Abstract
It is important to measure the rate of haemolysis in patients with sickle cell disease (SCD) to identify aplastic crises and indirectly assess the rate of vaso-occlusion and sequestration. The aim of this study was to assess whether end-tidal carbon monoxide (ETCOc) levels in children with sickle cell disease (SCD) could be measured reproducibly, reflected haemolysis and whether they were elevated compared to those of similarly aged, ethnic matched children without SCD (controls). ETCOc levels were measured non-invasively in 87 SCD children (age range 2.3–17.6 years) and 26 age and ethnic origin matched healthy controls using an electro-chemical sensor. The within- and between- occasion reproducibilities were assessed in ten and 15 SCD children respectively. ETCOc levels of 15 SCD children undergoing regular transfusions were related to carboxyhaemoglobin, haemoglobin and bilirubin levels. The within and between occasions’ mean intrasubject coefficients of reproducibility were 5% and 18% respectively. Positive correlations were found between the ETCOc and carboxyhaemoglobin ( P =0.007) and bilirubin ( P =0.02) levels, and a significant negative correlation between the ETCOc and haemoglobin ( P =0.0002) levels. The mean and SD ETCOc levels of the SCD children (4.9 ppm; SD 1.7 ppm) were significantly higher than that of the controls (mean 1.3 ppm; SD 0.4 ppm) (difference between means 3.60; 95% C.I. 2.93–4.28; P <0.0001). Conclusion:These results suggest that measurement of end-tidal carbon monoxide levels is a reliable and useful method to monitor haemolysis in children with sickle cell disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
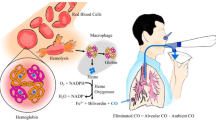
It is important to measure the rate of haemolysis in patients with sickle cell disease (SCD) to identify aplastic crises and indirectly assess the rate of vaso-occlusion and sequestration. Haemolysis can be assessed by analysing the carboxyhaemoglobin level of venous blood samples. The haem released from premature destruction of erythrocytes in patients with SCD, is degraded by the haem oxygenase (HO) enzyme complex to equimolar amounts of carbon monoxide (CO) and biliverdin [1,16] and CO combines with haemoglobin to form carboxyhaemoglobin. To assess carboxyhaemoglobin levels, a chromatographic technique [15] and closed rebreathing system have been used. Unfortunately, the latter technique is invasive and requires marked patient cooperation, making it unsuitable for use in young children. An alternative technique to assess haemolysis is to measure exhaled end-tidal breath carbon monoxide (ETCO) levels [6]. The catabolism of haem from erythrocytes accounts for approximately 70% of the body’s CO production [6]. ETCO levels have been shown to distinguish between children with haemolytic disorders, including beta-thalassaemia and hereditary spherocytosis, and healthy controls [3]. A volitional technique, however, was used which cannot be performed by all patients, particularly young children, and no patients with SCD were included. It was important, therefore, to establish whether a non-volitional, non-invasive technique to assess CO levels was well tolerated and reproducible in SCD children. Our aim was to provide such data and to test the hypothesis that ETCO levels would reflect haemolysis and be higher in SCD children than in similarly aged and ethnic origin matched controls. The CO-Stat device used to measure ETCO computes the ETCO value from the end-tidal carbon dioxide concentration and the mean concentrations of carbon dioxide and CO over the period of breath sampling. End-tidal carbon dioxide, however, only reflects alveolar carbon dioxide when there is a plateau in the tidal carbon dioxide curve. In subjects with a fast respiratory rate, the plateau may not be reached and, therefore, the ETCO level underestimated. Hence, in addition, we wished to determine if ETCO levels in children were affected by respiratory rate and/or age.
Subjects and methods
Subjects
A total of 72 SCD children in steady state with a median age of 9.5 years (range 2.3 to 17.6 years) and 26 controls with a median age of 8.1 years (range 2.6 to 17.8 years) were included in the study. In addition, 15 SCD children with a median age of 12.8 years (range 5.3 to 17.5 years) undergoing regular transfusions were studied. None of the children admitted to smoking. Four of the SCD children (none in the transfusion group) and five of the controls were asthmatic and taking regular medication.
Sample size
Recruitment of 15 patients for paired measurements was necessary to allow detection of a difference in ETCOc levels equivalent to one SD of the measurement, with 80% power at the 5% level. Recruitment, therefore, continued until there were 15 SCD children who had had paired measurements at least 2 weeks apart.
Methods
Children with SCD were recruited from two specialist clinics. Siblings without SCD and local school children of the same ethnic origin were recruited as controls. The study was approved by the research ethics committee of King’s College Hospital and parents gave informed written consent for their child to take part.
ETCO levels and respiratory rate were measured using an ETCO monitor (CO-Stat, Natus Medical Inc, San Carlos CA, USA). The system comprises two analysers, an electrochemical sensor, to measure CO and hydrogen, and an infrared analyser that measures carbon dioxide. The carbon dioxide signal from the infrared analyser is used to determine the end-tidal carbon dioxide level and the respiratory rate. The results of end-tidal carbon dioxide and the mean concentrations of carbon dioxide and CO over the period of breath sampling are used to compute the ETCO level. No child was measured within 2 weeks of an upper respiratory tract infection and the SCD children were not measured within 4 weeks of hospitalisation for a vaso-occlusive episode. To measure ETCO, a small sampling catheter was inserted 5 mm inside the child’s nostril and measurements made with the child seated. Testing was completed within 3 min of quiet tidal breathing. After each measurement, the instrument analysed the background, or inhaled CO level, which was then deducted from the patient’s value to give a corrected ETCO measurement (ETCOc). The monitor also measured the background hydrogen concentration, as this can interfere with measurement of CO levels. If the hydrogen concentration exceeded 50 ppm, the instrument terminated breath sampling and the ETCOc test was aborted.
To assess the reproducibility of the results of the ETCOc measurements, ten children each had two measurements on a single occasion and a further 15 SCD children were re-tested after at least a 2-week period. None of the 15 children had suffered a vaso-occlusive episode in the interim. None of the children in either group were receiving regular transfusion therapy.
To determine if the ETCOc levels accurately reflected haemolysis, measurements were made in SCD children undergoing regular transfusions to prevent recurrence of a major complication, such as stroke [11]. These children have blood samples immediately prior to the transfusion and thus it was possible to relate the ETCOc levels to carboxyhaemoglobin, haemoglobin, reticulocyte and bilirubin levels. As blood samples were not routinely measured during outpatient visits, such comparisons were not possible in non-transfused SCD children.
Statistical analysis
Normality testing using the Kolmogorov-Smirnov test demonstrated the data to be normally distributed. Differences were, therefore, assessed for statistical significance using the Student’s t test. Pearson correlation coefficients were calculated to determine the relationships between ETCOc levels and carboxyhaemoglobin, haemoglobin, reticulocyte and bilirubin levels and age.
Results
ETCOc measurements were well tolerated by all the children. The within occasion mean intra-coefficient of reproducibility of the ETCOc measurements was 5%. A group of 15 children had their ETCOc measurements repeated after a median of 8 weeks (range 2 to 36 weeks), their mean intra-coefficient of repeatability was 18% and the mean difference in the results of the paired measurements was 1.2 ppm, which was equivalent to one SD of the ETCOc levels of the SCD children. The mean intra-coefficient of repeatability was not greater in children aged 5 years or less (15%) compared to SCD children older than 5 years (19%). Although there was no significant correlation between the ETCOc and reticulocyte levels, the ETCOc levels of the transfused SCD children correlated positively with the carboxyhaemoglobin ( r 2=0.42, P =0.007) and bilirubin ( r 2=0.31, P =0.02) levels and negatively with the haemoglobin ( r 2=0.54, P =0.002) levels (Fig. 1). ETCOc levels were correlated with age in the SCD children ( r 2=0.28, P <0.0001) and their controls ( r 2=0.33, P =0.001) (Fig. 2). The age-related increase in ETCO levels was not associated with respiratory rate in either the SCD children ( r 2=-0.06, P =0.6) or healthy controls ( r 2=-0.23, P =0.3). SCD children had significantly higher mean ETCOc levels (4.9 ppm, SD 1.7 ppm) than that of the controls (1.3 ppm SD 0.4 ppm) (Difference between means 3.60; 95% C.I. 2.93–4.28; P <0.0001).
Discussion
We have demonstrated that ETCOc levels are significantly elevated in children with SCD compared to controls. The measurement technique was well tolerated, even in very young children with SCD. The ETCO analyser used has been shown to perform similarly to a previous device in adults and children [18]. It is important, however, when undertaking the measurement to analyse the room air as an index of inhaled CO and thus the rate of endogenously produced CO can be appropriately corrected [19]. There is a relatively long-lasting effect of CO exposure, as carboxyhaemoglobin has a half-life of up to 6 h; thus, it is also important to take into account prior exposure to high levels of CO from gas heaters, internal combustion engines and tobacco smoke. None of the children examined admitted to smoking. Passive smoking has a significant effect on exhaled CO levels, as levels are doubled in non-smoking adolescent subjects whose mothers smoke [8]. No parent admitted to smoking near their child but, as urinary cotinine levels were not assessed, the smoking status of the child or the possibility of the child passively smoking cannot be certain. Nevertheless, the repeated measurements at least 2 weeks apart were similar, suggesting environmental contamination had not been a problem. The similarity of the repeated measurements over time and the within occasion mean intra-coefficient of variation demonstrate that the technique gives reproducible results.
Our findings highlight that the ETCOc levels measured in the SCD children reflect haemolysis, as significant positive correlations were demonstrated between the ETCOc and both the carboxyhaemoglobin and bilirubin levels and a significant negative correlation with the haemoglobin level.
Two forms of HO have been described; the constitutive HO-2 and the inducible HO-1, which is ubiquitously distributed [13]. The latter is induced by a variety of pro-inflammatory cytokines [2], oxidants [9], nitric oxide [7], endotoxin [12] and reactive oxidant species [5] and is part of the protective response to oxidative stress. Patients with cystic fibrosis, particularly those not receiving treatment with steroids, have higher ETCOc levels compared to healthy subjects [13], which may reflect increased lung oxidation resulting in oxidation induced HO-1 hyperactivity [13]. ETCOc levels are also higher in children with persistent asthma, but are not raised in those with infrequent episodic asthma [17]. Very few of the children in this study were taking anti-asthma medication and, as the proportion was similar in the SCD children and the controls, we do not feel this biased the results. We also took care not to measure the children within 2 weeks of an upper respiratory tract infection, as ETCOc levels are raised in healthy children with this condition during the acute symptomatic phase [17]. Thus, we feel that the significant difference we demonstrate in ETCOc levels between the SCD children and the controls is genuine.
An explanation for the relationship we demonstrate between ETCOc levels and age which needs to be considered is that the younger children had a faster respiratory rate than the older children. In individuals with a very fast respiratory rate ETCO levels may be underestimated. We, however, demonstrated no significant relationship between ETCOc levels and respiratory rate and, hence, do not feel differences in respiratory rate do explain the relationship between ETCOc levels and age. We also demonstrated that younger SCD children did not have increased variability in their ETCOc measurements. In the absence of cotinine measurements, we cannot exclude the higher ETCOc levels in certain of the older children may at least in part be explained by them smoking, but again we emphasise none admitted to smoking. In addition, we saw a linear relationship with age and ETCOc levels and this seems very unlikely to be totally explained by covert cigarette smoking. Interestingly, the ETCOc levels in the SCD children appeared to increase at a greater rate with age than in the controls. A possible explanation for the relationship in the SCD children could be that the older children have more marked haemolysis but, as we did not undertake invasive monitoring in the steady state children, this must remain speculative. Our results, however, do suggest that age should be taken into account when interpreting ETCOc levels in SCD children.
The variability in the ETCOc measurements appeared to increase with age, which may reflect that the haemolytic rate differs considerably between patients [15]. A potential explanation is the persistence of fetal haemoglobin demonstrated in SCD children [10] and variability in their levels of fetal haemoglobin, which has been demonstrated in SCD adults [14]. Red blood cells are less prone to sickling when higher levels of fetal haemoglobin are present [4] and, hence, there will be lower levels of haemolysis.
ETCOc levels are raised in children with SCD and can be readily measured by an ETCO device at all ages. This technique may be useful to monitor children with SCD at risk of complications due to high levels of haemolysis.
Abbreviations
- CO :
-
carbon monoxide
- ETCO :
-
end-tidal carbon monoxide
- ETCOc :
-
corrected end-tidal carbon monoxide
- HO :
-
haem oxygenase
- SCD :
-
sickle cell disease
References
Berk, PD, Rodkey FL, Blaschke TF, Collison HA, Waggoner JG (1974) Comparison of plasma bilirubin turnover and carbon monoxide production in man. J Lab Clin Med 83: 29–37
Cantoni L, Rossi C, Rizzardini M, Gadina M, Ghezzi P (1991) Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J 279: 891–894
Chan GCF, Lau YL, Yeung CY(1998) End-tidal carbon monoxide concentration in childhood haemolytic disorders. J Paediatr Child Health 34: 447–450
Charache S, Conley CL (1964) Rate of sickling of red cells during deoxygenation of blood from persons with various sickling disorders. Blood 24: 25–48
Choi AM, Alam J (1996) Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 15: 9–19
Coburn RF, Williams WJ, Kahn SB (1966) Endogenous carbon monoxide production in patients with hemolytic anemia. J Clin Invest 45: 460–468
Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI (1997) Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res 80: 557–564
Gourgoulianis KI, Gogou E, Hamos V, Molyvdas PA (2002) Indoor maternal smoking doubles adolescents’ exhaled carbon monoxide. Acta Paediatr 91: 712–713
Keyse SM, Applegate LA, Tromvoukis Y, Tyrrell RM(1990) Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol Cell Biol 10: 4967–4969
Mason KP, Grandison Y, Hayes RJ, Serjeant BE, Serjeant GR, Vaidya S, Wood WG (1982) Post-natal decline of fetal haemoglobin in homozygous sickle cell disease: relationship to parenteral Hb F levels. Br J Haematol 52: 455–463
Ohene-Frempong K (2001) Indications for red cell transfusion in sickle cell disease. Semin Hematol 38[Suppl 1]: 5–13
Otterbein L, Sylvester SL, Choi AM(1995) Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol 13: 595–601
Paredi P, Shah PL, Montuschi P, Sullivan P, Hodson ME, Kharitonov SA, Barnes PJ (1999) Increased carbon monoxide in exhaled air of patients with cystic fibrosis. Thorax 54: 917–920
Serjeant GR (2001) Sickle cell disease. Oxford University Press, Oxford
Solanki DL, McCurdy PR, Cuttitta FF, Schechter GP (1988) Hemolysis in sickle cell disease as measured by endogenous carbon monoxide production. A preliminary report. Am J Clin Pathol 89: 221–225
Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394
Uasuf CG, Jatakanon A, James A, Kharitonov SA, Wilson NM, Barnes PJ (1999) Exhaled carbon monoxide in childhood asthma. J Pediatr 135: 569–574
Vreman HJ, Wong RJ, Harmatz P, Fanaroff AA, Berman B, Stevenson DK(1999) Validation of the Natus CO-Stat end tidal breath analyzer in children and adults. J Clin Monit Comput 15: 421–427
Vreman HJ, Wong RJ, Stevenson DK (2000) Exhaled carbon monoxide in asthma. J Pediatr 137: 889–891
Acknowledgements
Karl Sylvester and Richard Patey are supported by the Community Fund and Well Child Trust Medical Research Fund. All consumables were kindly donated by Natus Medical Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sylvester, K.P., Patey, R.A., Rafferty, G.F. et al. Exhaled carbon monoxide levels in children with sickle cell disease. Eur J Pediatr 164, 162–165 (2005). https://doi.org/10.1007/s00431-004-1605-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-004-1605-8