Abstract
We report two children with renovascular hypertension and fibromuscular dysplasia. They initially presented with severe hyponatremia, hypokalemia, polyuria, and transient proteinuria. This combination of symptoms is known to occur in patients with renovascular and malignant hypertension, and is known as hyponatremic-hypertensive syndrome (HHS), although it is considered rare in children. Since in both of our patients, the renal arterial stenosis was very severely or almost totally occlusive, we could not perform percutaneous transluminal renal artery angioplasty, and therefore nephrectomy was the only option. A histological study showed partial or complete occlusion with intimal hyperplasia and medial fibroplasia of intrarenal arteries such as the interlobular arteries. Conclusion: Both patients showed rapidly progressive renovascular hypertension and loss of function of the affected kidney. In order to preserve renal function in such cases, early invasive intervention appears to be necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of renovascular hypertension in the general population is not known precisely, but in children it probably accounts for between 5 and 25% of all cases of secondary hypertension [13]. The symptoms of renovascular hypertension vary, and sometimes it can be asymptomatic. In rare cases, the clinical manifestations can include electrolyte disorders including hyponatremia. The combination of hyponatremia and renovascular hypertension is known as hyponatremic-hypertensive syndrome [1, 3].
Among the causes of renovascular hypertension in children, atherosclerosis is rare and fibromuscular dysplasia is the most common, accounting for up to 60% [7]. Fibromuscular dysplasia is a nonatherosclerotic, noninflammatory vascular disease whose cause and nature remain unknown [15]. Here we report two young children with renovascular hypertension due to fibromuscular dysplasia who presented with severe hyponatremia and hypokalemia.
Case report
Case 1
A 4-year-old boy was referred to us because of drowsiness and a 2- to 3-month history of headache, abdominal pain, and frequent vomiting. Six months previously, transient proteinuria had been determined when the patient suffered acute enterocolitis, and for 2–3 months before presentation his mother had noticed enuresis and constant thirst. On arrival at the hospital, the patient suffered a sudden generalized clonic seizure, and at that time his blood pressure was 220/120 mmHg. The generalized convulsion was controlled by intravenous administration of diazepam. Brain CT showed hypodensity of the bilateral basal ganglia.
The laboratory data included serum sodium 123 mEq/l, potassium 2.8 mEq/l, chloride 78 mEq/l, plasma rennin activity 49.1 ng ml−1 h−1 (3.2–8.3 ng ml−1 h−1) [8], aldosterone 47.8 ng/dl (5–40 ng/dl) [8], antidiuretic hormone (ADH) 30.7 pg/ml (1.1±0.6 pg/ml) [11], urea nitrogen 18.4 mg/dl, creatinine 0.55 mg/dl, c-reactive protein 0.7 mg/dl, leukocyte count 14,200/μl and erythrocyte sedimentation rate 25 mm/h. Urinalysis showed 3+ microscopic hematuria, and 3+ proteinuria without glucosuria. The urinary protein level was 368 mg/dl and the urinary NAG level was 14.2 units/l.
Renal sonogram showed a small right kidney. Three-dimensional CT angiography showed occlusion of the right renal artery and disappearance of the inferior vena cava between the level of the bifurcation and the branching point of the left renal vein. In the area of the inferior vena cava abnormality, the perivertebral venous plexus and ascending lumbar vein had developed collateral veins. Injection of 99mTc-mercaptoacetyltriglycine (99mTc-MAG3) resulted in no RI uptake by the right kidney, showing that the kidney was nonfunctional.
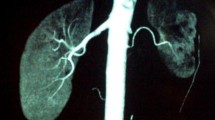
On admission, a nicardipine hydrochloride drip was started in order to maintain the blood pressure between 130 and 160 mmHg. Normal saline was immediately given to maintain a correct fluid intake and very slowly to modify the serum sodium concentration. On hospital days 8–9, diuresis and natriuresis peaked at 252.7 ml kg−1 day−1 and 16.7 mEq kg−1 day−1, respectively. At that time, fractional excretion of sodium was 2.21% and daily urinary calcium excretion was 240 mg/day (14.6 mg kg−1 day−1). After starting oral administration of enalapril, benidipine hydrochloride, and valsartan in addition to the nicardipine hydrochloride drip, the systolic blood pressure was stabilized to between 130 and 160 mmHg, and the serum sodium and potassium levels returned to within the normal range. Angiography performed on hospital day 34 showed complete occlusion of the right renal artery and a light nephrogram of the right kidney (Fig. 1), making it impossible to perform percutaneous transluminal renal artery angioplasty (PTRA). On hospital day 100, therefore, right nephrectomy was performed. After nephrectomy, the blood pressure normalized without use of antihypertensive drugs, and the serum sodium and potassium concentrations were maintained within the normal range. Brain CT showed disappearance of the basal ganglia hypodensity, and the patient had no neurological sequelae. A histological study of the nephrectomized kidney showed partial or complete occlusion of the arteries to the level of the arcuate artery due to intimal, medial and/or adventitial fibroplasia. Juxtaglomerular apparatus hypertrophy was observed in this specimen.
Case 2
A 4-year-old girl was brought to Minoh City Hospital with a 2-day history of fever, frequent vomiting, and a 1-kg loss of body weight. High blood pressure of 210/130 mmHg and moderate proteinuria (1–2 g/day) were demonstrated.
The laboratory data included serum sodium 130 mEq/l, potassium 3.4 mEq/l, chloride 95 mEq/l, plasma renin activity 22.0 ng ml−1 h−1 (3.2–8.3 ng ml−1 h−1) [8], aldosterone 142.6 ng/dl (5–40 ng/dl) [8], antidiuretic hormone (ADH) 4.5 pg/ml (1.1±0.6 pg/ml) [11], urea nitrogen 19 mg/dl, creatinine 0.3 mg/dl, c-reactive protein under 0.1 mg/dl, and leukocyte count 8,100/μl. Urinalysis showed 1+ glucosuria, microscopic hematuria (20–29 cells/HPF), and 4+ proteinuria. Urinary protein was 18,667 mg/g creatinine, and urine NAG was 18.9 unit/l. A nicardipine hydrochloride drip, spironolactone administration, and serum sodium and potassium correction were started. Despite sodium and potassium replacement therapy, daily urine volume remained between 1,200 ml/day (92.3 ml kg−1 day−1) and 2,400 ml/day (184.6 ml kg−1 day−1) and the serum sodium level remained at 120–130 mEq/l until around day 20 after onset. On hospital day 6, fractional excretion of sodium was 2.62% and daily urinary calcium was 183.6 mg (14.1 mg kg−1 day−1).
Enhanced abdominal CT showed severe stenosis of the left renal artery (Fig. 2). Angiography performed on hospital day 63 showed occlusion of the truncus of the left renal artery and blood supplementation from a collateral artery. A 99mTc-MAG3 renogram 31 days after onset showed that peak activity was observed in the delayed phase in the left kidney. The patient was transferred to Tokyo Women’s Medical University Hospital for surgical revascularization of the left renal artery. Preoperative evaluation with another 99mTc-MAG3 renogram on day 111 after onset showed no RI uptake and no function of the left kidney. At surgery, as the perfusion pressure of the left kidney was high, left nephrectomy was performed. Thereafter, plasma renin activity became normalized, and blood pressure was also normalized after administration of a calcium antagonist. A histological study of the nephrectomy specimen showed diffuse tubular atrophy, collapse and sclerosis of the glomeruli, and medial fibroplasia of the interlobar arteries. Juxtaglomerular apparati were hypertrophic in this specimen.
Discussion
Hyponatremic-hypertensive syndrome is a rare condition involving severe hypertension characterized by electrolyte abnormalities, such as hyponatremia and hypokalemia, with polyuria, proteinuria, and high renin activity. This syndrome was named by Brown et al. in 1965 and was originally reported in adults with renovascular hypertension [3]. As to the pathophysiology of HHS, Atkinson [1] has suggested that with critical renal ischemia, renin secretion is increased, resulting in high circulating angiotensin II levels, which in turn raise arterial pressure and stimulate aldosterone secretion from the adrenal glomerulosa. A sudden rise in arterial pressure can induce pressure natriuresis through the contralateral normal kidney, leading to volume depletion that may result in postural hypotension, further release of renin from the ischemic kidney, and heightening of the aldsterone response to angiotensin II. Potassium deficiency as a result of hyperaldosteronism may further stimulate renin secretion. The hyponatremia is presumed to result primarily from sodium depletion due to pressure natriuresis and the direct effect of angiotensin II on the kidney. In addition to the sodium depletion, the stimulation of thirst and release of ADH in response to the dual stimuli of exceedingly high levels of angiotensin II and volume depletion would make the hyponatremic condition more serious. According to this pathophysiological mechanism, the presence of a contralateral normal kidney would be necessary for onset of HHS. In other words, unilateral stenosis is the rule. In both of the present patients, renal artery stenosis was unilateral, not bilateral. Patient 1 had hyponatremia, hypokalemia, polydipsia, polyuria manifested as enuresis, and high plasma renin, aldosterone and ADH levels. Patient 2 had hyponatremia, hypokalemia, polyuria, polydipsia, body weight loss, and high levels of plasma renin, aldosterone, and ADH. These combinations of manifestations are compatible with HHS [1].
Generally, PTRA is appropriate in children with fibromuscular dysplasia and is almost always technically successful [12], with a reported success rate of between 90 and 100% [4, 7, 12, 14]. However, it was impossible to perform PTRA in our patients because their affected renal arteries were almost completely occluded due to fibromuscular dysplasia. We hypothesized that the symptoms of HHS would develop in children only when the stenosis in the affected artery became so severe that revascularization by PTRA was impossible. In the literature, only seven case reports of pediatric HHS with renovascular hypertension were found [2, 5, 6, 9, 10, 16]. Of the nine reported cases, including the present two, PTRA was technically successful in only one case [10]. Of the four cases that showed renovascular hypertension apparently associated with fibromuscular dysplasia, only one case was treated successfully with PTRA and the others were treated by nephrectomy of the affected kidney. The technical success rate of PTRA (25%, one of four cases) would likely be lower than that reported generally, and the frequency of severe cases would be high in patients with fibromuscular dysplasia, causing renovascular hypertension associated with HHS. These results would be compatible with our hypothesis.
In our patient 2, there was a period of only 2 months between the first 99mTc-MAG3 renography showing hypofunction of the affected kidney and preoperative 99mTc-MAG3 renography that showed no renal function, thus indicating extremely rapid loss of function of the affected kidney. In patient 1, there was a period of about 6 months between the chance detection of proteinuria and confirmation of the complete occlusion of the renal artery and failure of the affected kidney. Thus, renovascular fibromuscular dysplasia and loss of function of the affected kidney appear to be rapidly progressive in children, suggesting that early intervention is necessary in patients with this condition, in order to preserve renal function.
References
Atkinson AB, Brown JJ, Davies DL, Fraser R, Leckie B, Lever AF, Morton JJ, Robertson JIS (1979) Hyponatremic hypertensive syndrome with renal-artery occlusion corrected by captopril. Lancet ii:606–609
Blanc F, Bensman A, Baudon JJ (1991) Renovascular hypertension: a rare cause of neonatal salt loss. Pediatr Nephrol 5:304–306
Brown JJ, Davies DL, Lever AF, Robertson JIS (1965) Plasma renin concentration in human hypertension. 1: Relationship between renin, sodium, and potassium. Br Med J 2:144–148
Casalini E, Sfondrini MS, Fossali E (1995) Two-year clinical follow-up of children and adolescents after percutaneous transluminal angioplasty for renovascular hypertension. Invest Radiol 30:40–43
Dahlem P, Groothoff JW, Aronson DC (2000) The hyponatremic hypertensive syndrome in a 2-year-old child with behavioural symptoms. Eur J Pediatr 159:500–502
Dixit MP, Hughes JD, Theodorou A, DIxit NM (2004) Hyponatremic hypertensive syndrome (HHS) in an 18-month-old child presenting as malignant hypertension: a case report. BMC Nephrol 5:5
Estepa R, Gallego N, Orte L, Puras E, Aracil E, Ortuño (2000) Renovascular hypertension in children. Scand J Urol Nephrol 35:388–392
Fiselier TJW, Lijnen P, van Munster P, Jansen M, Peer P (1983) Levels of renin, angiotensin I and II, angiotensin-converting enzyme and aldosterone in infancy and childhood. Eur J Pediatr 141:3–7
Gouyon JB, Bernardini S, Semama DS, Françoise M (1997) Salt depletion and dehydration in hypertensive preterm infants. Pediatr Nephrol 11:201–204
Kaneko K, Shimazaki S, Ino T, Yabuta K, Nakazawa T, Takahashi H, Kaneko K (1994) Severe hyponatremia in a patient with renovascular hypertension: case report. Nephron 68:252–255
Kluge M, Riedl S, Hofmann BE, Hartmann J, Waldhauser F (1999) Improved extraction procedure and RIA for determination of arginin8-vasopressin in plasma: role of premeasurement sample treatment and reference values in children. Clin Chem 45:103–198
McLaren CA, Roebuck DJ (2003) Interventional radiology for renovascular hypertension in children. Techniques Vasc Intervent Radiol 6:150–157
Ng CS, de Bruyn R, Gorden I (1997) The investigation of renovascular hypertension in children: the accuracy of radio-isotopes in detecting renovascular disease. Nucl Med Commun 18:1017–1028
Norling LL, Chevalier RL, Gomez RA, Tegtmeyer CJ (1992) Use of interventional radiology for hypertension due to renal artery stenosis in children. Child Nephrol Urol 12:162–166
Slovut DP, Olin JW (2004) Fibromuscular dysplasia. N Eng J Med 350:1862–1871
Trivelli A, Ghiggeri GM, Canepa A, Oddone M, Bava G, Perfumo F (2005) Hyponatremic-hypertensive syndrome with extensive and reversible renal defects. Pediatr Nephrol 20:102–104
Acknowledgements
We thank Dr. Masahiro Kiai at Minoh City Hospital, Drs. Tamao Watanabe and Shigeyuki Echigo at the National Cardiovascular Center, Professor Youji Katsuoka and Haruhito Azuma at Department of Urology of Osaka Medical College, and Professor Hiroshi Toma and Dr. Kazunari Tanabe at Department of Urology of Tokyo Women’s Medical University Hospital for clinical assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashida, A., Matsumura, H., Inoue, N. et al. Two cases of hyponatremic-hypertensive syndrome in childhood with renovascular hypertension. Eur J Pediatr 165, 336–339 (2006). https://doi.org/10.1007/s00431-005-0048-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-005-0048-1