Gierke disease (OMIM: +232200), is a congenital carbohydrate disorder caused by glucose-6-phosphatase (G6Pase) deficiency (EC 3, 1, 3, 9) [4, 9]. G6Pase deficiency is associated with glycolysis and hence production of acetyl coenzyme A (CoA) (Fig. 1). This leads, directly or indirectly, possibly through signaling actions of G6Pase, to stimulation of de novo lipogenesis and cholesterogenesis but also to inhibitions of both liver fatty acid oxidation and ketogenesis by promoting production of malonyl-CoA, which blocks carnitine palmitoyltransferase I (CPT-I) (Fig. 1) [2–4, 8, 13, 14].
Carbohydrate and lipid metabolisms in glycogen storage disease type Ia. (1) Transport system of long-chain fatty acids dependent of the carnitine system. (2) Transportation of medium- and short-chain fatty acids independent of the carnitine system. G6Pase glucose-6-phosphatase, CPT-I carnitine palmitoyltransferase-I, CPT-II carnitine palmitoyltransferase II
Accordingly, besides profound hypoglycemia together with hyperlacticemia, patients with GSD Ia often present with prominent hypertriglyceridemia [2, 4, 8, 13]. A considerable suppression of liver fatty acid oxidation in these patients has also been documented [3, 4, 7, 12, 13]. Dietary treatment of GSD Ia with carbohydrate-rich, lipid-poor meals such as cornstarch has been shown to prevent hypoglycemia and to lower plasma lactate levels, but hypertriglyceridemia is often resistant to dietary treatment [2, 4, 8, 13].
Medium-chain triglycerides (MCT) are absorbed via the portal vein without being incorporated into chylomicrons, and in turn, the released medium-chain free fatty acids are subjected to liver fatty acid oxidation without the carnitine system, which is essential to beta- oxidation of long-chain fatty acids (Fig. 1) [15]. MCT milk has been used to treat children with profound hypertriglyceridemia and has often been shown to dramatically lower serum triglyceride levels [5, 10]. Furthermore, MCT has been administered for fatty acid beta-oxidation disorders [15]. Nevertheless, the efficacy of MCT milk in GSD Ia has never been investigated sufficiently.
In this report, we document three Japanese children with GSD Ia who were given treatment with MCT milk. The feasibility of MCT milk in the clinical management of GSD Ia is discussed.
Subjects and methods
Subjects
Patient 1
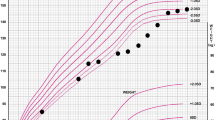
A boy, now age 13 years, was born to healthy parents with no consanguinity as a first child at 39 weeks of gestational age weighing 2,794 g. There was no family history of liver or metabolic disease and no episode during the first year of his life. At the age of 1 year 1 month, he presented with pyrexia, cough, and nasal discharge and visited a neighboring hospital. He was then found to have prominent hepatomegaly palpable at 10 cm below the right costal margin but not splenomegaly, and he was referred to our hospital to receive a precise examination. Biochemical tests on admission showed moderate increases in transaminase [alanine amino transferase (ALT) 147 U/l, aspartate amino transferase (AST) 150 IU/l], fasting hypoglycemia (fasting glucose level, 3.11 mmol/l) with a concomitant striking increase in blood lactate level (6 mmol/l), and prominent hypertriglyceridemia without hypercholesterolemia (triglycerides 7.41 mM, total cholesterol 4.65 mM). Two months later, needle liver biopsy was performed. Liver histology proved glycogen accumulation in liver cells, and enzyme examination revealed that G6Pase activity of the liver was strongly reduced. Under the diagnosis of GSD Ia, he began to receive dietary treatment with uncooked cornstarch. Since then, his fasting blood glucose level has been maintained at between 4.05 and 4.66 mmol/l, and his lactate and triglycerides showed lower levels (lactate 1.5–3.2 mmol/l, triglycerides 3.16–3.74 mM). His liver dysfunction also improved substantially within a few months of the initiation of cornstarch. The gene analysis at the age of 6 years revealed that he is homozygous for G727T mutation in the G6Pase gene, which is highly prevalent in Japanese patients [1, 9]. His growth and development were normal (Fig. 2). From the age of 12 years, 200 mg/day of bezafibrate, a fibric acid, was administered for his hypertriglyceridemia, but the effect was not sufficient. At the age of 13 years 1 month, the cornstarch was replaced by MCT milk with a high sugar content, designated as carbohydrate-rich MCT milk (Table 1).
Patient 2
A boy, now age 1.5 years, was born to healthy parents with no consanguinity at 40 weeks of gestational age weighing 3,010 g. His older sister, now age 6 years, presented with convulsions based on hypoglycemia at the age of 6 months and was diagnosed as GSD Ia by liver G6Pase activity and genetic analysis. Until the age of 4 months, the boy was fed with breast milk seven to eight times daily. At the age of 5 months, following a request by his parents, he underwent a thorough examination for GSD. At the time of examination, his liver was palpable at 6 cm below the costal margin, but his spleen was not palpable. In addition, hypoglycemia in an absence of ketosis, hyperlacticemia, and hypertriglyceridemia were noted. Soon after, the boy was frequently fed carbohydrate-rich, lipid-poor milk under the suspicion of GSD Ia. His fasting glucose level was maintained at between 3.33 and 4.44 mmol/l, whereas his lactate and triglyceride levels decreased substantially but remained high. Beginning at the age of 7 months, carbohydrate-rich, lipid-poor milk was replaced by carbohydrate-rich MCT milk (Table 1). The patient’s height and body weight standard deviation (SD) scores at present are −0.2 SD and −0.1 SD, respectively, indicating normal growth. Gene analysis revealed that he is homozygous for G727T mutation, as is his older sister.
Patient 3
A boy, now age 2 years 2 months, was born to healthy parents with no consanguinity at 37 weeks of gestational age weighing 2,550 g. His two older brothers are entirely healthy. There was no episode during the newborn period. At the age of 5 months, the boy developed general tonic and clonic convulsions and subsequent unconsciousness. He was transferred to our hospital and was then found to have profound hypoglycemia (0.56 mmol/l) without an elevation of blood ketone level, severe hyperlacticemia, and hypertriglyceridemia. In addition, prominent hepatomegaly (palpable 8 cm below the costal margin) without splenomegaly was noted. Glucose was administered intravenously, and the patient recovered from unconsciousness. Liver specimen obtained by needle biopsy at the age of 6 months showed glycogen accumulation in liver cells. Thereafter, the boy was frequently fed carbohydrate-rich, lipid-poor milk. Fasting glucose level was maintained at between 3.33 and 4.56 mmol/l, while lactate and triglyceride levels decreased substantially but remained high. Beginning at the age of 8 months, carbohydrate-rich, lipid-poor milk was replaced by carbohydrate-rich MCT milk (Table 1). His weight and height scores at present are −0.7 SD and −0.2 SD, respectively, indicating normal growth. Recent gene analysis revealed that he is homozygous for G727T mutation.
Study design
Carbohydrate, lipoprotein, and fatty acid metabolism in patient 1 under the dietary treatment with carbohydrate-rich MCT milk were examined and were compared with those under the treatment with uncooked cornstarch. His daily energy intake corresponded to the requirement for his age. The energy intake from lipids in the former treatment was increased approximately 5% compared with the latter treatment, while the energy intake from carbohydrate was decreased approximately 5% (Table 1).
For patients 2 and 3, carbohydrate, lipoprotein, and fatty acid metabolism under treatment with carbohydrate-rich MCT milk and under treatment with carbohydrate-rich, lipid-poor milk were serially examined. Their daily energy intakes also corresponded to the requirement for their ages. In comparison with these dietary treatments, the energy intake from lipids in the former treatment was 5% higher than that in the latter treatment, while the energy intake from carbohydrate was 5% lower (Table 1).
Blood samples were collected from a vein directly before breakfast (7:00 A.M.) as follows: patient 1, about 5.5 h after the intake of 2.0 g/kg (8 Kcal/kg) of uncooked cornstarch or 1.7 g/kg (8 Kcal/kg) of carbohydrate-rich MCT milk; patients 2 and 3, 5 h after the intake of 1.7 g/kg of carbohydrate-rich MCT milk or 1.87 g/kg (8 Kcal/kg) of carbohydrate-rich, lipid-poor milk (Table 1).
Serum levels of glucose, lactate, and insulin were determined chronologically. To evaluate lipoprotein metabolism, serum levels of total cholesterol, triglycerides, and HDL cholesterol were determined. To assess fatty acid oxidation, serum levels of free fatty acids, ketone bodies, total carnitine, and free carnitine were determined. Concomitantly, the acylcarnitine profile was examined. Age-matched control levels for patient 1 were obtained from 225 boys aged 13 years, and those for patients 2 and 3 were obtained from seven boys and six girls aged 6–10 months.
Biochemical assays
Serum insulin level was determined by an enzyme immunoassay using a commercial kit (TOSOH-II, Tosoh Co, Ltd., Tokyo, Japan). Serum levels of total cholesterol and triglycerides were determined by enzymatic methods using commercial kits (Kyowa Medex Co. Ltd., Tokyo, Japan). Serum levels of free fatty acids and total ketone bodies were measured by enzymatic methods using the NEFA-SS kit EIKEN (Eiken Chemicals Co. Ltd., Tokyo, Japan) and the total ketone body kit (Kainos Laboratories Inc., Tokyo, Japan), respectively. Serum total carnitine and acylcarnitine levels were determined by an enzymatic cycling method described by Takahashi et al. [18]. Acylcarnitine profiles were examined by tandem mass spectrometry according to a method described by Shigematsu et al. [16].
Statistic analysis
Comparisons of values of age-matched controls and those of patients were performed with the Mann–Whitney U test, and significances in changes in patients’ data were assessed by the two-factor analysis of variance (ANOVA) test. Any p < 0.05 was considered significant.
Results
Mean values of the markers for carbohydrate and lipoprotein metabolism and fatty acid oxidation are shown in Fig. 3.
Blood lactate level of patient 1 under cornstarch administration was 2.56 ± 0.39 mmol/l (mean±SD), higher than the normal range (0.7–1.2 mmol/l). This level was decreased to 2.00 ± 0.30 mmol/l after the initiation of dietary treatment with MCT (p < 0.01), while the glucose level remained unchanged (4.44 ± 0.37 under cornstarch vs. 4.5 ± 0.41 mmol/l under MCT milk). Lactate levels of patients 2 and 3 before MCT treatment were 2.55 ± 0.45 and 2.67 ± 0.52 mmol/l, respectively, and decreased to 1.89 ± 0.24 and 2.00 ± 0.15 mmol/l (p < 0.05), respectively. Their glucose levels before MCT treatment were significantly lower than those of age-matched healthy controls: 3.90 ± 0.20 for patient 2 and 3.71 ± 0.31 mmol/l for patient 3 vs. 4.34 ± 0.35 mmol/l for age-matched controls (p < 0.05). After initiation of MCT milk, their glucose levels increased to 4.50 ± 0.54 and 4.33 ± 0.42 mmol/l, respectively (p < 0.01) (Fig. 3a). The insulin/glucose ratio increased in all patients after initiation of MCT treatment: patient 1, 0.013 ± 0.003 to 0.016 ± 0.003 (p < 0.01); patient 2, 0.010 ± 0.002 to 0.013 ± 0.002 (p < 0.05); patient 3, 0.011 ± 0.002 to 0.013 ± 0.002 (p < 0.05) (Fig. 3b).
Triglyceride level of patient 1 under cornstarch administration was 3.00 ± 0.27 mmol/l, which was high compared with age-matched healthy controls (0.93 ± 0.27 mmol/l). The level after initiation of MCT milk was 2.05 ± 0.27 mmol/l, showing significant decrease, with p value <0.001. Triglyceride levels of patients 2 and 3 under carbohydrate-rich, lipid-poor milk administration were also high, the values being 2.74 ± 0.21 and 3.62 ± 0.35 mmol/l, respectively. After initiation of MCT milk, these levels returned to 2.13 ± 0.2 and 2.70 ± 0.33 mmol/l, respectively, showing significant decrease, with p values <0.001 (Fig. 3c).
Total cholesterol level of patient 1 never differed with dietary treatment (4.68 ± 0.22 for cornstarch and 4.34 ± 0.25 mmol/l for MCT treatment). Those of patients 2 and 3 under carbohydrate-rich, lipid-poor milk were 4.69 ± 0.42 and 6.00 ± 0.52 mmol/l, respectively, and these levels decreased to 4.16 ± 0.22 and 5.12 ± 0.65 mmol/l, respectively. These changes were statistically significant, with p values <0.05.
HDL cholesterol level of patient 1 under treatment with MCT milk was significantly higher than during cornstarch therapy (0.91 ± 0.12 vs. 0.67 ± 0.09 mmol/l, p < 0.01). HDL cholesterol levels of patients 2 and 3 under MCT milk treatment were also high compared with those under carbohydrate-rich, lipid-poor milk (patient 2: 0.62 ± 0.06 vs. 0.85 ± 0.09, p < 0.01; patient 3: 0.52 ± 0.06 vs. 0.72 ± 0.08 mmol/l, p < 0.01) (Fig. 3c).
Administration of MCT milk resulted in significant increases in serum free fatty acids levels (μmol/l) of patients 1 and 2 but not of patient 3: patient 1, 312 ± 49 to 382 ± 43, p < 0.05; patient 2, 414 ± 42 to 504 ± 61, p < 0.05; patient 3, 483 ± 43 to 484 ± 61, p > 0.1). Ketone body levels (μmol/l) of patients 1, 2, and 3 were 131 ± 31, 111 ± 25 and 171, respectively, and these values were lower than those of healthy controls (240 ± 40 for healthy boys aged 13 years; 454 ± 79 for healthy infants aged 4–6 months). Their ketone body levels under MCT milk administration increased to 283 ± 55, 400 ± 66, and 451 ± 69 mmol/l, respectively, indicating substantial increases (p < 0.001) (Fig. 3d).
Total carnitine levels (normal range, 44–70 mg/dl) increased in all patients before MCT treatment: patient 1, 107 ± 11 mg/dl; patient 2, 107 ± 9 mg/dl; patient 3, 99 ± 5 mg/dl. Those under MCT treatment were 84 ± 10 mg/dl for patient 1, 84 ± 8 mg/dl for patient 2, and 77 ± 8 mg/dl for patient 3—lower than before initiation of MCT (p < 0.01). Inversely, acylcarnitine levels significantly increased: patient 1, 12 ± 3 mg/dl to 23 ± 4 mg/dl, p < 0.01; patient 2, 9 ± 1 to 23 ± 5 mg/dl, p < 0.01; patient 3, 10 ± 2 to 25 ± 5 mg/dl, p < 0.001 (Fig. 3e). Tandem mass spectrometry revealed that actetoacetyl carnitine and butyryl carnitines accounted for most of the increased acylcarnitine.
Discussion
Hypoglycemia is often a life-threatening problem in patients with GSD Ia [4, 13]. Furthermore, it has been postulated that hypoglycemia accounts for most of the hypertriglyceridemia and hyperlacticemia [2, 4, 8, 13]. From this context, nutritional management of this disorder has been based on high-carbohydrate diets, such as starches, which counteract the rapidly falling blood glucose level [2, 4, 13]. However, hypertriglyceridemia often remains unresolved and may lead to pancreatitis [2, 4, 8, 13].
Along with carbohydrates, fatty acids are also important fuels for humans, as demonstrated by congenital fatty acid oxidation disorders that cause hypoglycemia, which in some cases may be profound [6, 11, 15]. A few precedent reports have described impairment of fatty acid beta-oxidation in patients with GSD Ia [3 4, 7, 13]. It has been postulated that an overproduction of malonyl-CoA resulting from promoted hepatic glycolysis inhibits the activity of CPT-I, a key enzyme for beta-oxidation of long-chain fatty acids [7, 14, 15].
Unlike long-chain triglycerides, MCT is not incorporated into chylomicrons, and the released medium-chain fatty acids enter into mitochondria without the carnitine system that is essential for long-chain fatty acids to pass the mitochondrial membrane [7, 14, 15]. MCT milk has often been used to treat children with profound hypertriglyceridemia and was shown to effectively lower triglyceride levels [5, 10]. This milk has also been used to treat patients with fatty acid oxidation disorders [15]. However, there is no report in the literature describing the efficacy of MCT in GSD Ia. This report is the first.
The present study showed that despite the increase in daily lipid intake at the expense of carbohydrate, MCT milk exerted the effects of lowering blood triglyceride levels and raising HDL cholesterol levels in all patients. Considering that hypertriglyceridemia in GSD Ia is often resistant to various treatments, MCT might be used to treat hypertriglyceridemia in GSD Ia.
Despite the decreased intake of carbohydrate, a substantial increase in glucose level with a substantial decrease in lactate level was observed in patients 2 and 3, and these results allowed us to assume that MCT milk counteracted falling blood glucose levels and suppressed glycolysis. The lactate level of patient 1 also decreased after initiation of MCT milk, suggesting suppressed glycolysis, although the concurrent glucose level remained unchanged.
Marked increases in serum total and free carnitine levels without a substantial increase in serum acylcarnitine levels, characteristic findings of CPT-I deficiency that is a disorder of fatty acid beta-oxidation, were observed in all patients before initiation of MCT milk [6, 11, 15]. Furthermore, the patients’ blood ketone body levels at the fasting state were low compared with those of age-matched controls, consistent with the findings of disturbed fatty acid oxidation [6, 11, 15]. Accordingly, it is likely that these patients have considerable impairments in fatty acid oxidation. Alternatively, low ketone levels might reflect good metabolic compensation.
MCT milk produced a considerable rise in blood ketone levels, reflecting the promoted beta-oxidation of medium-chain fatty acids [6, 11, 15]. Simultaneously, the serum acylcarnitine level increased while the serum-free carnitine level decreased. Acylcarnitine analysis by tandem mass spectrometry revealed that the increases in acetoacetyl carnitine and butyryl carnitine, which are formed from ketone bodies, accounted for most of the increase acylcarnitine (data not shown), thus confirming the ability of MCT milk to increase ketogenesis.
It has been shown that an increase in serum acylcarnitine level suppresses renal carnitine uptake [17]. The decreases in total and free carnitine levels after initiation of MCT milk were possibly explained by this notion. Alternatively, decrease of malonyl-CoA production resulting from suppression of glycolysis by MCT milk and the resultant recovery of the carnitine system led to decreases in total and free carnitine levels (Fig. 1). Promoted glycolysis and the concomitant increase of malonyl-CoA production in the liver leads to promoted production of triglycerides (Fig. 1). We speculate that suppression of glycolysis after initiation of MCT contributed, at least in part, to improvement of hypertriglyceridemia.
The insulin/glucose ratio was significantly increased after initiation of MCT milk in all patients, suggesting increases in their insulin secretions. Insulin exerts many metabolic effects, such as a stimulatory effect on activity of lipoprotein lipase that hydrolyze triglycerides on chylomicrons and very low-density lipoprotein [19]. Therefore, increased insulin might also participate in the metabolic changes that occurred in our patients.
The present study showed that MCT milk exerts the effects to lower blood triglyceride levels and to raise HDL cholesterol levels in our patients. All patients were homozygous for G747T mutation, which is highly prevalent among Japanese patients and is associated with a somewhat milder phenotype [1, 9]. Therefore, whether the results obtained from our patients could be applied to other patients with different mutations must be explored.
Abbreviations
- GSD Ia:
-
glycogen storage disease type Ia
- G6Pase:
-
glucose-6-phosphatase
- MCT:
-
medium-chain triglyceride
- AST:
-
aspartate aminotransferase
- ALT:
-
alanine aminotransferase
- HDL:
-
high-density lipoprotein
References
Akanuma J, Nishigaki T, Fujii K, Matsubara Y, Inui K, Takahashi K, Kure S, Ohura T, Miyabayashi S, Ogawa E, Iinuma K, Okada S, Narisawa K (2000) Glycogen storage disease type Ia: molecular diagnosis of 51 Japanese patients and characterization of splicing mutations by analysis of ectopically transcribed mRNA from lymphoblastoid cells. Am J Med Genet 81:107–111
Bandsma RH, Smith GP, Kuipers F (2002) Disturbed lipid metabolism in glycogen storage disease type I. Eur J Pediatr 161:S65–S69
Binkiewics A, Senior B (1973) Decreased ketogenesis in von Gierke’s disease. J Pediatr 83:973–978
Chen YT (2001) Glycogen storage diseases. In: Scriver CR, Baudet AL, Valle D, Sly WS . The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill. New York, p. 1521–1551
Feoli-Fonseca JC, Levy E, Godard M, Lambert M (1998) Familial lipoprotein lipase deficiency in infancy: clinical, biochemical, and molecular study. J Pediatr 133:417–423
Fingerhut R, Roschinger W, Muntau AC, Dame T, Kreischer J, Arnecke R, Superti-Furga A, Troxler H, Liebl B, Olgemoller B, Roscher AA (2001) Hepatic carnitine palmitoyltransferase I deficiency: acylcarnitine profiles in blood spots are highly specific. Clin Chem 47:1763–1768
Foster DW (2004) The role of the carnitine system. Ann NY Acad Sci 6:402–413
Greene HL, Swift LL, Knapp HR (1991) Hyperlipidemia and fatty acid composition in patients treated for type IA glycogen storage disease. J Pediatr 119:398–403
Matern D, Seydewitz HH, Bali D, Lang C, Chen YT (2002) Glycogen storage disease type I: diagnosis and phenotype/genotype correlation. Eur J Pediatr 161:S10–S19
Nagasaka H, Kikuta H, Chiba H, Murano T, Harashima H, Ohtake A, Senzaki H, Sasaki N, Inoue I, Katayama S, Shirai K, Kobayashi K (2003) Two cases with transient lipoprotein lipase (LPL) activity impairment: evidence for the possible involvement of an LPL inhibitor. Eur J Pediatr 162:132–138
Olpin SE (2004) Implications of impaired ketogenesis in fatty acid oxidation disorders. Prostaglandins Leukot Essent Fatty Acids 70:293–308 Review
Pettersen JE, Winsnes A (1981) Dicarboxylic aciduria during during ketotic phases in various type of glycogen storage disease. Acta Pediatr Scand 70:309–313
Rake JP, Visser G, Labrune P, Leonard JV, Ullrick K, Smith GP (2002) Glycoge storage disease type I: diagnosis, management, clinical course and outcome. Results of the European study on glycogen storege disease type I (ESGSD I). Eur J Pediatr 161:S20–S34
Rasmussen BB, Holmback UC, Volpi E, Morio-Liondore B, Paddon-Jones D, Wolfe RR (2002) Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest 110:1687–1693
Roe CR, Ding JH (2001) Mitochondria fatty acid oxidation disorders. In: Scriver CR, Baudet AL, Valle D, Sly WS. The metabolic and molecular bases of inherited disease, 8th edn. NMcGraw-Hill, New York, p 2297–2326
Shigematsu Y, Hata I, Kikawa Y, Mayumi M, Tanaka Y, Sudo M, Kado N (1999) Modifications in electrospray tandem mass spectrometry for a neonatal-screening pilot study in Japan. J Chromatgr B Biomed Sci Appl 731:97–103
Stanley CA, Berry GT, Bennett MJ, Willi SM, Treem WR, Hale DE (1993) Renal handling of carnitine in secondary carnitine deficiency disorders. Pediatr Res 34:849–897
Takahashi M, Ueda S, Misaki H, Sugiyama N, Matsumoto K, Matsuno N, Murao S (1994) Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem 40:817–821
Teruel T, Hernandez R, Rial E, Martin-Hidalgo A, Lorenzo M (2005) Rosiglitazone up-regulates lipoprotein lipase, hormone-sensitive lipase and uncoupling protein-1, and down -regulates insulin-induced fatty acid synthetase gene expression in brown adipocytes of Wistar rats. Diabetologia 48:1180–1188
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagasaka, H., Hirano, Ki., Ohtake, A. et al. Improvements of hypertriglyceridemia and hyperlacticemia in Japanese children with glycogen storage disease type Ia by medium-chain triglyceride milk. Eur J Pediatr 166, 1009–1016 (2007). https://doi.org/10.1007/s00431-006-0372-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0372-0