Abstract
Henoch-Schönlein purpura is the most common systemic vasculitis during childhood. Many antigenic stimuli and infectious agents have been proposed as a trigger of the disease. Group A beta-hemolytic streptococcus is the most common proposed infectious agent as a trigger for Henoch-Schönlein purpura. We report a 9-year-old boy who has Henoch-Schönlein purpura and acute rheumatic fever with complete atrioventricular block.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Henoch-Schönlein purpura (HSP) is the most common systemic vasculitis seen in children [1]. It occurs more frequently between the ages of 3 and 15 and is more common in boys than in girls. Many antigenic stimuli and infections have been proposed as a trigger for HSP [16]. Cases of HSP with acute rheumatic fever (ARF) were reported in the literature [8, 9, 13]. In this article we discuss a case of HSP and acute rheumatic fever with complete atrioventricular block.
Case report
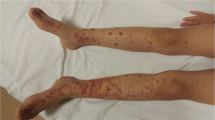
A 9-year-old boy was admitted to the hospital with the complaints of rash on his lower extremities, arthralgia, and difficulty in walking. His history revealed that he had had an upper respiratory tract infection 20 days earlier. He was observed for 3 days in a local hospital. Upon physical examination, he had arthritis in both ankles and purpuric rash on the lower extremities. Cardiac examination revealed grade 3/6 pansystolic murmur. His previous medical history was normal. His father had hyperlipidemia and had undergone a cardiac bypass operation 2 years ago. On the basis of the clinical findings, acute rheumatic fever and HSP were diagnosed and salicylate therapy was started in the local hospital. Then the patient was referred to our research hospital for cardiac evaluation.
Physical examination on admission: weight was 26 kg (25–50 percentile), height was 131 cm (50–75 percentile), blood pressure was 115/70 mmHg (75–90 percentile), pulse rate was 52 bpm and rhythmic. He had a petechial and purpuric rash on his lower extremities, especially at the areas exposed to pressure. He had no arthritis and cardiac auscultation showed grade 2/6 systolic murmur over the mitral region. Eye examination was normal. Laboratory examination showed a hemoglobin level of 13.2 g/dl, leukocyte count of 9.5×109/l, and platelet count of 292×103/mm3; liver and renal functions were normal. Antistreptolysin O titer was 1,220 IU/l, C-reactive protein (CRP) was 0.67 mg/dl, rheumatoid factor was negative, and erythrocyte sedimentation rate (ESR) was 69 mm/h. Throat culture taken on admission revealed group A beta-hemolytic streptococcus (GABHS). Urine examination showed microscopic hematuria, and stool examination was negative for occult blood. Immunological analyses showed C3 99 mg/dl (normal: 77–195), C4 19.6 mg/dl (normal: 7–40), IgG 1,380 mg/dl (normal: 608–1,572), IgA 220 mg/dl (normal: 33–236), IgM 118 mg/dl (normal: 52–242); tests for antinuclear antibody, anti-dsDNA, perinuclear antineutrophil cytoplasmic antibody (p-ANCA), and cytoplasmic ANCA were negative. Hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb) were also negative. Lyme antibody IgM and IgG were negative. The light microscopic examination of skin biopsy showed leukocytoclastic vasculitis. Immunofluorescent study for IgA was not performed.
His pulse rate was between 82 and 93 bpm on admission to the local hospital. At admission to our research hospital, his pulse rate was 52 bpm; Mobitz type 2 atrioventricular block and complete atrioventricular block were detected by electrocardiography (Fig. 1). Holter monitoring during 24 h revealed an atrial rate of 120/min and a ventricular rate of minimum 38/min, maximum 75/min. Ventricular or supraventricular tachycardia was not recorded during monitoring. Diagnosis of complete atrioventricular block was confirmed. Abdominal ultrasonography was normal. Echocardiographic examination showed first-degree aortic insufficiency and a mild degree of mitral insufficiency, left ventricular ejection fractions (74%) and fractional shortening (43%) were within normal limits, and left ventricular diameter was mildly enlarged. The patient had no signs and symptoms of congestive heart failure. Salicylate therapy was started at the dosage of 90 mg/kg as well as benzathine penicillin prophylaxis. At the 3rd week of follow-up, acute phase reactant levels regressed. Microscopic hematuria and skin rash disappeared.
At the 3rd month of follow-up, left ventricular diameter regressed to normal limits. Holter monitoring showed no improvements in the rhythm, atrial rate was 110/min, and ventricular rate was minimum 36/min, maximum 61/min. Rare premature ventricular contractions were recorded.
Discussion
Henoch-Schönlein purpura is one of the most common vasculitides of childhood. It is characterized by nonthrombocytopenic purpura, arthritis and arthralgia, abdominal pain and gastrointestinal hemorrhage, and renal involvement [18]. Acute rheumatic fever is a multisystemic disorder that mainly affects the heart, joints, and central nervous system. Even though epidemiological studies do not support the hypothesis that GABHS can cause HSP [15], there are studies that show HSP is associated with GABHS [4, 8, 9, 13, 14].
In 1947 Gardner et al. first reported two patients, 15 and 40 years old, who developed rheumatic fever 2 weeks after HSP [9]. When HSP-associated acute rheumatic fever cases were studied, it was reported that rheumatic complications occurred 5 days to 12 weeks after the rash of HSP [4]. Considering cardiac lesions seen in HSP, myocarditis is more common than valvulitis [10, 14]. The existence of valvulitis in our patient is in favor of acute rheumatic fever. Typical purpuric rash and nonmigratory arthritis of HSP occurred 10 days after the upper respiratory infection in our patient. Cardiac lesions were detected at the same time. Arthritis seen in HSP is mild and nonmigratory, recovers spontaneously, and is responsive to anti-inflammatory therapy [5]. Our patient had nonmigratory arthritis at the ankles during the first 2 days before salicylate therapy was started. Even though nonmigratory arthritis is not in the Jones criteria [16], early salicylate therapy prevents progression of typical migratory arthritis. Good response to salicylate therapy supports the diagnosis of acute rheumatic fever. Due to existence of arthritis and carditis associated with high acute phase reactants, ECG abnormality, and positive throat culture for GABHS infection, acute rheumatic fever was diagnosed according to modified Jones criteria [16] and therapy with salicylates was started.
HSP with dysrhythmia was not reported in the literature before. On the other hand, some cases of permanent and transient complete atrioventricular block, lasting from a few days to a year, associated with acute rheumatic fever were reported [2, 3, 6, 11, 12, 15, 17, 19]. The atrioventricular complete block which developed in our patient was thought to be related with rheumatic carditis. Before bradycardia was first detected in the local hospital, his pulse rate was between 82 and 93 bpm. His pulse rate decreased to 36–65 bpm during the next 2 weeks. This decrease was thought to be related with the inflammation of the atrioventricular (AV) conduction system. At the 3rd month of follow-up, he had a different degree of AV block; Mobitz type 2 block was detected on ECG taken at rest and complete AV block was detected on ECG taken after exercise. This finding detected in our patient shows us that vasculitis-related inflammation in the cardiac conduction system can be variable. Even though we have no ECG recorded during the healthy state of our patient, the decrease in the recorded pulse rate over the course of the illness proves that the heart block is more likely to be acquired rather than congenital. At the end of 1 year of follow-up, there was no indication for a permanent cardiac pacemaker according to American Heart Association guidelines [7]. As a result, HSP and acute rheumatic fever are distinct disorders with different clinical findings which can be rarely seen together like in our patient. GABHS may play a role in the etiology of both of these disorders. This case showed that HSP together with rheumatic carditis and AV block could have developed during the time of childhood.
Abbreviations
- HSP:
-
Henoch-Schönlein purpura
- GABHS:
-
Group A beta-hemolytic streptococcus
- ARF:
-
Acute rheumatic fever
References
Bagga A, Dillan M (2001) Leukocytoclastic vasculitis. In: Cassidy JT, Petty RE (eds) Textbook of pediatric rheumatology. Saunders, Philadelphia, pp 569–579
Budianskii MV, Isamukhamedov SZ (1971) Effectiveness of dexamethasone in the treatment of complete atrioventricular block of rheumatic origin. Ter Arkh 43(2):56–58
Campana A, Cappuccio L, De Santis M, Petrone M, Di Mauro M (1986) Total atrioventricular block in recurrent rheumatic fever. G Ital Cardiol 16:95–98
Eisenstein EM, Navon-Elkan P (2002) Acute rheumatic fever associated with Henoch-Schonlein purpura: report of three cases and review of the literature. Acta Paediatr 91:1265–1267
Emery H, Larter W, Schaller JG (1977) Henoch-Schönlein vasculitis. Arthritis Rheum 20(Suppl):385
Friedland SW (1969) Pacemaker in rheumatic myocarditis. N Engl J Med 281(26):1489
Gregoratas G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, Kerber RE, Naccarelli GV, Schoenfeld MH, Silka MJ, Winters SL, Gibbons RJ, Antman EM, Alpert JS, Gregoratos G, Hiratzka LF, Faxon DP, Jacobs AK, Fuster V, Smith SC Jr (2002) ACC/AHA/NASPE (2002) guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Guidelines). Circulation 106:2145
Gulati T, Kumar P, Dewan V, Anand VK (2004) Henoch Schönlein purpura with rheumatic carditis. Indian J Pediatr 71:371–372
Imai T, Matsumoto S (1970) Anaphylactoid purpura with cardiac involvement. Arch Dis Child 45:727–729
Kereiakes DJ, Ports TA, Finkbeiner W (1984) Endomyocardial biopsy in Henoch-Schonlein purpura. Am Heart J 107:382–385
Malik JA, Hassan C, Khan GQ (2002) Transient complete heart block complicating acute rheumatic fever. Indian Heart J 54:91–92
Mohindra R, Pannu HS, Mohan B, Kumar N, Dhooria HS, Sehgal A, Avasthi G (2004) Syncope in a middle aged male due to acute rheumatic fever. Indian Heart J 56(6):668–669
Ocal B, Karademir S, Oðuz D, Öner A, Şenocak F (2000). Acute rheumatic carditis in Henoch-Schönlein purpura. Int J Cardiol 74:97–98
Osman A, McCreery CJ (2000) Cardiac vasculitis in Henoch-Schonlein purpura. Circulation 101:69–70
Shah CK, Gupta R (1993) Persistent complete heart block following acute rheumatic fever in a 12 year old girl. J Assoc Physicians India 41:389–390
Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association (1992) Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. JAMA 268:2069–2073
Thomas DB (1971) Complete heart block complicating rheumatic carditis. Aust Paediatr J 7:108–110
Vernier RL, Worthern HG, Peterson RDA, Cole A, Good RA (1961) Anaphylactoid purpura: pathology of the skin and kidney and frequency of streptococcal infection. Pediatrics 27:181–193
Zalstein E, Maar R, Zucker N, Katz A (2003) Advanced atrioventricular conduction block in rheumatic fever. Cardiology Young 13(6):491–494
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Güven, H., Özhan, B., Bakiler, A.R. et al. A case of Henoch-Schönlein purpura and rheumatic carditis with complete atrioventricular block. Eur J Pediatr 165, 395–397 (2006). https://doi.org/10.1007/s00431-006-0094-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0094-3