Abstract
Innate immunity defends against infection but also mediates immunoregulatory effects shaping innate and adaptive responses. Studies of murine cytomegalovirus (MCMV) infections have helped elucidate the mechanisms inducing, as well as the elicited soluble and cellular networks contributing to, innate immunity. Specialized receptors are engaged by infection-induced structures to stimulate production of key innate cytokines. These then stimulate cytokine and cellular responses such as activation of natural killer (NK) cells to mediate elevated killing by type 1 interferon (IFN) and/or to produce the pro-inflammatory and antiviral cytokine IFN-γ by interleukin 12 (IL-12). An inter-systemic loop, with IL-6 inducing glucocorticoid release, negatively regulates these early cytokine responses. As infections advance into periods of overlapping innate and adaptive responses, however, the cells are intrinsically conditioned to modify the biological effects of exposure to individual cytokines. Some pathways are turned off to inhibit an existing, whereas others are broadened for acquisition of a new, response function. Remarkably, extended NK cell proliferation during MCMV infection is associated with epigenetic modifications shifting the state of the inhibitory cytokine IL-10 gene from closed to open and results in their becoming equipped to produce this cytokine. When induced, NK cell IL-10 negatively regulates the magnitude of adaptive responses to protect against immune pathology. Thus, innate immunoregulatory cytokine networks are integral to pro-inflammatory and defense functions, but responding cells have the flexibility to undergo cell intrinsic conditioning with changing network characteristics to result in a new negative immunoregulatory function, and consequently, both promote beneficial and limit detrimental immune responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune system’s major functions are to sense a wide range of infectious agents and to elicit the endogenous immune responses most beneficial for protecting the host against the particular infection encountered as well as any disease that might result from the infection itself and/or the immune response to it. Given these critical responsibilities, the system’s complexity should come as no surprise. The still incomplete understanding of the molecular mechanisms in place to carry out these functions has taken more than 100 years to develop. Margret Gladys Smith’s isolation of the murine cytomegalovirus (MCMV) and initial characterization of infections of mice with this agent over 60 years ago provided a powerful approach for studying endogenous immune responses to viruses [1]. Numerous research groups have built on Smith’s work, and important breakthroughs in knowledge have resulted. Because of their unique characteristics, infections of individual hosts with different agents elicit particular responses. There are shared molecular and cellular constituents, however, and the studies using MCMV have helped provide a framework for what is now known about much of innate and adaptive immunity.
The advances include characterization of the innate sensors in place to identify infectious threats and their stimulation by engagement to result in the induction of innate cytokine networks important in resistance to microbial infection and in immune regulation. Much of the work leading to the identification of these pathways was first carried out in the MCMV system, and the importance of natural killer (NK) cell-mediated cytotoxicity as well as NK cell IFN-γ production in antiviral defense and immunoregulation was first identified during MCMV infection. More recently, MCMV studies have moved into the characterization of events as infection progresses into periods of overlapping innate and adaptive responses. These are revealing a surprising flexibility in innate cellular responses resulting from the induction of intrinsic changes as the cells experience the conditions of infections. The cells demonstrate changing biological responses elicited by exposure to particular cytokines, with some effects turned off and others broadened. The acquisition of a new negative regulatory function delivered by NK cells is an example of this. Here, NK cells already mediating pro-inflammatory functions by producing IFN-γ acquire the ability to produce the inhibitory cytokine IL-10. An overview of the specifics of these innate responses as they unfold during MCMV infections, as well as the implications of these to the general understanding of how the individual constituents of immunity are accessed to mediate particular functions as needed, is the focus of this review.
Innate sensors of infection
Although the details of the innate cytokine cascades elicited during infections were being filled in prior to the understanding of how they were induced, characterization of the pathways to their stimulation led to the identification of germ-line gene families coding for receptors sensing nonself or inappropriately expressed determinants indicative of infection. A brief overview of these receptors as they function in MCMV infection is helpful to the understanding of the cytokine networks being reviewed here.
The first ligands for these receptors identified were pathogen-associated molecular patterns (PAMPs) expressed by particular infectious agents but not by host cells, and the receptors for these were called pattern recognition receptors (PRRs) [2]. The Toll-like receptors (TLRs) are a class of these sensors expressed in membranes either on the cell surface or in endosomes [3]. They are largely expressed on dendritic cells (DCs) and monocyte/macrophages of the innate immune system, face out to survey the environment and as a result, sense a threat prior to infection of the cell. Once engaged, TLRs activate intracellular signaling pathways to stimulate elevated transcription and production of pro-inflammatory cytokines. This can lead to the release of: type 1 interferons (IFNs), products of a family including one α and multiple β genes; interleukin (IL-12), a protein dimer with two different chains; tumor necrosis factor (TNF) and IL-6. The molecular pathways to expression of some of these products are better characterized than those to others. During in vivo replication of MCMV with its DNA genome and RNA transcription to express viral proteins, TLR9-sensing DNA motifs and the RNA-sensing TLR7 play important roles in initiating innate cytokine cascades (Fig. 1a) [4–9].
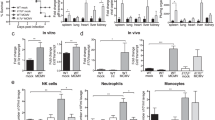
Induction of innate cytokine networks during MCMV infection. a, b Specialized sensors recognize viral products or virus-induced changes on/in infected cells to signal a threat. These are found on many cell types. The TLRs and cytosolic receptors expressed on membranes in uninfected DCs and infected monocyte/macrophages survey the environment to stimulate the production of the type 1 IFNs, IL-12, TNF and IL-6 during MCMV infections. A cytosolic AIM2 receptor is also stimulated in infected cells to induce the processing of biologically active IL-18. Once these cytokines are induced, they promote pro-inflammatory responses. c The type 1 IFNs have an important role in inducing elevated NK cell-mediated killing, and IL-12 is a potent inducer of NK cell IFN-γ production. Once induced, IL-6 leads to HPA axis activation to stimulate glucocorticoid release and provide feedback inhibition of cytokine expression (based on the studies reported in [15, 16, 26, 27], model modified from Ref. [4])
In addition to the TLRs, there are many cytosolic PRRs to sense microbial products in infected cells, and some of these stimulate transcription to induce type 1 IFNs. The best characterized are receptors for RNA structures not usually found in host cell cytoplasm. In addition, however, there are now a number of these sensors for cytosolic DNA. Recent work is focusing on the cyclic-GMP-AMP (cGAMP) synthase (cGAS) [10]. These intracellular PRRs have the potential to be engaged by DNA and RNA produced during viral infections, but the pathways to their effects must be blocked in MCMV-infected cells because the TLR sensors account for most of the type 1 IFN production in response to this virus. Finally, there is a unique group of cytosolic receptors in place that stimulate the production of biologically active IL-1 and IL-18 by activating enzymes to process their precursor protein molecules [11]. One example, the protein absent in melanoma 2 (AIM2) plays an important role in the induction of IL-18 during MCMV infection [12].
Initial cytokine responses
Innate cytokines are elicited in coordinated cytokine cascades following engagement of the innate sensors (Fig. 1a). Because both TLRs and AIM2 initially recognize products of MCMV, the pro-inflammatory and antiviral cytokines, type 1 IFNs, IL-12, TNF, IL-6 and IL-18 are all induced after infection with this virus [4, 13–17] (Fig. 1b). For unknown reasons, only low or undetectable levels of released IL-1 are detected even though IL-1 gene transcription is induced [16, 18]. The IL-10 cytokine with negative immunoregulatory functions is not initially induced to significant levels, but can be found at later points under conditions of high viral challenge [19, 20]. Remarkably, the pro-inflammatory cytokines are detected with peak production at 36–44 h, or at approximately 1.5 days, after infection regardless of the viral dose, but TNF and IL-18 are produced for more extended periods of time [16, 17]. The tight kinetics of production is in part a result of the fact that plasmacytoid dendritic cells (pDCs) are the major producers of many of these cytokines, particularly the type 1 IFNs and IL-12, and their frequencies decline as the infection progresses [21–24]. The extended TNF and IL-18 production is a result of the fact that other cell types can contribute to these responses [12, 25]. Certain of the cytokines are known to amplify the expression of themselves or other members of the pro-inflammatory cytokine family to accelerate the kinetics and elevate the magnitude of the innate responses.
Early innate cytokine stimulation of NK cells
The induced cytokines play important roles in many antiviral and pro-inflammatory events, but NK cells are major innate cell targets of their effects [15, 26, 27] (Fig. 1c). Because NK cells have granules containing the perforin and granzyme molecules required to kill target cells, they are potent at cell-mediated cytotoxicity. In addition to inducing antiviral states by stimulating the expression of multiple proteins directly inhibiting viral replication [28], the type 1 IFNs induce elevated NK cell-mediated killing, and this function is important in defense against MCMV because it acts to eliminate the cells serving as viral factories [29–31]. In addition, the type 1 IFNs promote IL-15 expression [27], and at times of type 1 IFN induction in vivo, this factor contributes to an early NK cell blastogenesis and proliferation [27, 32–34]. The IL-12 response leads to the induction of NK cell IFN-γ production [26, 27]. This cytokine also has pro-inflammatory and antiviral effects, and IL-12-induced IFN-γ production by NK cells promotes these during MCMV infection [35–38].
Regulation of innate cytokine signaling
Type 1 IFNs and IL-12 are members of the JAK-STAT cytokine family having receptors signaling through tyrosine kinases and signal transducers and activators of transcript molecules. There are seven different STAT molecules with high degrees of homology, and individual JAK-STAT cytokine receptors have preferred and alternative use of these [39–42]. Both type 1 IFN receptor (IFNR) and the IL-12 receptor (IL-12R) can activate STAT4, important for the induction of IFN-γ gene transcription [43–45]. It is a preferred signaling molecule for the IL-12R, but because the IFNR has a higher affinity for STAT1, it uses STAT4 as an alternative signaling molecule. NK cells have high basal STAT4 expression levels [46] and can initially respond to either type 1 IFN [46, 47] or IL-12 [27] with STAT4 activation and IFN-γ production, particularly in the presence of IL-18 [17, 48]. High STAT1 levels, however, are induced by type 1 IFN exposure. Because IFNR has a higher affinity for STAT1, the shift in relative concentrations blocks type 1 IFN STAT4 activation while promoting both STAT1-dependent activation of killing by NK cells [27] and STAT1-dependent direct antiviral effects in most cells [28, 40]. The block in STAT4 activation protects against unregulated IFN-γ production and a resulting IFN-γ-dependent cytokine-mediated disease during infections with sustained production of the type 1 IFNs [46]. The changing relative STAT concentrations is one example of cell conditioning during infection and helps explain the IL-12 requirement for achieving a strong NK cell IFN-γ response: the IL-12R has a preference for activating STAT4 even in the presence of elevated STAT1. Hence, at times of systemic innate cytokine responses to MCMV infection, type 1 IFNs are largely responsible for elevated NK cell-mediated killing, whereas IL-12 is inducing their IFN-γ production.
Network to glucocorticoid production for protection against disease mediated by innate cytokines
When elicited at high enough levels for long enough periods of time, the innate pro-inflammatory cytokine cascade, with TNF, IL-12 and IFN-γ, can induce disease [49, 50]. Because systemic pro-inflammatory cytokines are the mediators of septic shock, with wasting and even death, it is critical to control the magnitude of these responses. Changing STAT concentrations can contribute to the regulation of IFN-γ production in response to type 1 IFNs, but there are other extracellular mechanisms in place to control the pro-inflammatory cytokines. During MCMV infection at sufficiently high doses, there is a limited TNF-dependent liver necrosis [51]. Remarkably, however, systemic diseases that can be promoted by TNF, IL-12 and IFN-γ are largely controlled in the context of MCMV infections of immune competent mice. This is in part a result of the fact that there is a regulatory pathway between the innate cytokines and the neuroendocrine system to induce steroids for feedback inhibition. In particular, IL-6 produced during MCMV infections has non-redundant communication with the brain to activate the hypothalamic–pituitary–adrenal axis (HPA) such that the production of corticotropin-releasing hormone (CRH) by the hypothalamus stimulates pituitary release of adrenocorticotropin hormone (ACTH) to induce adrenal gland production of glucocorticoids. In the mouse, the natural glucocorticoid steroid is corticosterone and detected at early times during MCMV infection [16] (Fig. 1c). The loop is critical for limiting the levels of TNF production under conditions of comparable MCMV burdens to protect from a TNF-dependent wasting and death at the very earliest times of infection [18]. Thus, although early innate cytokine responses have amplification loops, there is an independent circuit to the neuroendocrine system to negatively regulate these and protect from the detrimental consequences resulting from unregulated pro-inflammatory cytokine expression.
NK cell receptors
In addition to having their innate cytokine receptors, NK cells express a composite of receptors from germ-line families for recognizing molecules on other cell surfaces [52, 53]. Some of these are activating and stimulate, whereas others deliver negative signals and inhibit NK cell responses. At the site of NK cell engagement with a target cell, the balance of positive and negative signals determines the outcome. With few exceptions, the NK receptors are highly polymorphic and representatives are even polygenic with differences in the presence or absence of genes between individuals of the same species. The majority of receptors are stochastically expressed on high frequencies of NK cell subsets. Net stimulatory signals through these are required for delivery of NK cell-mediated cytotoxicity but may also induce responses overlapping cytokine receptor stimulation, i.e., NK cell IFN-γ production and proliferation. Because their ligands can be induced on virus-infected target cells, NK activating receptors have characteristics of innate sensors. A brief overview of the classes of these activating receptor-ligand pairs reported to be in place during viral infections is helpful here.
A variety of modifications on the surfaces of virus-infected cells can be ligands for NK activating receptors. To date, the known ligands fall into three classes: viral protein products, with only a couple of examples; virus-induced changes in the major histocompatibility class 1 molecules to result in their recognition by particular NK receptors; and host stress molecules induced in infected cells and recognized by a broadly expressed and evolutionarily conserved NK activating receptor, NKG2D [53]. The NKG2D–stress molecule pairs appear to be highly effective because both human CMV and MCMV have evolved potent mechanisms to inhibit the expression of these ligands on infected cells [54]. A well-characterized mouse NK activating receptor directly recognizing a viral protein product is Ly49H. This receptor is expressed in some but not all strains of mice [55–58]. It interacts with the m157 protein generally expressed by MCMV [58]. By mediating the killing of virus-infected cells, the Ly49H-m157 activating receptor–ligand pair plays a significant role in controlling MCMV burdens when present, and NK cells expressing the activating receptor are proliferating and increasing in frequency during high-dose infections with strains of MCMV expressing m157 [59, 60].
Activating receptor-dependent NK cell proliferation and maintenance during sustained viral infection
Studies using mice deficient in the perforin protein required to deliver killing molecules to target cells, in the Ly49H activating receptor required for recognition of target cells or in both have demonstrated roles for the Ly49H activating receptor independent of its function in NK cell-mediated killing [60]. Under the conditions of sustained and elevated MCMV replication resulting from the inability to kill virus-infected cells, NK cell subsets expressing the Ly49H activating receptor undergo profound proliferation and expansion into periods overlapping adaptive immunity on day 5 of infection. In comparison with those in immunocomplete mice, the splenic frequencies of the NK cells in perforin-deficient mice are predominantly Ly49H expressing, the overall NK cell frequencies are increased up to fivefold such that they represent approximately 25 % of the leukocyte populations, and the total NK cell yields are up approximately threefold. In addition to facilitating proliferation, the activating receptor plays an important role in maintaining the cells because NK cell subsets are greatly diminished in the absence of both perforin and Ly49H with only small numbers of immature NK cell detectable. Thus, activating receptors recognizing a virus-induced ligand are important not only for delivering NK cell-mediated killing for antiviral defense but also for driving proliferation of the NK cell subsets expressing the activating receptor recognizing molecular changes on infected cells and for preserving the presence of mature NK cells into periods overlapping adaptive immunity.
NK cell IL-10 production for regulation of adaptive immune responses
During sustained MCMV infections in the absence of the antiviral defense delivered by cell-mediated killing, the presence of the Ly49H activating receptor and the resulting maintenance of NK cells protect from a wasting disease first detected on days 4–6 after low-dose, and an infection-induced death after high-dose challenge [60]. The protection is independent of viral burden because perforin deficiency alone and deficiencies in both perforin and Ly49H lead to similar high levels of viral replication. In the absence of NK cell maintenance resulting from the Ly49H deficiency, the adaptive CD8 T cell response to the sustained infection mediates the pathologies. A potent inhibitory cytokine made by many cell types is IL-10 [61, 62]. With the Ly49H-dependent NK cell expansion and maintenance, protection is afforded by NK cell production of the inhibitory IL-10 cytokine [60]. Although stimulation through the Ly49H receptor can induce modest levels of IL-10 production, particular cytokines are better inducers of IL-10 production by the NK cells prepared at day 4 of infection. These observations demonstrate that NK cells, conditioned during their activating receptor-dependent expansion and maintenance under conditions of profound viral replication, have acquired a new negative immunoregulatory function and that this function is important for protecting against pathology mediated by the adaptive immune system.
Proliferation-dependent conditioning of NK cells for negative immunoregulatory function
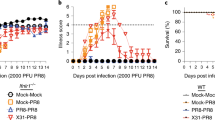
The conditioning of NK cells to produce IL-10 and deliver a negative immunoregulatory function can also be induced during infections of immunocompetent mice [20]. Although both low- and high-dose MCMV infections elicit the NK cell IFN-γ production at times of innate cytokine responses on day 1.5 of MCMV, the NK cell IL-10 response only appears on and after day 3.5 of high-dose MCMV infection (Fig. 2a, b). High doses result in sustained MCMV replication in the spleen with extended and elevated proliferation of Ly49H NK cells into days 2.5 and 3.5 of infection. Although the direct mechanism inducing NK cell IL-10 production has not been identified, it is clear that the population has been changed in its ability to respond to a variety of cytokines with delivery of this negative immunoregulatory function. The NK cells taken on day 3.5 of infection, but not those from uninfected mice, respond to both IL-12 and IL-21 ex vivo to produce IL-10, and in contrast to the early NK cell IL-12 response limited to IFN-γ production, NK cells from later times of infection respond to IL-12 with both IFN-γ and IL-10 production ex vivo. Moreover, NK cells prepared on day 3.5 of infection respond to the endogenously produced IL-12 with IL-10 expression when they are transferred into mice for the day 1.5 response of this cytokine. Therefore, the NK cells have been intrinsically altered in their responsiveness to IL-12 with a shift to include the production of IL-10 for acquisition of a negative immunoregulatory function as well as IFN-γ.
Conditioning of NK cells during MCMV for changing NK cell function. a Serum measurements of IL-10 and IFN-γ by cytometric bead assay during low (5000 PFU)-or high (70,000 PFU)-dose infection with MCMV. b IL-10 production from media conditioned for 24 h with total splenic leukocytes from day 0, uninfected or 3.5 MCMV-infected mice, or with FACS-purified NK (CD49b+TCR-β-) and T (CD49b-TCRβ+) cells prepared from day 3.5 MCMV infection. c IL-10 expression, evaluated by induction of the IL-10-GFP reporter gene, among CD49b+TCRβ-NK cells taken from day 0, 1.5, 2.5 or 3.5 infected mice, in culture with IL-2, with or without IL-12 and with or without mitomycin C pre-treatment to block proliferation. d Distribution of histone methylation marks H3K4 (open), H3K27 (closed) and H3K36 (open) from purified NK cells on day 0, 1.5 and 3.5 of infection for the IFN-γ, IL-10 and Myod1 genes, as assessed by chromatin immunoprecipitation and massive parallel sequencing (figures reproduced with permission from Ref. [20]: panel A from Fig. 1A, 1F, panel B from Fig. 7B and panel C from Fig. 6)
The development of this new function occurs at times of NK cell proliferation during MCMV infection, but it can be induced in culture with high IL-2 doses supporting NK cell expansion with cells from humans [63] or cells prepared from uninfected mice [20]. The IL-2-driven expansion of mouse NK cells demonstrates the independence of the change on infection and also on the Ly49H activating receptor [20]. The proliferation requirement for the IL-10 response has been demonstrated by blocking expansion in response to IL-2 ex vivo with mitomycin C treatment. NK cells, reporting IL-12 induction of IL-10, prepared from uninfected mice and mice on day 1.5, as well as on days 2.5 and 3.5 of MCMV infection, need to proliferate to IL-2 in culture to acquire IL-12 induction of IL-10 if they have not already proliferated during the infection in vivo but not if they have (Fig. 2c). NK cells are known to have IFN-γ expression available basally [64], and the NK cell IFN-γ gene appears in an open and accessible conformation as characterized by histone methylations both before and during MCMV infection [20]. In contrast, the NK cell IL-10 gene goes from a closed to the open state required for transcription as the infection progresses [20] (Fig. 2d). Thus, proliferation plays a role in intrinsically altering NK cells by promoting the conditions supporting epigenetic modifications for the expression of IL-10 (Fig. 3) and, as a result, allows a flexibility in the population for the acquisition of a new negative immunoregulatory function.
A proliferation-dependent conditioning of NK cells to acquire the ability to express IL-10 and mediate negative immunoregulatory function. Because their IFN-γ gene has histone methylations in a configuration open for gene expression but those for the IL-10 gene in a closed configuration, NK cells in uninfected mice are initially prepared to respond to IL-12 with IFN-γ but not IL-10 production, and they do so at early times after MCMV infection. These events are occurring as the adaptive T cells responses are slowly being induced. Under conditions of extended and elevated MCMV replication, subsets of NK cells expressing the Ly49H activating receptor, which recognizes the m157 viral protein as its ligand, are stimulated to undergo preferential expansion. As a result of these events, the IL-10 gene is shifted from a closed to an open state for expression. Conditioned by their experiences, these NK cells now produce IL-10 during high-dose infections to negatively regulate adaptive T cell responses and can respond to IL-12 exposure with both IFN-γ and IL-10 production. Hence, proliferation promotes the flexible use of this innate cell type for pro-inflammatory/antiviral and negative immunoregulaotry functions as needed (based on the studies reported in Ref. [20])
Discussion
To summarize the current understanding of endogenous immune responses, a large and growing number of innate sensors, coded for by germ-line genes in all cells and/or in specialized innate cell populations, are distributed to survey intracellular and extracellular environments for the presence of microbial and host structures that are out of place and thus foreign to a normal, healthy condition. Once engaged, these receptors stimulate the production of innate cytokines, with some receptors acting in concert to independently promote the production of different cytokines that act either in parallel or in synergy to promote resistance to infection. In the case of MCMV infections (Fig. 1) [4], TLR sensors, particularly in pDCs, lead to transcriptional activation for the production of type 1 IFN, IL-12, TNF and IL-6, with a wider range of cell types expressing TNF and the AIM2 receptor to induce the processing of biologically active IL-18. The type 1 IFNs can directly induce antiviral mechanisms in all nucleated cells, but also deliver immunoregulatory effects in subsets of immune cells. NK cells are basally prepared to respond to either type 1 IFN or IL-12 with the pSTAT4 activation leading to IFN-γ production [46], but during MCMV infection, they do so primarily in response to IL-12. Thus, a downstream innate cytokine in the cascade of responses is IFN-γ. Because IL-18 can act synergistically with either IL-12 or type 1 IFN, the engagement of independent sensors provides a mechanism to dramatically enhance the IFN-γ response. The virus carries molecules to interfere with detection and cytokine functions and is more effective at inhibiting some rather than others. When the system is dramatically elevated, IL-6 communicates with the neuroendocrine system to induce glucocorticoid release and regulate the magnitude of the TNF expression to protect from innate cytokine-mediated immune pathology [16, 18].
In addition to the negative feedback loop delivered by steroids, exposure to type 1 IFN induces STAT1 expression in all cells. This can enhance stimulation of the STAT1-dependent antiviral gene targets activated by the cytokines, because it is a preferred target of the cytokine receptor, which also acts to block the alternative stimulation of STAT4 by type 1 IFNs themselves [46]. Thus, there is an intracellular loop in place to negatively regulate type 1 IFN enhancement of IFN-γ. The IL-12 receptor has a preferred, strong interaction with STAT4 and can be stimulated to induce IFN-γ even in the presence of high STAT1. Consequently, one pathway of conditioning cellular responses is altering the relative intracellular concentrations of the STAT signaling molecules to change the biological effects of exposure to particular cytokines.
Recent studies of NK cells as their responses are extended into longer periods of MCMV infection [20, 60] have identified another mechanism of intrinsically altering cellular function, i.e., changing access to target genes by epigenetic modification of histone methylations to vary their states in open or closed configurations [20]. As a result, known and potential innate cytokine networks are altered through the experience of infection. Specifically, although NK cells have the IFN-γ gene open under basal conditions, the IL-10 gene shifts from a closed to an open state as the cells are stimulated to proliferate [20]. Because IL-10 has potent negative effects on immune responses, the NK cells acquire the potential to mediate negative immunoregulatory functions. The ability of NK cells to produce IL-10 is dependent on their proliferation and can occur in infection-independent conditions when the cell proliferation is driven with high doses of IL-2 [20]. During the infection, however, the expansion of NK cells is dependent on their expression of the innate NK receptor Ly49H [59, 60]. Because the NK receptors are a family of innate sensors and because the ligand for the Ly49H receptor, m157, is a viral protein product, the in vivo conditioning of NK cells to acquire the negative regulatory functions mediated by IL-10 is a result of sensing the magnitude of the infection as it is sustained into periods of adaptive immunity and, in the context of unrelenting viral infection, leads to the limiting of damaging adaptive responses by the innate immune system.
The link between proliferation and changes at the epigenetic level may provide an explanation for the reported NK cell IL-10 production during chronic hepatitis C virus infections in humans and sustained Toxolasma gondii infections in the mouse [65–67]. Previous work in T cells has demonstrated that proliferating is linked to the acquisition of cytokine production through TCR stimulation [68], and this occurs at the level of epigenetic modification [69]. In the T cell system, however, the activating receptor signaling for proliferation and signaling for cytokine gene accessibility have not been untangled. Other innate cell populations may be behaving in a similar manner to NK cells. Macrophages are also a high-frequency cell with functional diversity and have recently been observed to undergo local proliferation in the context of inflammation [70, 71]. Recently, it has been shown that human monocyte/macrophage subsets driven through expansion into different functional lineages in culture have differences in histone methylations associated with the open and closed states of particular genes [72]. Thus, the link between NK cell proliferation and changing function may be a general mechanism by which the host can rapidly utilize a limited pool of innate cells for different functions.
Where are we now? As is generally the case, new questions arise from new understanding. Major advances have been made in characterizing innate cytokine networks during immune responses to infection, but understanding of the mechanisms regulating the conditioning responses as needed is in its infancy. Changing relative levels of different STAT molecules are likely to be important in modulating the effects of a number of cytokines because there are seven different STAT molecules and numerous receptors using these with particular preferred and alternative STAT signaling pathways [41, 42]. Likewise, conditioning of innate immune cell subsets for differences in gene expression states has the potential to mediate a variety of mechanisms in a range of innate cell types. There will be, however, many other unanticipated regulatory pathways in the complex intercellular and intracellular communication mediated by cytokines. Margret Gladys Smith originally isolated MCMV because she was looking for an agent causing pathology with a cellular appearance similar to that seen in the human. No one could have foreseen how her work would set the foundation for much of what is now known about immunoregulatory cytokine networks. The next 60 years of research in this system promises to unlock many other important secrets on the interactions shaping a broad range of immune responses.
References
Smith MG (1954) Propagation of salivary gland virus of the mouse in tissue cultures. Proc Soc Exp Biol Med 86(3):435–440
Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54(Pt 1):1–13
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11(5):373–384. doi:10.1038/ni.1863
Dalod M, Biron CA (2013) Immunoregulatory cytokine networks discovered and characterized during murine cytomegalovirus infections. In: Reddenhase MJ (ed) Cytomegaloviruses: from molecular pathogenesis to therapy, vol II. Caister Academic Press, Nerfolk, pp 232–258
Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M (2004) TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21(1):107–119. doi:10.1016/j.immuni.2004.06.007
Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA 101(10):3516–3521. doi:10.1073/pnas.0400525101
Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F (2005) MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol 175(10):6723–6732
Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, Dalod M (2008) Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int Immunol 20(1):45–56. doi:10.1093/intimm/dxm119
Zucchini N, Bessou G, Traub S, Robbins SH, Uematsu S, Akira S, Alexopoulou L, Dalod M (2008) Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J Immunol 180(9):5799–5803
Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339(6121):786–791. doi:10.1126/science.1232458
Kanneganti TD (2010) Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol 10(10):688–698. doi:10.1038/nri2851
Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11(5):395–402. doi:10.1038/ni.1864
Grundy JE, Trapman J, Allan JE, Shellam GR, Melief CJ (1982) Evidence for a protective role of interferon in resistance to murine cytomegalovirus and its control by non-H-2-linked genes. Infect Immun 37(1):143–150
Chong KT, Gresser I, Mims CA (1983) Interferon as a defence mechanism in mouse cytomegalovirus infection. J Gen Virol 64(Pt 2):461–464
Orange JS, Biron CA (1996) Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol 156(12):4746–4756
Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA (1997) Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med 185(7):1185–1192
Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA (2000) Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J Immunol 165(9):4787–4791
Ruzek MC, Pearce BD, Miller AH, Biron CA (1999) Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol 162(6):3527–3533
Oakley OR, Garvy BA, Humphreys S, Qureshi MH, Pomeroy C (2008) Increased weight loss with reduced viral replication in interleukin-10 knock-out mice infected with murine cytomegalovirus. Clin Exp Immunol 151(1):155–164. doi:10.1111/j.1365-2249.2007.03533.x
Tarrio ML, Lee SH, Fragoso MF, Sun HW, Kanno Y, O’Shea JJ, Biron CA (2014) Proliferation conditions promote intrinsic changes in NK cells for an IL-10 response. J Immunol 193(1):354–363. doi:10.4049/jimmunol.1302999
Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2(12):1144–1150. doi:10.1038/ni736
Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA (2002) Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med 195(4):517–528
Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA (2003) Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med 197(7):885–898. doi:10.1084/jem.20021522
Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M (2011) Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med 208(12):2367–2374. doi:10.1084/jem.20110654
Hanson LK, Slater JS, Karabekian Z, Virgin HW 4th, Biron CA, Ruzek MC, van Rooijen N, Ciavarra RP, Stenberg RM, Campbell AE (1999) Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis. J Virol 73(7):5970–5980
Orange JS, Biron CA (1996) An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol 156(3):1138–1142
Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA (2002) Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol 169(8):4279–4287
Sadler AJ, Williams BR (2008) Interferon-inducible antiviral effectors. Nat Rev Immunol 8(7):559–568. doi:10.1038/nri2314
Tay CH, Welsh RM (1997) Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 71(1):267–275
Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HW (2005) Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79(1):661–667. doi:10.1128/JVI.79.1.661-667.2005
Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, Degli-Esposti MA (2009) The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol 87(7):559–566. doi:10.1038/icb.2009.41
Biron CA, Welsh RM (1982) Blastogenesis of natural killer cells during viral infection in vivo. J Immunol 129(6):2788–2795
Biron CA, Turgiss LR, Welsh RM (1983) Increase in NK cell number and turnover rate during acute viral infection. J Immunol 131(3):1539–1545
Biron CA, Sonnenfeld G, Welsh RM (1984) Interferon induces natural killer cell blastogenesis in vivo. J Leukoc Biol 35(1):31–37
Orange JS, Wang B, Terhorst C, Biron CA (1995) Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med 182(4):1045–1056
Boehm U, Klamp T, Groot M, Howard JC (1997) Cellular responses to interferon-gamma. Annu Rev Immunol 15:749–795. doi:10.1146/annurev.immunol.15.1.749
Salazar-Mather TP, Hamilton TA, Biron CA (2000) A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Investig 105(7):985–993. doi:10.1172/JCI9232
Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A (2012) NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36(6):1047–1059. doi:10.1016/j.immuni.2012.03.026
Darnell JE Jr, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264(5164):1415–1421
Garcia-Sastre A, Biron CA (2006) Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312(5775):879–882. doi:10.1126/science.1125676
van Boxel-Dezaire AH, Rani MR, Stark GR (2006) Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25(3):361–372. doi:10.1016/j.immuni.2006.08.014
Kallal LE, Biron CA (2013) Changing partners at the dance: variations in STAT concentrations for shaping cytokine function and immune responses to viral infections. Jak-Stat 2(1):e23504. doi:10.4161/jkst.23504
Kaplan MH, Sun YL, Hoey T, Grusby MJ (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382(6587):174–177. doi:10.1038/382174a0
Rogge L, D’Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, Adorini L, Sinigaglia F (1998) The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol 161(12):6567–6574
Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA (2002) Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297(5589):2063–2066. doi:10.1126/science.1074900
Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA (2007) High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med 204(10):2383–2396. doi:10.1084/jem.20070401
Mack EA, Kallal LE, Demers DA, Biron CA (2011) Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. mBio 2 (4). doi:10.1128/mBio.00169-11
Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T (2008) Cutting edge: priming of NK cells by IL-18. J Immunol 181(3):1627–1631
Beutler B, Cerami A (1988) The common mediator of shock, cachexia, and tumor necrosis. Adv Immunol 42:213–231
Trinchieri G (1998) Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol 16(3–4):365–396. doi:10.3109/08830189809043002
Orange JS, Salazar-Mather TP, Opal SM, Biron CA (1997) Mechanisms for virus-induced liver disease: tumor necrosis factor-mediated pathology independent of natural killer and T cells during murine cytomegalovirus infection. J Virol 71(12):9248–9258
Yokoyama WM, Seaman WE (1993) The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol 11:613–635. doi:10.1146/annurev.iy.11.040193.003145
Vidal SM, Khakoo SI, Biron CA (2011) Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr Opin Virol 1(6):497–512. doi:10.1016/j.coviro.2011.10.017
Jonjic S, Polic B, Krmpotic A (2008) Viral inhibitors of NKG2D ligands: friends or foes of immune surveillance? Eur J Immunol 38(11):2952–2956. doi:10.1002/eji.200838823
Scalzo AA, Fitzgerald NA, Simmons A, La Vista AB, Shellam GR (1990) Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med 171(5):1469–1483
Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM (2001) Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292(5518):934–937. doi:10.1126/science.1060042
Lee SH, Girard S, Macina D, Busa M, Zafer A, Belouchi A, Gros P, Vidal SM (2001) Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet 28(1):42–45. doi:10.1038/88247
Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL (2002) Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296(5571):1323–1326. doi:10.1126/science.1070884
Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM (2001) Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2(10):951–956. doi:10.1038/ni714
Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA (2009) Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med 206(10):2235–2251. doi:10.1084/jem.20082387
Saraiva M, O’Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10(3):170–181. doi:10.1038/nri2711
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109. doi:10.1146/annurev-immunol-031210-101312
Mehrotra PT, Donnelly RP, Wong S, Kanegane H, Geremew A, Mostowski HS, Furuke K, Siegel JP, Bloom ET (1998) Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J Immunol 160(6):2637–2644
Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM (2003) Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 198(7):1069–1076. doi:10.1084/jem.20030630
De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L (2007) Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol 37(2):445–455. doi:10.1002/eji.200635989
Perona-Wright G, Mohrs K, Szaba FM, Kummer LW, Madan R, Karp CL, Johnson LL, Smiley ST, Mohrs M (2009) Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe 6(6):503–512. doi:10.1016/j.chom.2009.11.003
Wagage S, John B, Krock BL, Hall AO, Randall LM, Karp CL, Simon MC, Hunter CA (2014) The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J Immunol 192(4):1661–1670. doi:10.4049/jimmunol.1300497
Gett AV, Hodgkin PD (1998) Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA 95(16):9488–9493
Denton AE, Russ BE, Doherty PC, Rao S, Turner SJ (2011) Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proc Natl Acad Sci USA 108(37):15306–15311. doi:10.1073/pnas.1112520108
Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332(6035):1284–1288. doi:10.1126/science.1204351
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19(9):1166–1172. doi:10.1038/nm.3258
Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JW, Joosten LA, Wijmenga C, Martens JH, Xavier RJ, Logie C, Netea MG, Stunnenberg HG (2014) Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345(6204):1251086. doi:10.1126/science.1251086
Acknowledgments
Work from the Biron laboratory is supported by the National Institutes of Health, USA. The authors thank the many contributors to the advances in knowledge reviewed here.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Special Issue on Cytomegalovirus.
Rights and permissions
About this article
Cite this article
Biron, C.A., Tarrio, M.L. Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Med Microbiol Immunol 204, 345–354 (2015). https://doi.org/10.1007/s00430-015-0412-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-015-0412-3