Abstract
Introduction
Nephrotic children are prone to develop thromboembolic complications secondary to an acquired hypercoagulable state. Cerebral sinovenous thrombosis (CSVT) is increasingly recognised in this population, but clinical characteristics and outcome are not well documented.
Patients and methods
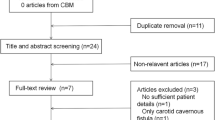
The database of the Canadian Pediatric Ischemic Stroke Registry (Toronto Site) containing prospectively enrolled children from 1992–2004 with CSVT identified four children with NS. A pooled literature analysis retrieved 17 additional cases reports.
Results
CSVT presented in the majority of cases during the first flare or within 6 months after the onset of NS and was found to occur more often in SSNS/SDNS (n=13) than in SRNS (n=4). Clinical manifestations were non-specific and consisted primarily of seizures (n=8) and signs of raised intracranial pressure (n=16). Imaging studies revealed a predilection for superior sagittal sinus involvement (n=21) and rare parenchymal lesions (n=4). The most consistent biological risk factors were a severe hypoalbuminaemia (n=14) and, to a lesser extent, decreased antithrombin (AT) levels (n=9/16). Deficiency of other coagulation inhibitors (protein S, protein C) was not identified. Inherited thrombophilia was documented in a single case, suggesting that acquired, more than genetic, coagulation factors are involved. Anticoagulation was safe, and the outcome was good in most patients, and no recurrence of thrombotic event was reported.
Discussion
In conclusion, CSVT is now a well-described complication of NS with potential morbidity. A high index of suspicion is required, especially in young children with NS presenting neurological symptoms. Reliable biological predictors of CSVT are lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotic syndrome (NS) is a common renal disorder in childhood characterised by severe proteinuria, hypoalbuminaemia and oedema. The majority of cases are attributable to minimal change glomerulopathy and are referred as idiopathic nephrotic syndrome (INS). Less frequently in children, NS is secondary to a variety of systemic disorders [13].
NS is associated with a hypercoagulable state and thrombosis in both children and adults [30]. To date, no predictors for thrombosis have been established in children with NS, and prophylactic anticoagulation remains controversial [22]. The hypercoagulable state is due to several potential mechanisms, including the urinary loss of anticoagulants such as antithrombin, a raised plasma fibrinogen concentration, increased platelets aggregability and intravascular volume depletion [8, 34]. However, their respective roles and other potential factors of hypercoagulability, such as inherited thrombophilia, have not been well documented [43].
Thrombosis is either venous or, less commonly, arterial and may be more frequently associated with steroid-resistant NS [2]. Renal vein thrombosis is the most common site of thrombosis [47]. Although cerebral sinovenous thrombosis (CSVT) is infrequent in nephrotic children, it may carry increased morbidity compared to other locations [7, 19].
The purpose of this present article is to describe the clinical characteristics and pattern of coagulation alterations in four consecutive children from a single centre with CSVT and INS as well as to review existing cases from the literature.
Patients and methods
The database of the Canadian Pediatric Ischemic Stroke Registry (Toronto Site) containing prospectively enrolled children from 1992–2004 with CSVT [11] was searched to identify children (aged 1 month to 18 years) with confirmed CSVT and NS. This involved ICD-9 health record searches supplemented by a mail request for additional patients sent to all nephrologists at our institution.
Sinovenous thrombosis was documented either by computer tomography with venography (CT with CTV) and/or magnetic resonance imaging (MRI) with or without magnetic resonance venography (MRV). Nephrotic syndrome is characteristically defined as heavy proteinuria (>40 mg/m2 per hour), hypoalbuminaemia (<25 g/l), and clinical oedema. In our institution, 24-h proteinuria is not always quantified in young children, and, therefore, the diagnosis of nephrotic syndrome relies on persistent positive results of the urine dipstick test, hypoalbuminaemia and clinical oedema. Exclusion criteria were:
-
1.
NS secondary to systemic disorders including systemic lupus erythematosus, vasculitis and others
-
2.
Congenital nephrotic syndrome
Medical charts from our institution were reviewed for detailed clinical, radiographic and laboratory data obtained at the time of the thrombosis. Special effort was made to collect data regarding the degree of proteinuria, prothrombotic conditions and radiological studies at the time of diagnosis. Clinical features, presumed diagnosis, modality of imaging, treatment and long-term outcome were obtained for all patients.
For the pooled literature analysis we searched English and French medical literature from 1970 to the present time, using Medline database and cross-referencing. Patients aged 1 month to 18 years with sufficient clinical and laboratory data to satisfy our criteria for INS and CSVT were included.
Comparison of our own data was made with existing reports from the literature survey. In addition, the collected patients were divided into those with steroid-sensitive nephrotic syndrome (SSNS), steroid-dependant nephrotic syndrome (SDNS) and steroid-resistant nephrotic syndrome (SRNS) according to accepted criteria [22].
Results
Patients’ characteristics
Over a period of 12 years a total of 85 children with CSVT beyond the neonatal period was enrolled in the registry at the Toronto site. We identified four children (two boys and two girls) with CSVT and INS aged 2.5 years to 8.5 years. Two nephrotic patients (aged 12 years and 14 years) with lupus nephropathy and CSVT, as well as one patient with Wegener’s granulomatosis (aged 15 years), were excluded from the current study.
We found an additional 17 cases reported in the literature from 1980 to 2005 who met our criteria for inclusion in the pooled literature analysis.
Therefore, we included a total of 21 cases in the analysis. The clinical characteristics of those patients are summarised in Tables 1 and 2. There were 13 male and seven female subjects. Gender was not available in one case. Mean age was 4.8 years (range 2–9 years). Nephrotic syndrome was steroid-sensitive in seven patients, steroid-dependant in six and steroid-resistant in four. In the remaining four cases, the information was not available. In nine children CSVT developed during the first flare of NS, and, in the majority of children (13/19), it developed within 6 months after the initial manifestation of NS.
Nineteen patients were on steroids (oral prednisone in 17) at time of diagnosis; four were receiving diuretics and two were under prophylactic anticoagulation, one on dypiridamole and the second on LMWH.
Clinical presentation
Clinical manifestations included seizures in eight children. In addition, signs of raised intracranial pressure including headache, vomiting, lethargy and irritability were present in the majority of cases (n=16). Papilloedema was observed in half of the patients (n=11). Focal neurological deficits including cranial nerve palsy (n=7) and hemiparesis (n=4) were reported less often.
CSVT was not suspected prior to imaging in three of our four Toronto patients, and presumptive diagnoses pre-imaging consisted of pseudotumour cerebri, gastroenteritis, and hypertensive encephalopathy. This information was not available in the literature cases.
Imaging
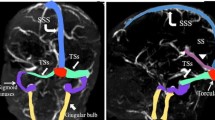
Neuroimaging consisted of CT in 20 patients, with contrast enhancement in 17. In nine cases, MRI was also performed, of which patients seven had venography (MRV). In one case from our institution, MRI was the only modality. Conventional angiography was performed in one case. In all patients (n=21), thrombosis of the superior sagittal sinus was present. Deep venous system involvement was reported in only two patients.
Parenchymal lesions were present in none of the four Toronto patients and were reported in four of the 17 literature patients and included bland venous infarcts (n=2) or haemorrhages (n=2).
Management
Of the 21 patients, 20 received anticoagulant therapy, which consisted of initial LMWH in seven patients and standard unfractionated heparin (UFH) in 13 patients, of whom two received additional fresh frozen plasma (FFP). Duration of treatment was variable and ranged in the Toronto patients from 3 months to 5 months. Literature cases did not provide sufficient information to determine anticoagulation duration. No bleeding complications were reported.
Outcome
Outcome, with a mean follow-up of 2.7 years, was available for the four Toronto patients of our institution and was excellent in all four. One patient had a temporary strabismus, which resolved within 6 months, and no other residual neurological deficits or seizures were observed.
Among cases from the literature, 15 out of 17 had full recovery. One child died secondary to massive pulmonary emboli, which developed subsequently [9] and an other had residual cognitive impairment probably in relation to a bilateral venous thalamic infarct. [28] None of the patients had recurrence of a thrombotic event.
Laboratory findings
Data regarding laboratory testing results were variable among the literature cases. The laboratory data are presented in Table 2.
Proteinuria
Heavy proteinuria was present in the majority of patients and was reflected in severe hypoalbuminaemia (less than 20 g/l) in 14 reported patients. Two patients had minimal or absent proteinuria at the time of CSVT diagnosis but had documented nephrotic range proteinuria prior to steroid treatment.
Prothrombotic risk factors
Levels of AT were reported in 16 patients. Nine patients had at least transiently decreased AT, and one had a borderline low level. None of the patients tested had protein C or protein S deficiency (0/11). Fibrinogen was increased in a minority of tested patients (3/13). In addition, increased factor VIII (n=3), low plasminogen (n=2) and microcytic anaemia (n=2) were rarely reported.
Screening for the inherited thrombophilias factor V Leiden and prothrombin G20210A gene mutation was performed in all four patients in our institution and was negative. Factor V Leiden mutation was reported in one single case from the literature. Lipoprotein (a) assay was performed in two patients, and the level was increased (2/2). Platelets count was reported in 13 patients and was elevated in one patient, who had coincidental microcytic anaemia. [29] No platelets function tests were performed.
In total, in almost half of the patients (n=10), prothrombotic abnormalities were either not found or not reported. In contrast, a combination of multiple prothrombotic risk factors was present in at least five patients.
Discussion
In the current study, we analysed the largest series of children with nephrotic syndrome and cerebral sinus venous thrombosis. We found that CSVT occurs in both SSNS and SRNS and that, while the antithrombin level was decreased relatively frequently, its level was normal in almost half of the patients and that other contributing prothrombotic abnormalities were rarely observed.
The current incidence of CSVT in children based on the Canadian registry is 0.67/100,000 which is probably an underestimate of the true frequency [46]. Diagnosis remains difficult due to often variable and subtle presentation, but early diagnosis is necessary to prevent serious morbidity [5, 11]. The clinical presentation of CSVT in our population did not differ from previous paediatric series, was non-specific and included seizures and signs and symptoms of raised intracranial pressure such as headache, vomiting, altered level of consciousness and papilloedema [5, 11, 20, 45]. Despite increased awareness, the diagnosis was not considered initially in three of our four patients, which delayed brain imaging studies and management.
Brain CT is usually performed as the primary imaging modality in children and adults with neurological symptoms [27, 46]. However, conventional CT techniques can miss the presence of CSVT in up to 40% of children and underestimate both the extent of the thrombus and the presence of venous infarcts [46]. In addition, there is an increased risk of acute renal failure due to contrast nephropathy, and only isosmotic contrast media should be used. MRI with MRV is now acknowledged to be the method of choice in childhood CSVT. In contrast to CT imaging, MR imaging does not raise the concern of radiation or nephrotoxicity, can visualise flow and thrombus, and is sensitive to parenchymal changes, making it a reliable and safe tool [46, 48]. In equivocal cases, high resolution CT venography can be required [45, 46] .In our analysis, we found no report of contrast nephropathy. We also found an increasing use of MRI with MRV in the past 10 years (n=8), usually as a complementary confirmative tool. In the majority of the patients (18/21), the diagnosis was based upon CT scans. However, in two of our Toronto patients, initial non-contrast CT scans were reported as being normal, and CSVT was diagnosed on subsequent contrast-enhanced imaging. Similarly, in the patient of Lin et al. [28], the initial CT scan was normal and the diagnosis was made 5 days later with MRI, which stresses the importance of additional imaging studies when there is a high index of suspicion.
Interestingly, in comparison to recent published cohort studies of children with cerebral venous thrombosis, all patients in our survey had involvement of the superior sagittal sinus (SSS) and a decreased rate of associated parenchymal brain lesions. The latter possibly accounts for the general good outcome in this population compared to previously documented outcomes of childhood CSVT [5, 11, 20, 45]. Death resulting from sinus venous thrombosis in a nephrotic child remains, to our knowledge, exceptional [40].
CSVT is a rare complication of NS, but recent increase in the frequency of case reports suggests that it could have been underdiagnosed in the past [3, 26]. The exact incidence is unknown, but this entity apparently represents between 4.7 % (our study) and 6% of all cases of childhood CSVT (non-neonates) [45]. In a large survey including 3,177 children with NS, Egli et al. found 1.8% of various thromboembolic complications, including two patients with CSVT, and reported on one of them [14, 15]. However, the prevalence of thromboembolism in NS is thought to be underestimated [23, 52]. Divekar et al. have observed a single case of CSVT among 700 hundred consecutive patients with NS [12].
The hypercoagulable state in NS is thought to be multifactorial, due to variable abnormalities in the haemostatic system, resulting in an imbalance between the clotting activator system and the inhibitor system [43, 47]. These include increased procoagulatory activity (fibrinogen, factors V and VIII), urinary loss of anticoagulants (AT, protein C, total and free protein S), altered fibrinolytic system, thrombocytosis and enhanced platelet activation and aggregability [43]. Hyperlipidaemia per se could also be prothrombotic [8]. The contribution of steroids to CSVT risk is controversial [44, 50]. Other factors, such as the use of diuretics, haemoconcentration, could also play a role [44].
The pathogenesis of CSVT in nephrotic patients is unknown but is hypothesised to be secondary to urinary loss of coagulant inhibitors [1, 19, 45]. Despite the obvious limitation of our study, related to the limited number of patients in our institution and the lack of complete laboratory data in the literature cases, our study demonstrates that a deficiency in AT is the most frequently encountered coagulation abnormality. However AT deficiency is not a constant feature, as it was reported in only nine of 16 patients, including one Toronto patient. In addition, free/total protein S or protein C deficiencies, when tested for, were not found. Finally, hyperfibrinogenaemia was present in only a small subset of patients, making it also unlikely to be a major risk factor. The single most consistent biological risk factor was the presence of severe hypoalbuminaemia confirming previous data [43]. Hypoalbuminaemia probably reflects not only intravascular volume depletion, causing increased blood viscosity, but may also be directly related to platelet hyperaggregability and alteration in the fibrinolytic system [47]. These dual actions make hypoalbuminaemia potentially an important prothrombotic factor.
CSVT in children is often associated with an inherited prothrombotic state [4, 11, 51]. Some authors have suggested that inherited thrombophilia could increase the risk of thrombosis in patients with NS, and, therefore, a thorough work-up should be performed [2, 37]. In contrast, inherited thrombophilia was considered only a weak risk factor for thrombosis in children with NS in one well-documented study [16], but this question has not specifically been addressed in CSVT. None of our four children had a genetic prothrombotic state, and factor V Leiden is reported in a single case report [17]. Unfortunately, data regarding inherited thrombophilia were not available in most case reports. Again, despite this limitation, it seems unlikely that genetic prothrombotic factors play a major role in the genesis of CSVT in NS, and acquired, rather than inherited, alterations are more likely to be involved in the development of thrombosis.
Additional prothrombotic factors to consider include increased lipoprotein (a) [Lp(a)]. The major component of Lp(a) is apolipoprotein (a), which has structural homology close to that of plasminogen and competes with fibrin binding, causing impaired fibrinolysis. Increased Lp(a) has recently been shown to be an independent risk factor for venous thromboembolism in children [33] as well as a significant risk factor for CSVT in children [20]. Increased Lp(a) level has been demonstrated in children with relapsing NS and could, therefore, be a particularly relevant prothrombotic risk factor in children with NS [38, 43]. In our survey, since Lp(a) testing has only been recently available, Lp(a) values were reported in only two cases and were elevated in both cases [17, 38]. If present, iron deficiency anaemia and increased factor VIII could also be associated risk factors for the development of CSVT, as noted by Sebire et al. [45], and were identified in our survey, respectively, in two and three patients.
Acute anticoagulation, with either UFH or LMWH, was administered to all but one patient and appeared to be very safe, with no bleeding complications reported. Anticoagulation therapy in children with CSVT is now widely implemented in the absence of major intracranial haemorrhage, aiming to prevent further propagation of the existing thrombus and to decrease associated morbidity and mortality [11, 24]. Current recommendations are either UFH or LMWH for 5 to 7 days followed by LMWH or vitamin K antagonists for 3 to 6 months [31]. As efficient heparin therapy requires adequate levels of AT [31], which is often reduced in nephrotic patients, direct AT replacement or adjunction of fresh frozen plasma could be necessary [1].
Prophylactic anticoagulation is recommended in adult nephrotic patients with idiopathic membranous nephropathy and is administered so long as the patient has nephrotic proteinuria and/or albumin level below 20 g/l [42]. Prophylactic anticoagulation in children with steroid-resistant or frequent relapsing NS has been suggested by some authors, owing to presumed greater risk of thrombosis [2, 35]. Interestingly in our survey there was a higher number of SSNSs, and the incidence of thrombosis seems greater in the first flare-up of NS, contrasting with the above statement. Other authors have suggested selecting for prophylactic anticoagulation by assessing for prothrombotic risk. Different biological markers have been suggested, such as hypoalbuminaemia lower than 20 g/l, high fibrinogen levels (above 6 g/l), and AT deficiency (<70%), [32]. However, so far, there is no reliable biological predictor [13, 43]. Owing to the lack of reliable predictors, and to the inherent risk of anticoagulation, prophylactic anticoagulation is usually not administered to nephrotic children unless there has been a prior thrombotic event [13, 22]. The use of low-dose aspirin (2–5 mg/kg per dose) has also been considered, but no controlled trials have been performed to demonstrate its efficacy in preventing thromboses [21, 32].
In summary, children with INS (SSNS or SRNS) are at risk of thromboembolic complications, including CSVT. Careful attention should be paid to any neurological symptoms developing in this population, and a high index suspicion of CSVT is important to avoid delay in diagnosis. If available, MRI coupled with MRV is the imaging modality of choice. The use of CT venography is useful in equivocal cases. However, the risk of contrast nephropathy should be considered. Acute anticoagulation, with either UFH or LMWH, appears safe and is recommended to prevent potential morbidity. The aetiology of CSVT in NS appears multifactorial, more likely to be related to variable acquired disturbances in the haemostatic system rather than consecutive to inherited thrombophilia. Further studies are necessary to identify patients at risk and biological predictors of thrombosis.
Abbreviations
- NS :
-
Nephrotic syndrome
- CSVT :
-
Cerebral sinovenous thrombosis
- SSNS :
-
Steroid-sensitive nephrotic syndrome
- SDNS :
-
Steroid-dependant nephrotic syndrome
- SRNS :
-
Steroid-resistant nephrotic syndrome
- UHF :
-
Unfractionated heparin
- LMWH :
-
Low molecular weight heparin
- AT :
-
Antithrombin
- Lip(a) :
-
Lipoprotein (a)
References
Akatsu H, Vaysburd M, Fervenza F, Peterson J, Jacobs M (1997) Cerebral venous thrombosis in nephrotic syndrome. Clin Nephrol 48:317–320
Andrew M, Brooker LA (1996) Hemostatic complications in renal disorders of the young. Pediatr Nephrol 10:88–99
Barthelemy M, Bousser MG, Jacobs C (1980) Cerebral venous thrombosis, complication of the nephrotic syndrome. Presse Med 9:367–369
Bonduel M, Sciuccati G, Hepner M, Pieroni G, Torres AF, Mardaraz C, Frontroth JP (2003) Factor V Leiden and prothrombin gene G20210A mutation in children with cerebral thromboembolism. Am J Hematol 73:81–86
Carvalho KS, Bodensteiner JB, Connolly PJ, Garg BP (2001) Cerebral venous thrombosis in children. J Child Neurol 16:574–580
Casteels K, Demaerel P, Proesmans W (1995) Clinical quiz. Cerebral venous thrombosis. Pediatr Nephrol 9:247–249
Chan AK, deVeber G, Monagle P, Brooker LA, Massicotte PM (2003) Venous thrombosis in children. J Thromb Haemost 1:1443–1455
Citak A, Emre S, Sairin A, Bilge I, Nayir A (2000) Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr Nephrol 14:138–142
Delmas MC, Cochat P, Ranchin B, Negrier C, Barre P, Bouvier R, David L (1992) Thrombosis of superior longitudinal sinus and pulmonary embolism in nephrotic syndrome. Pediatrie 47:31–35
de Saint-Martin A, Terzic J, Christmann D, Knab MC, Peter MO, Fischbach M (1997) Superior sagittal sinus thrombosis and nephrotic syndrome: favourable outcome with low molecular weight heparin. Arch Pediatr 4:849–852
deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, Camfield CS, David M, Humphreys P, Langevin P, MacDonald EA, Gillett J, Meaney B, Shevell M, Sinclair DB, Yager J (2001) Cerebral sinovenous thrombosis in children. N Engl J Med 345:417–423
Divekar AA, Ali US, Ronghe MD, Singh AR, Dalvi RB (1996) Superior sagittal sinus thrombosis in a child with nephrotic syndrome. Pediatr Nephrol 10:206–207
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–639
Egli F, Elmiger P, Stalder G (1973) Thrombosis as a complication of nephrotic syndrome. Helv Paediatr Acta 30 (Suppl): 20–21
Egli F, Eimiger P, Stalder G (1974) Thromboembolism in the nephrotic syndrome. European Society for Pediatric Nephrology. Pediatr Res 8:903
Fabri D, Belangero VM, Annichino-Bizzacchi JM, Arruda VR (1998) Inherited risk factors for thrombophilia in children with nephrotic syndrome. Eur J Pediatr 157:939–942
Fluss J (2005) Syndrome néphrotique de l’enfant et thrombose veineuse cérébrale. Thesis. Faculty of Medicine, University of Geneva, Geneva
Freycon MT, Richard O, Allard D, Damon G, Reynaud J, Freycon F (1992) Intracranial venous sinus thrombosis in nephrotic syndrome. Pediatrie 47:513–516
Gangakhedar A, Wong W, Pitcher LA (2005) Cerebral thrombosis in childhood nephrosis. J Paediatr Child Health 41:221–224
Heller C, Heinecke A, Junker R, Knofler R, Kosch A, Kurnik K, Schobess R, von Eckardstein A, Strater R, Zieger B, Nowak-Gottl U (2003) Cerebral venous thrombosis in children: a multifactorial origin. Circulation 108:1362–1367
Hodson E (2003) The management of idiopathic nephrotic syndrome in children. Paediatr Drugs 5:335–349
Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J (2000) Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105:1242–1249
Hoyer PF, Gonda S, Barthels M, Kroch HP, Brodehl J (1986) Thromboembolic complications in children with nephrotic syndrome. Risk and incidence. Acta Paediatr Scand 75:804–810
Johnson MC, Parkerson N, Ward S, de Alarcon PA (2003) Pediatric sinovenous thrombosis. J Pediatr Hematol Oncol 25:312–315
Lasry F, Damane H, Oumlil M, Hadj Khalifa H (2004) Esotropia revealing a cranial sinus thrombosis in a child with nephrotic syndrome. Arch Pediatr 11:258
Lau SO, Bock GH, Edson JR, Michael AF (1980) Sagittal sinus thrombosis in the nephrotic syndrome. J Pediatr 97:948–950
Lee SK, terBrugge KG (2003) Cerebral venous thrombosis in adults: the role of imaging evaluation and management. Neuroimaging Clin N Am 13:139–152
Lin CC, Lui CC, Tain YL (2002) Thalamic stroke secondary to straight sinus thrombosis in a nephrotic child. Pediatr Nephrol 17:184–186
Meena AK, Naidu KS, Murthy JM (2000) Cortical sinovenous thrombosis in a child with nephrotic syndrome and iron deficiency anaemia. Neurol India 48:292–294
Mehls O, Andrassy K, Koderisch J, Herzog U, Ritz E (1987) Hemostasis and thromboembolism in children with nephrotic syndrome: differences from adults. J Pediatr 110:862–867
Monagle P, Chan A, Massicotte P, Chalmers E, Michelson AD (2004) Antithrombotic therapy in children: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:645S–687S
Niaudet P (2004) Steroid-sensitive idiopathic nephrotic syndrome in children. In: Avner ED, Harmon WE, Niaudet P (eds) Pediatric nephrology, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 543–554
Nowak-Gottl U, Junker R, Hartmeier M, Koch HG, Munchow N, Assmann G, von Eckardstein A (1999) Increased lipoprotein(a) is an important risk factor for venous thromboembolism in childhood. Circulation 100:743–748
Orth SR, Ritz E (1998) The nephrotic syndrome. N Engl J Med 338:1202–1211
Palcoux JB, Gaspard F, Campagne D (2003) Cerebral sinus thrombosis in a child with steroid-resistant nephrotic syndrome. Pediatr Nephrol 18:610–611
Papachristou FT, Petridou SH, Printza NG, Zafeiriou DI, Gompakis NP (2005) Superior sagittal sinus thrombosis in steroid-resistant nephrotic syndrome. Pediatr Neurol 32:282–284
Petaja J, Jalanko H, Holmberg C, Kinnunen S, Syrjala M (1995) Resistance to activated protein C as an underlying cause of recurrent venous thrombosis during relapsing nephrotic syndrome. J Pediatr 127:103–105
Pillekamp F, Hoppe B, Roth B, Querfeld U (1997) Vomiting, headache and seizures in a child with idiopathic nephrotic syndrome. Nephrol Dial Transplant 12:1280–1281
Pirogovsky A, Adi M, Dagan A, Sinai L, Sthoeger D, Barzilai N, Tabachnik E (2001) Superior sagittal sinus thrombosis: a rare complication in a child with nephrotic syndrome. Pediatr Radiol 31:709–711
Report of the International Study of Kidney Disease in Children (1984) Minimal change nephrotic syndrome in children: deaths during the first 5 to 15 years’ observation. Pediatrics 73:497–501
Rodrigues MM, Zardini LR, de Andrade MC, Mangia CM, Carvalhaes JT, Vilanova LC (2003) Cerebral sinovenous thrombosis in a nephrotic child. Arq Neuropsiquiatr 61:1026–1029
Sarasin FP, Schifferli JA (1994) Prophylactic oral anticoagulation in nephrotic patients with idiopathic membranous nephropathy. Kidney Int 45:578–585
Schlegel N (1997) Thromboembolic risks and complications in nephrotic children. Semin Thromb Hemost 23:271–280
Schnaper HW (2001) Nephrotic syndrome. In: Schrier R (ed) Diseases of the kidney and the urinary tract, 7th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1807–1811
Sebire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, Husson B, Williams AN, Wade A, Kirkham FJ (2005) Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain 128:477–489
Shroff M, deVeber G (2003) Sinovenous thrombosis in children. Neuroimaging Clin N Am 13:115–138
Singhal R, Brimble KS (2006) Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res (in press)
Stam J (2005) Thrombosis of the cerebral veins and sinuses. N Engl J Med 352:1791–1798
Tullu MS, Deshmukh CT, Save SU, Bhoite BK, Bharucha BA (1999) Superior sagittal sinus thrombosis: a rare complication of nephrotic syndrome. J Postgrad Med 45:120–122
Ueda N (1990) Effect of corticosteroids on some hemostatic parameters in children with minimal change nephrotic syndrome. Nephron 56:374–378
Vielhaber H, Ehrenforth S, Koch HG, Scharrer I, van der Werf N, Nowak-Gottl U (1998) Cerebral venous sinus thrombosis in infancy and childhood: role of genetic and acquired risk factors of thrombophilia. Eur J Pediatr 157:555–560
Weisz W, Kemper MJ, Weil J, Muller-Wiefel DE (2002) Asymptomatic intracardiac thrombus in steroid-sensitive nephrotic syndrome. Pediatr Nephrol 17:287–289
Acknowledgement
The authors gratefully acknowledge the data management assistance of Ann-Marie Pontigon in the preparation of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fluss, J., Geary, D. & deVeber, G. Cerebral sinovenous thrombosis and idiopathic nephrotic syndrome in childhood: report of four new cases and review of the literature. Eur J Pediatr 165, 709–716 (2006). https://doi.org/10.1007/s00431-006-0147-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0147-7