Abstract
Data about the prevalence of human papillomaviruses (HPV) in African women with normal and abnormal cervical cytology are still scarce. Current HPV vaccines contain HPV types, which mainly represent the HPV epidemiology of industrial countries. As further developments of HPV vaccines are going on, it is necessary to regard regional differences in HPV type prevalence to ensure optimal protection by the vaccine. Vaginal swabs of Ghanaian pregnant women, routinely collected before delivery to rule out bacterial infections causing early onset sepsis, were screened for 12 high-risk (HR), 13 probably/possibly (pHR), and 18 low-risk (LR) HPV types. Most pregnant women come for delivery to the hospital. This was considered as appropriate possibility to have an unselected group of women. HPV DNA were detected in 55/165 women (33.3, 95 % CI 26.3–41.1 %). Thirty-four out of fifty-five (61.8, 95 % CI 47.7–74.3 %) of HPV-positive women were infected with HR and/or pHR HPV types. The five most prevalent HR or pHR HPV types were HPV-52 and HPV-67 (7 women each, 4.2, 95 % CI 1.9–8.9 %), HPV-53 (six women, 3.6, 95 % CI 1.5–8.1 %), HPV-45 (five women, 3.0, 95 % CI 1.1–7.3 %), and HPV-18 (four women, 2.4, 95 % CI 0.8–6.5 %), respectively. HPV-16 was found in two women only (1.2, 95 % CI 0.2–4.8 %). Future HPV vaccine research may devote special interest to HPV-67 and HPV-53 provided further studies confirm their high prevalence in the general population of Sub-Saharan African countries. The true carcinogenic potential of HPV-67, which is a member of species alpha9 including HPV-16, and so far categorized as pHR, should be clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human papillomaviruses (HPV) of the genus alpha commonly infect the anogenital tract without necessarily causing pathologic lesions. In women, 90 % of infections clear spontaneously. However, following persistent infection with one or more high-risk (HR) HPV types, precursor cervical intraepithelial neoplasias (CIN) and cervical cancer might develop. Additional risk factors, such as a high number of sexual partners (e.g. in prostitution and with promiscuity) or immunosuppression (e.g. in HIV-positive persons or transplant recipients), are well-known cofactors for higher HPV susceptibility and persistence [1]. During pregnancy, a physiological Th1–Th2 shift occurs leading to a systemic dominance of humoral immunity and suppression of cell-mediated immunity, which may favour the increased susceptibility to intracellular pathogens like HPV [2, 3].
Cervical cancer is the fourth most common cancer in women worldwide [4], and is estimated to be the most frequent cause of malignant disease in women of all ages in Ghana, a lower-middle-income country. The estimated age-standardized incidence rate amounts to 35.4 per 100,000 women, which is the fourth highest rate compared to all 15 Western African countries [5].
Cervical cancer is also estimated to be the first cause of deaths from malignant disease in Ghanaian women. Regarding all 15 Western African countries, Ghana takes the seventh highest position with an age-standardized mortality rate of 18.9 per 100,000 women [5].
Epidemiologic data on HPV prevalence among African women with normal and abnormal cervical cytology [6–8] as well as those obtained with histological specimens from the cervix [9–11] are still scarce compared to data available from other parts of the world [12–15]. According to the International Agency for Research on Cancer (IARC) in 2012, HR HPV types with sufficient evidence causing cancer of the cervix are HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59 (group 1 = carcinogenic to humans, HR). In addition, HPV-68, classified as probably carcinogenic (group 2A), and HPV-26, -30, -34, -53, -66, -67, -69, -70, -73, -82, -85, and -97, classified as possibly carcinogenic to humans (group 2B), are grouped as pHR HPV [1].
The current vaccines contain HPV-16 and -18, which have the highest oncogenic potential [16], and are responsible for up to 70 % of all cervical cancers in patients worldwide [17]. HPV-31, -33, -35, -45, -52, and -58 contribute to another 20 % of cervical cancers [17]. Nevertheless, these 20 % of cervical cancers show regional differences in HR HPV type prevalence [10, 18, 19]. Furthermore, HPV-16 and -18 are found in up to 38 % of low-grade and in up to 64 % of high-grade cervical lesions, respectively [20, 21].
With availability of the first generation HPV vaccines, quadrivalent Gardasil® (Merck & Co., Inc.) and bivalent Cervarix® (GlaxoSmithKline Biologicals), for almost 10 years now, there is an excellent tool for primary prevention of HPV infections and HPV-related diseases [22, 23]. The use of the second generation, nonavalent HPV vaccine GARDASIL® 9 (Merck & Co., Inc.), which was approved by the US Food and Drug Administration in 2014 and by the European Medicines Agency in 2015, in clinical practice has started. This new vaccine contains virus-like particles of low-risk (LR) HPV-6 and HPV-11 as well as the HR HPV types HPV-16, -18, -31, -33, -45, -52, and -58.
As further developments of HPV vaccines are going on, it is necessary to regard regional differences in HPV type prevalence to ensure the optimal protection by the vaccine. Our study was designed to contribute to improve the knowledge of the HPV type distribution in Ghana.
Materials and methods
Period and place of study
Between October 2011 and January 2012, a cross-sectional study investigating the HPV prevalence in pregnant women before delivery was conducted at St. Martin de Porres Hospital in Eikwe, Ghana. This hospital is a partner institution of the University Medical Center Göttingen (UMG). Since the year 2000 the Institute for Medical Microbiology of the UMG supports the running of the bacteriology laboratory [24].
The St. Martin de Porres Hospital in Eikwe is a general hospital located in a rural coastal village in the Western region of Ghana with 175 beds serving a population of about 380,000 people. Hospital service includes obstetrics and gynaecology (approximately 150 deliveries/month), general surgery, internal medicine, paediatrics, preventive care services, and a regional HIV treatment centre. At the time of the study, medical staff consisted of two specialists for obstetrics and gynaecology and four physicians in training.
HPV screening of vaginal swabs
On the initiative of the chief medical officer of the St. Martin de Porres Hospital, a gynaecologist, the Institute for Medical Microbiology of the UMG was requested for support to screen pregnant women for HPV infection to get information about the local HPV prevalence. Many women especially from rural areas do not attend antenatal clinic regularly but most of them come to the hospital for delivery. This was considered as appropriate possibility to have an almost unselected group of women at almost the same stage of pregnancy.
Vaginal swabs of Ghanaian pregnant women, routinely collected before delivery to rule out bacterial infections, which can cause early onset sepsis, were screened for 12 HR, 13 pHR and 18 LR HPV types.
The study was approved by the ethical committee of the University Medical Center Göttingen, Germany (number 14/10/11), and the authority of the St. Martin de Porres Hospital in Eikwe, Ghana.
Patient selection and inclusion criteria
Pregnant women to be included in this study were admitted from Monday till Friday between 8 am and 4 pm. During this time, a translator (trained nurse) for the two most common local languages, Nzema and Akan, was available to obtain informed consent and to fill out a questionnaire together with the pregnant women asking for origin, journey time, demographic data, and obstetric history. Inclusion criteria were gestational age ≥39 weeks or delivery within ≤1 week. The study population consisted of 177 pregnant women attending the antenatal clinic of the hospital.
Vaginal swab collection
Part of antenatal care is a microbiological screening for pathogenic bacteria like B streptococci by vaginal swab taken by a trained nurse. The vaginal specimen was obtained approximately 2 cm cranial of the introitus vaginae by using the ESwab system (Copan Liquid Amies Elution Swab; Copan Italia S.P.A.). It consists of an applicator swab with flocked nylon fibre tip and a polypropylene screw-cap tube containing 1 mL of modified liquid amies preservation medium. Following collection, the swab was put in the tube, immediately sent to the laboratory, and there inoculated and streaked at a chocolate and a blood agar plate for bacteriological culture. Thereafter, the swab was put again in the tube and kept at 4 °C until further processed for HPV testing. No additional swab was collected for HPV testing.
Human papillomavirus typing
A portion (200 μL) of the swab specimen was subjected to nucleic acid purification by using the MagNA Pure LC 2.0 instrument in combination with the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics GmbH) according to the instructions of the manufacturer.
To obtain the highest sensitivity, HPV DNA detection and typing were done using two different broad spectrum PCRs (A6/A8- and BSGP5 + 6 + -PCR), which are based on different primers and amplification protocols (nested vs. single round PCR) with slight variations in their sensitivity for the detection of different HPV types.
Hybridization of PCR products was performed to 43 (12 HR, 13 pHR, and 18 LR) type-specific probes by using bead-based multiplex genotyping on a Luminex Lx100 as described before [25–28] (M. Schmitt, personal communication). The probe sequences are summarized in Supplementary Table 1. The tested HR HPV types were: HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59. The tested pHR HPV types were -26, -30, -34, -53, 66, -67, -68, -69, -70, -73, -82, -85, and -97. The tested LR HPV types were: HPV-6, -11, -27, -40, -42, -43, -44, -54, -55, -57, -61, -71, -72, -81, -83, -84, -89, and -177. Any A6/A8 PCR sample, that tested positive in agarose gel electrophoresis, but did not hybridize in the Luminex assay, was sequenced as described before [29].
Quantification of the β-globin gene by real-time PCR was performed to ensure that sufficient cellular material for investigation had been collected and additionally serving as an internal control to rule out the presence of inhibitory factors [28]. A vaginal smear with four (=2 cells) or less β-globin gene copies was considered as not evaluable.
Statistical analysis
Statistical analysis was performed with STATISTICA, version 10, for Windows (StatSoft GmbH, Hamburg, Germany). Pairwise comparisons between groups were conducted, using Student’s t test for continuous variables, Chi-Quadrat-test for categorical variables and Fishers exact test for dichotomous variables.
Kendall’s tau (τ) coefficient was used to test for dependency of non-parametric variables.
P values were regarded as significant at P < 0.05 and Bonferroni Holms procedure was used to adjust the P value to obtain an overall significance of P < 0.05.
Results
Human papillomavirus prevalence
Of the 177 vaginal swabs from pregnant women, 165 (93.2 %) were of good quality (ß-globin-positive) and were included in the study. HPV was found in 55 of the 165 vaginal swabs representing a prevalence of 33.3 % (95 % CI 26.3–41.1 %). The only HIV-positive woman in the study was HPV-negative.
Human papillomavirus type prevalence
The vaginal swabs were investigated for 43 different HPV types, which included 12 HR, 13 pHR, and 18 LR HPV types (Supplementary Table 1).
Ninety-three HPV infections, caused by nine different HR HPVs, nine different pHR HPVs, and 12 different LR HPVs, respectively, were detected in 55 females (Supplementary Table 2).
Infection with a single HPV type occurred in 35 women (21.2; 95 % CI 15.4–28.4 %), and infections with multiple HPV types were identified in 20 women (12.1; 95 % CI 7.7–18.3 %; Table 1).
In 23 women (13.9, 95 % CI 9.2–20.4 %), 30 HR HPVs were detected [single infection in eight women (4.9, 95 % CI 2.3–9.7 %); multiple infections in 15 women (9.1, 95 % CI 5.4–14.8 %)]. In 22 women (13.3, 95 % CI 8.7–19.7 %), 23 pHR HPVs were detected [single infection in eight women (4.9, 95 % CI 2.3–9.7 %); multiple infections in 14 women (8.5, 95 % CI 4.9–14.1 %)]. In 31 (18.8, 95 % CI 13.3–25.8 %) women, 40 LR HPVs were detected [single infections in 19 women (11.5, 95 % CI 7.3–17.6 %); multiple infections in 12 women (7.3, 95 % CI 4.0–12.6 %)] (Table 1).
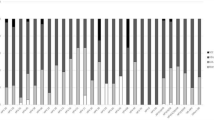
The most prevalent HPV types were LR HPV-6 (eight patients, 4.8, 95 % CI 2.3–9.7 %), HR HPV-52, pHR HPV-67, and LR HPV-61 (seven patients each, 4.2, 95 % CI 1.9–8.9 %), pHR HPV-53 and LR HPV-54 (six patients each, 3.6, 95 % CI 1.5–8.1 %), HR HPV-45 (five patients, 3.0, 95 % CI 1.1–7.3 %), and HR HPV-18 and LR HPV-42 (four patients each, 2.4, 95 % CI 0.8–6.5 %). HR HPV-16 was found in two patients only (1.2, 95 % CI 0.2–4.8 %) (Fig. 1). HR HPV-33, -39, -56, pHR HPV-26, -70, -85 and 97, and LR HPV-11 were not detected.
Prevalence (%) of HR, pHR, and LR HPV types in 165 pregnant women. aClassification of HPV types according to the International Agency for Research on Cancer (IARC) in 2012; group 1, carcinogenic to humans, HR; group 2A, probably carcinogenic to humans, pHR; group 2B, possibly carcinogenic to humans, pHR; group 3, not classifiable as to their carcinogenicity to humans, LR [1]. The bars show the prevalence of each HPV type listed in the respective line. HPV types included in the nonavalent vaccine Gardasil 9 are marked in bold. HPV human papillomavirus, HR high-risk, pHR probably/possibly high-risk, LR low-risk, IARC International Agency for Research on Cancer
Human papillomavirus status and age
The age of 146 (88.5 %) of the 165 women was available. Of these 146 women, 49 were HPV positive and 97 were HPV negative. The median age was 26 years (range 14–48 years; mean age 26.3 ± 6.6). The median age of the 49 HPV-positive and the 97 HPV-negative mothers was 23 years (range 14–39 years; mean age 23.9 ± 5.5) and 27 years (range 15–48 years; mean age 27.5 ± 6.8), respectively. HPV-positive women were significantly younger than HPV-negative women (P = 0.0018; Fig. 2). The HPV frequency in women <25 years (n = 60) was 45.0 % (95 % CI 32.3–58.3 %) compared to 25.6 % (95 % CI 17.1–36.3 %) in women ≥25 years (n = 86). There was a negative association between the number of identified HR, pHR, and LR HPV types in a woman and her age (τ = −0.16, τ = −0.15, and τ = −0.23, respectively), and the infections were significantly more frequent in younger women (P = 0.0032, P = 0.0084 and P < 0.0001, respectively). Triple infections were only detected in women <25 years.
Age distribution of HPV-negative and HPV-positive women. The lower and the upper end of the boxes represent the 25th and 75th percentile, respectively. The minimum age was 15 years in HPV-negative and 14 years in the HPV-positive group. ***P = 0.0018, according to Student’s t test. HPV human papillomavirus
The frequency of LR HPV-6 and pHR HPV-67 was particularly high in women <25 years compared to women ≥25 years (7:1 and 6:1, respectively). Both HPV types were detected mainly in co-infections, which were found only in women <25 years. The HPV type distribution in co-infections is detailed in Table 1.
Human papillomavirus status, parity, and age
Among 148 (89.7 %) of the 165 pregnant women, information about their parity was available. Primipara (n = 47) were significantly more frequent HPV positive with 53.2 % (95 % CI 38.2–67.6 %) compared to multipara (n = 101) with 21.8 % (95 % CI 14.4–31.3 %; P = 0.0001). Among 131 (79.4 %) of the 165 mothers, both age and parity were recorded. HPV-positive multipara (n = 21; mean age: 21.2 ± 4.5) were significantly younger than HPV-negative multipara (n = 69; mean age: 29.5 ± 6.2; P = 0.0086). There were no significant age differences in HPV-negative (n = 20; mean age: 21.2 ± 4.5) and HPV-positive primipara (n = 21; mean age: 21.0 ± 3.9; P = 0.9702).
Discussion
This study investigated the HPV status in Ghanaian pregnant women from a rural area before delivery. Routine vaginal swabs were screened for 12 HR, 13pHR, and additionally for 18 LR HPV types [1].
The prevalence of HR HPV types in vaginal and cervical specimens has been shown to be similar as carcinogenic HPV types infect the entire lower genital tract equally [30]. A vaginal swab can be considered as alternative to a cervical swab [30, 31] and may be preferred by the woman, as it is less invasive since no speculum is needed.
In our study, 93.2 % of the swabs contained sufficient cellular material as demonstrated by ß-globin DNA detection. LR HPV types are known to be more prevalent in the vaginal squamous cell epithelium than in the metaplastic or columnar tissue of the cervix [30]. This may explain the frequent detection of LR HPV types in the present study. HPV-6 was the most prevalent HPV type with 4.8 %. HPV-61 with 4.2 % and HPV-54 with 3.6 % belong also to the most frequent detected HPV types (Fig. 1; Supplementary Table 2).
A meta-analysis by Liu et al. [14] of the risk of HPV infection in pregnant women from Asia, Europe and North America revealed a significantly increased risk (summary odds ratio 1.42) resulting in a prevalence of 16, 13, and 30 %, respectively. Furthermore, a higher HPV prevalence in the younger age group of pregnant women was shown (24 % in women <25 years of age and 14 % in women ≥25 years of age) [14], which is in line with the widely accepted higher prevalence in younger women. Our results confirmed the findings of the meta-analysis. After sexual debut, HPV prevalence rises sharply up to a region specific maximum and then declines rapidly after the age of 20–25 years as a result of developing immunity and elimination of HPV as well as less sexual activity [32, 33].
Brandful et al. [7] found a HPV prevalence of 64.5 % in healthy, HIV-negative women between 18 to 41 years of age at various stages of pregnancy attending the antenatal clinic from the university hospital in Accra, Ghana. This prevalence is much higher than we detected in pregnant women with rural residence. Brandful et al. did not discuss the results in relation to other prevalence studies in pregnant women but only in reference to Attoh et al. [9] a study performed on cervix carcinomas in Ghanaian women. The higher prevalence could be explained to some extent by a younger mean age compared to our study population. However, the mean age is not provided [7]. According to the Ghana Demographic and Health Survey 2014, the percentage of women who reported having in the past 12 months a sexually transmitted infection, genital discharge, sore or ulcer was 27.2 % in women with urban residence and 21.8 % in women with rural residence, respectively [34]. This might have been also contributed partly to the higher HPV prevalence in the study of Brandful et al. [7]. Ultimately, the reasons for the higher HPV prevalence remain largely unclear.
Taking into account the about 1.4-fold increased risk of HPV infection in pregnant women calculated in the meta-analysis by Liu et al. [14], the prevalence of 33.3 % observed in our study correlates with a prevalence of 25 and 27 % described for non-pregnant women with normal cytological findings from Nigeria [35], and Benin [36], respectively. Recent data by Ogembo et al. 2015 estimate the HPV prevalence in Western Africa (Benin, Côte d’Ivoire, Gambia, Ghana, Guinea, Mali, Nigeria, Senegal) with 28.7 % (95 % CI 27.1–30.8 %) [21].
Yar et al. [8] investigated cervicovaginal swabs of non-pregnant HIV-positive and HIV-negative women from metropolitan Kumasi, and found a HPV prevalence of 86.9 and 56.0 %, respectively. HIV is a long known risk factor for a higher HPV prevalence [1]. The higher HPV prevalence in HIV-negative women (mean age 40.9 ± 11.3 years), compared to the prevalence we found in pregnant women (mean age 26.3 ± 6.6 years), might be explained in part through a higher prevalence of sexually transmitted infection, genital discharge, sore or ulcer in women with urban compared to rural residence (27.2 vs. 21.8 %) [34]. Yar et al. [8] did not specify any underlying disease in the study group of HIV-negative women. It is only mentioned that these women attended the outpatient department of the hospital. It is conceivable that these women might have suffered from non-communicable diseases, which may lead to immunosuppression to some degree, contributing to a higher HPV prevalence. However, it remains speculation.
The prevalence of identified HR HPV types in Ghanaian pregnant women differs considerably from the worldwide ranking [13]. The most frequently detected HR and pHR HPV types in our study were HPV-52 and HPV-67 (4.2 % each) and HPV-53 (3.6 %). In contrast, HPV-16 and HPV-18 were relatively rare (Fig. 1 and Supplementary Table 2), which is in line with the findings of Brandful et al. from Ghana placing HPV-16 and HPV-18 on rank eight [7].
Yar et al. [8] found in cervicovaginal swabs of HIV-negative, non-pregnant women the following most prevalent HR and pHR HPV types in descending order: HPV-58 (10.9 %), -35, -70, -82 (10.0 % each), and -31 (7.0 %). Except pHR HPV-70, which was not detected in our study, and pHR HPV-67, which was not investigated by Yar et al., all the other HPV types were also detected in our study but less prevalent. The prevalence of HR HPV-52 and pHR-53 (5.0 % each) and the low prevalence of HPV-16 (2.0 %) and HPV-18 (1.0 %) [8], are similar to our results from the rural coastal Eikwe. Differences in HPV type distribution may be partially due to the different geographical locations.
Three studies performed on cervical cancer specimens, all from Accra, Ghana, from 2003 by Attoh et al. [9], 2004–2006 by Awua et al. [11] and 2007–2010 by Denny et al. [10], respectively, found a HPV prevalence of 98.0, 89.8, and 93.9 %, respectively. HPV type-specific prevalence was quite different. Attoh et al. [9] detected most frequently HPV-18 (84 %), HPV-16 (24 %), HPV-45 (6 %), and HPV-39 (4 %). Awua et al. found HPV-18 (47.4 %), HPV-59 (42.2 %), HPV-45 (37.4 %), and HPV-16 (10.0 %) to be the most prevalent HPV types [11]. In contrast, Denny et al. detected most common HPV-16 (55.4 %), HPV-18 (19.0 %), HPV-35, and -45 (6.6 % each) [10]. The different prevalence of HPV-16 and HPV-18 is striking, but cannot be explained by different squamous cell carcinoma/adenocarcinoma ratios either by the geographical location. However, differences in HPV genotyping might have been contributed.
Irrespective of the HPV-16/-18 difference, the high ranking of HPV-16, -18, and -59 in cervical cancers in comparison to HPV-52, -53, and -67 in the pregnant women of our study is not unexpected because of the enrichment of particularly high risk types in cancers [37, 38]. This may also explain the fact that HPV-53 belonged to the most frequently detected HPV types in our study, but was found as single infection in only one cervical cancer from Nigeria in the study by Denny et al. [10].
HPV-67 was not included in the studies of cervical cancer in Ghana [9–11]. The carcinogenic potential of HPV-67 is still poorly defined. Its classification as pHR type is based on a limited number of HPV-67-positive CIN and cervical cancers [39–42]. One reason for the limited data on HPV-67 is most probably the lack of HPV-67-specific probes in commercially available HPV genotyping systems used in major studies on HPV prevalence [10]. In a recent meta-analysis by Bzhavala et al., HPV-67 was detected in 0.2 % of normal cervical specimens versus 0.3 % in invasive cervical cancers [38], which may hint to a certain carcinogenic potential. HPV-67 is the only member of species alpha9 including HPV-16, which so far is not categorized as carcinogenic [1]. HPV-67 is not included in the just recently licensed nonavalent HPV vaccine (Gardasil 9) [43], but may be a candidate to be added in future HPV vaccines, if more information about its prevalence and carcinogenic potential is available.
Although the number of women included in the study was relatively low, this is to the best of our knowledge the first systematic study on prevalence of different HPV types in rural Ghana.
In conclusion, investigations of vaginal swabs from pregnant women taken during routine health programs, like antenatal clinic, may be a good option to screen for HPV and get more information about the local HPV type epidemiology in resource constrained countries. In attempts to make future HPV vaccines more effective for regions with a high prevalence of cervical cancer like Sub-Saharan Africa countries, more prevalence studies should be performed ideally on a national basis. The genotyping in HPV prevalence studies should include HPV-67 to get more information about the real prevalence.
References
International Agency for Research on Cancer (IARC) (2012) Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100:1–441
Jamieson DJ, Theiler RN, Rasmussen SA (2006) Emerging infections and pregnancy. Emerg Infect Dis 12:1638–1643
Szekeres-Bartho J (2002) Immunological relationship between the mother and the fetus. Int Rev Immunol 21:471–495
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Muñoz J, Bosch FX, de Sanjosé S, Castellsagué X. ICO Information Centre on HPV and Cancer (HPV Information Centre) (2016) Human papillomavirus and related diseases in Ghana. Summary Report 2016-02-26. http://www.hpvcentre.net/statistics/reports/GHA.pdf. Accessed 2016-08-24
Czegledy J, Rogo KO, Evander M, Wadell G (1992) High-risk human papillomavirus types in cytologically normal cervical scrapes from Kenya. Med Microbiol Immunol 180:321–326
Brandful JAM, Bonney EY, Asmah RH, Apea-Kubi KA (2014) Oncogenic human papillomavirus (HPV) in women from Ghana. J Cancer Res Exp Oncol 6:31–38
Yar DD, Salifu SP, Darko SN, Annan AA, Gyimah AA, Buabeng KO, Owusu-Dabo E (2016) Genotypic characterisation of human papillomavirus infections among persons living with HIV infection; a case–control study in Kumasi, Ghana. Trop Med Int Health 21:275–282
Attoh S, Asmah R, Wiredu EK, Gyasi R, Tettey Y (2010) Human papilloma virus genotypes in Ghanaian women with cervical carcinoma. East Afr Med J 87:345–349
Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, Snyman L, Wiredu E, Molijn A, Quint W, Ramakrishnan G, Schmidt J (2014) Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer 134:1389–1398
Awua AK, Sackey ST, Osei YD, Asmah RH, Wiredu EK (2016) Prevalence of human papillomavirus genotypes among women with cervical cancer in Ghana. Infect Agents Cancer 11:4
de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, Bosch FX (2007) Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 7:453–459
Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 202:1789–1799
Liu P, Xu L, Sun Y, Wang Z (2014) The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol Infect 142:1567–1578
Wagner M, Bennetts L, Patel H, Welner S, de Sanjose S, Weiss TW (2015) Global availability of data on HPV genotype-distribution in cervical, vulvar and vaginal disease and genotype-specific prevalence and incidence of HPV infection in females. Infect Agents Cancer 10:13
Bernard E, Pons-Salort M, Favre M, Heard I, Delarocque-Astagneau E, Guillemot D, Thiebaut AC (2013) Comparing human papillomavirus prevalences in women with normal cytology or invasive cervical cancer to rank genotypes according to their oncogenic potential: a meta-analysis of observational studies. BMC Infect Dis 13:373
de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menendez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andujar M, Castellsague X, Sanchez GI, Nowakowski AM, Bornstein J, Munoz N, Bosch FX (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11(11):1048–1056
Castellsague X, de Sanjose S, Aguado T, Louie KS, Bruni L, Munoz J, Diaz M, Irwin K, Gacic M, Beauvais O, Albero G, Ferrer E, Byrne S, Bosch FX (2007) HPV and cervical cancer in the world: 2007 report. Section I. Continents and regions. Vaccine 25(Suppl 3):C1–C26
Louie KS, de Sanjose S, Mayaud P (2009) Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Trop Med Int Health 14:1287–1302
Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, Clifford GM (2012) Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 131:2349–2359
Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, Maranda L, Ogembo JG (2015) Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PLoS One 10:e0122488
Doerr HW, Berger A (2014) Vaccination against infectious diseases: what is promising? Med Microbiol Immunol 203:365–371
Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, Saah AJ, Drury R, Das R, Velicer C (2016) Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 63:519–527
Gross U, Amuzu SK, de Ciman R, Kassimova I, Gross L, Rabsch W, Rosenberg U, Schulze M, Stich A, Zimmermann O (2011) Bacteremia and antimicrobial drug resistance over time, Ghana. Emerg Infect Dis 17:1879–1882
Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T (2006) Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 44:504–512
Schmitt M, Dondog B, Waterboer T, Pawlita M (2008) Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5 + and GP6+ primers. J Clin Microbiol 46:1050–1059
Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T (2010) Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol 48:143–149
Silling S, Kreuter A, Hellmich M, Swoboda J, Pfister H, Wieland U (2012) Human papillomavirus oncogene mRNA testing for the detection of anal dysplasia in HIV-positive men who have sex with men. J Clin Virol 53:325–331
Wieland U, Ritzkowsky A, Stoltidis M, Weissenborn S, Stark S, Ploner M, Majewski S, Jablonska S, Pfister HJ, Fuchs PG (2000) Communication: papillomavirus DNA in basal cell carcinomas of immunocompetent patients: an accidental association? J Invest Dermatol 115:124–128
Castle PE, Rodriguez AC, Porras C, Herrero R, Schiffman M, Gonzalez P, Hildesheim A, Burk RD (2007) A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis 34:849–855
Roberts CC, Liaw KL, Skjeldestad FE, Jansen KU, Bryan JT (2009) Importance of specimen type in detecting human papillomavirus DNA from the female genital tract. J Med Virol 81:1620–1626
Gravitt PE (2011) The known unknowns of HPV natural history. J Clin Invest 121:4593–4599
Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Pearse A, Montoya GD, Robertson M, Shearman CA, Castle PE (2013) A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 132:198–207
Ghana Statistical Service (GSS), Ghana Health Service (GHS), and ICF International (2015) Ghana Demographic and Health Survey 2014. Rockville, Maryland, USA: GSS, GHS, and ICF International. https://dhsprogram.com/pubs/pdf/FR307/FR307.pdf. Accessed 2016-08-24
Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A, Oladepo O, Smith JS, Arslan A, Munoz N, Snijders PJ, Meijer CJ, Franceschi S (2004) Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. Br J Cancer 90:638–645
Piras F, Piga M, De Montis A, Zannou AR, Minerba L, Perra MT, Murtas D, Atzori M, Pittau M, Maxia C, Sirigu P (2011) Prevalence of human papillomavirus infection in women in Benin, West Africa. Virol J 8:514
Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM (2007) Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 121:621–632
Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G (2013) A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 445:224–231
Andersson S, Mints M, Sallstrom J, Wilander E (2005) The relative distribution of oncogenic types of human papillomavirus in benign, pre-malignant and malignant cervical biopsies. A study with human papillomavirus deoxyribonucleic acid sequence analysis. Cancer Detect Prev 29:37–41
Gudleviciene Z, Didziapetriene J, Ramael M, Uleckiene S, Valuckas KP (2006) Human papillomavirus and p53 polymorphism in Lithuanian cervical cancer patients. Gynecol Oncol 102:530–533
Gargiulo F, De Francesco MA, Schreiber C, Ciravolo G, Salinaro F, Valloncini B, Manca N (2007) Prevalence and distribution of single and multiple HPV infections in cytologically abnormal cervical samples from Italian women. Virus Res 125:176–182
Wentzensen N, Schiffman M, Dunn T, Zuna RE, Gold MA, Allen RA, Zhang R, Sherman ME, Wacholder S, Walker J, Wang SS (2009) Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer 125:2151–2158
Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE (2015) Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 64:300–304
Acknowledgments
We would like to thank Nabila Ristow and Monika Junk from the German National Reference Centre for Papilloma- and Polyomaviruses, University of Cologne, for their excellent technical assistance. We are also grateful to Klaus Jung, Ph.D., and his colleagues from the Department of Medical Statistics, University of Göttingen, for their valuable support in statistical analysis of the data. This study was presented in part as a poster at the 30th International Papillomavirus Conference, Lisbon, Portugal, 17–21 September 2015, in part as a talk at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, United States, 17–21 September 2015, and in part as a poster at the 26th Annual Meeting of the Society of Virology, Münster, Germany, 6–9 April 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethical committee of the University Medical Center Göttingen, Germany (number 14/10/11), and the authority of the St. Martin de Porres Hospital in Eikwe, Ghana.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Marco H. Schulze and Fabian M. Völker have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schulze, M.H., Völker, F.M., Lugert, R. et al. High prevalence of human papillomaviruses in Ghanaian pregnant women. Med Microbiol Immunol 205, 595–602 (2016). https://doi.org/10.1007/s00430-016-0475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-016-0475-9