Abstract
Like apoptosis, necroptosis is an innate immune mechanism that eliminates pathogen-infected cells. Receptor-interacting protein kinase (RIP)3 (also called RIPK3) mediates necrotic death by phosphorylating an executioner protein, MLKL, leading to plasma membrane leakage. The pathway is triggered against viruses that block caspase 8. In murine CMV, the viral inhibitor of caspase 8 activation prevents extrinsic apoptosis but also has the potential to unleash necroptosis. This virus encodes the viral inhibitor of RIP activation to prevent RIP homotypic interaction motif (RHIM)-dependent signal transduction and necroptosis. Recent investigations reveal a similar mechanism at play in the human alpha-herpesviruses, herpes simplex virus (HSV)1 and HSV2, where RHIM competitor function and caspase 8 suppression are carried out by the virus-encoded large subunit of ribonucleotide reductase (R1). In human cells, R1 inhibition of caspase 8 prevents TNF-induced apoptosis, but sensitizes to TNF-induced necroptosis. The RHIM and caspase 8 interaction domains of R1 collaborate to prevent RIP3-dependent steps and enable both herpesviruses to deflect host cell death machinery that would cut short infection. In mouse cells, HSV1 infection by itself triggers necroptosis by driving RIP3 protein kinase activity. HSV1 R1 contributes to the activation of RIP3 adaptor function in mice, a popular host animal for experimental infection. Based on these studies, infection of RIP3-kinase inactive mice should be explored in models of pathogenesis and latency. The necrotic death pathway that is suppressed during infection in the natural host becomes a cross-species barrier to infection in a non-natural host.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed cell death contributes to innate immune control over intracellular pathogens, eliminating infected cells and restricting dissemination within the host organism. DNA viruses, in particular, encode functions that preserve cell viability to ensure sustained production of progeny from infected host cells [1, 2]. The cell-intrinsic (mitochondrial) pathway of apoptosis is responsible for the developmental elimination of cells, relying on pro-apoptotic BCL2 family proteins BAX and BAK to control the release of mediators such as cytochrome c from mitochondria [3]. Intrinsic pathway adaptors are disarmed by virus-encoded mitochondrial suppressors [1, 4], several of which have shown a critical role in viral pathogenesis [5–11]. Mitochondrial apoptosis forms a cross-species barrier in human cells to prevent replication of murine cytomegalovirus (CMV) that is relieved dependent on the species origin of virus-encoded cell death suppressor [12]. In addition to mitochondrial cell death, cell autonomous death occurs through the assembly of preexisting cytosolic components into a caspase (Casp)8-containing signaling complex. The existence of a cytosolic complex was first demonstrated in the context of TNF family death receptor signaling [13] where receptor-interacting protein kinase (RIP)1 (also called RIPK1), Fas-associated protein with death domain (FADD), Casp8, and the long form of FLICE inhibitory protein (FLIPL) associate as shown in (Fig. 1). A similar complex, called a ripoptosome, is key to signal transduction downstream of pathogen sensors and genotoxic stress [14–16] in addition to death receptors. No matter how this complex is triggered, the ripoptosome is now known to act as a regulator of alternate Casp8-dependent apoptosis and RIP3-dependent necroptosis outcomes (Fig. 2). Necrotic signaling from the ripoptosome has been implicated in inflammatory disease [2, 17]; however, whether necroptosis drives inflammation [18, 19] or results from inflammation [20] remains unresolved. Cell death signaling outcomes suggest that this complex acts as a broad pathogen supersensor [21] with demonstrated activity against vaccinia and reovirus [2, 22–25], the natural mouse pathogen, murine CMV [17, 26–28] and human herpesviruses, herpes simplex virus (HSV)1 and HSV2 [29–31] as well as bacteria [32, 33]. Murine CMV, HSV1, and HSV2 encode suppressors of RIP homotypic interaction motif (RHIM) signal transduction to prevent RIP3 activation (Fig. 1), providing insights into the host defense role of necroptosis [27–29].
Formation of the ripoptosome from cytosolic components. FADD becomes activated by signal transduction downstream of death receptor (DR), Toll-like receptor (TLR), interferon receptor (IFNR) as well as T cell receptor (TCR) activation or intracellular genotoxic stress and virus infection. When activated, FADD recruits cFLIPL–Casp8 heterodimer via death effector domain (DED) interactions, and RIP1 is recruited via death domain (DD) interactions. Associated RIP1 recruits RIP3 via a RHIM interaction (red rectangle). Some R1 proteins of herpesviruses, such as murine CMV (MCMV), HSV1, and HSV2, act as RHIM competitors to disrupt the formation of the ripoptosome
Regulation of Casp8-mediated apoptosis and RIP3-mediated necroptosis by the ripoptosome. cFLIPL–Casp8 association prevents autocleavage activation of Casp8 and maintains sufficient basal protease activity to prevent necroptosis. Casp8-mediated apoptosis requires homodimerization, which leads to Casp8 autocleavage to execute apoptosis under conditions such as low cFLIP levels. RIP3-mediated necroptosis is an alternate death pathway, triggered when Casp8 activity is blocked. In this setting, the RIP1–RIP3 heterodimer is activated by phosphorylation, and then phosphorylated RIP3 recruits and activates mixed lineage kinase domain-like (MLKL) protein. The RIP1–RIP3–MLKL complex, called a necrosome, localizes to membranes and directs the final steps in necroptosis leading to membrane leakage
The potency and delicate balance of apoptotic and necrotic pathways was first brought to light in studies that revealed a RIP1–RIP3-dependent process dictating midgestational embryonic lethality of Casp8-deficient mice [34, 35] and FADD-deficient mice [36, 37]. This was further reinforced when RIP1 deficiency [38–40] was shown to unleash a combination of Casp8- and RIP3-mediated pathways that are normally held in check via RIP1 RHIM signaling through the final stages of gestation and during parturition, a time during development when tonic levels of TNF, interferons, and nucleic acids combine to activate the ripoptosome [38]. The propensity for a ripoptosome to dictate apoptotic or necrotic outcomes was extended by studies of RIP3 mutant-bearing cells and mice [41, 42] and, in particular, the behavior of RIP3 kinase inhibitors, where viability of cells and mice was undermined by RHIM-dependent signal transduction associated with robust ripoptosome formation [41]. It is now recognized that, in addition to death receptor signaling [23, 43, 44], a ripoptosome regulates necroptosis downstream of Toll-like receptors [14], T cell receptors [45], interferon receptors [41, 46, 47], and intracellular genotoxic stress [15] in addition to the well-understood contribution in death receptor signaling (Fig. 1). While less well characterized, similar principles dictate RIP1-independent RIP3-mediated necroptosis via RHIM-signaling adaptors TIR-domain-containing adapter-inducing interferon-β (TRIF) [48, 49] and DNA inducer of interferon activation (DAI, also called ZBP1) [27, 28]. We have argued that the ability to assemble a ripoptosome in such diverse settings evolved to facilitate the mammalian response to pathogens [18, 21], particularly those that inhibit mitochondrial and Casp8 death pathways. One consequence of such pathogen–host détente is a propensity for untoward developmental and immunological defects arising from an imbalance in key ripoptosome components [35, 38, 41, 42]. Necroptosis very likely evolved as a “trap door” host adaptation to eliminate cells infected with pathogens that inhibit Casp8 [2, 18, 21]. It has become increasingly clear that, through the ripoptosome and virus-encoded countermeasures that target this complex, Casp8-dependent apoptosis and RIP3-dependent necroptosis execute cells in order to reduce the burden of infection.
Viral inhibition of RHIM signal transduction in mouse and human cells
A ripoptosome (Fig. 1) is nucleated via activation of FADD, Casp8, cFLIPL, RIP1, or RIP3 and forms through death effector domain (DED)-dependent, death domain (DD)-dependent, and RHIM-dependent interactions [16]. The RIP1–RIP3 necrosome is crucial for execution of necroptosis (Fig. 2). The murine CMV M45-encoded viral inhibitor of RIP activation (vIRA) brought both the remarkable potency of necroptosis and the importance of virus-encoded countermeasures to light [17, 26–28]. Without vIRA RHIM competitor function, this virus fails to infect its natural mouse host due to the induction of DAI–RIP3 necroptosis that eliminates infected cells [28]. The potency of this cell autonomous host defense pathway is reinforced by the remarkable attenuation of M45-deficient viruses for immunocompetent as well as immunodeficient mice [27, 28, 50, 51]. Cell death is triggered by DAI-mediated recruitment and activation of RIP3, leading to the execution step involving MLKL phosphorylation and subsequent membrane permeabilization, as shown in Fig. 3 [27, 28, 49]. Virus-infected cells are eliminated within a few hours, and virus fails to sustain infection of the host animal. Elimination of either RIP3 or DAI from the mouse germ line reverses the phenotype; however, elimination of other RHIM adaptors, TRIF or RIP1, does not. In addition to its natural role in preventing this virus-induced necroptosis, vIRA RHIM competitor can also prevent consequences of RIP1–RIP3 interaction downstream of TNF receptor (TNFR)1 [27] and TRIF–RIP3 interaction in Toll-like receptor (TLR)3- and TLR4-dependent necroptosis [49], as shown in Fig. 3.
RIP3-mediated necroptosis is activated by three distinct RHIM-containing host adaptors (RIP1, TRIF, DAI) and blocked by R1 proteins of MCMV (shown) and HSV (not shown). RIP1 is a key pro-necrotic kinase acting downstream of TNF family death receptor (TNFR1, Fas, TRAIL), forming a RIP1–RIP3 complex. Virus-induced DAI–RIP3 necrosis is characterized by MCMV M45 mutant virus infection. In addition to RIP1 and DAI, TRIF, the key TLR3- and TLR4-signaling adaptors, activates RIP3 via TRIF–RIP3 interaction. RIP3 is specifically activated by RIP1, DAI, or TRIF in a RHIM-dependent manner, when it autophosphorylates at S277 and targets MLKL via phosphorylation at T357 and S358. The RHIM competitor MCMV M45 functions during infection to prevent RIP3 association with DAI (solid line), but experimentally can also inhibit association with RIP1 or TRIF (dashed lines)
Like all beta-herpesviruses, murine CMV M45 encodes an enzymatically inactive homolog of the large subunit (R1) of ribonucleotide reductase (RNR) [52]. This gene is conserved in alpha- and gamma-herpesviruses as well, where it encodes a subunit of an enzymatically active RNR responsible for converting ribonucleotides to deoxyribonucleotides in support of viral DNA synthesis [52]. Herpes simplex virus (HSV)1 and HSV2 regulate cell death early during infection [53, 54], suppressing necrosis [55] as well as apoptosis [56–62]. Cell death suppression requires the regulatory protein ICP4 as well as the protein kinase US3 [54, 63–65]. Early viral gene products gD [62, 66], US3 [64, 65, 67], and R1 [60, 68–71] are all able to inhibit apoptosis in defined settings [72, 73]. Furthermore, reactivation of HSV1 from latency in rodent animal models is tied to suppression of apoptosis by the viral latency transcript [74, 75]. The mechanism of cell death suppression mediated by the R1 proteins, HSV1 ICP6 and HSV2 ICP10, has become the best understood at a mechanistic level. The large C-terminal RNR domain interacts directly with Casp8 DED [71] to prevent Casp8-mediated apoptosis [60, 70, 71, 76], a pattern of cell death suppression that is comparable to the beta-herpesvirus-encoded inhibitor of caspase activation (vICA) [2, 4, 77, 78] or, possibly, gamma-herpesvirus-encoded FLIP (vFLIP) homologs [77].
TNF as well as TLR3 pathways trigger cell death and contribute to host defense against HSV in mice [79–82]. Humans with mutations in innate signaling pathways exhibit marked susceptibility to HSV1 encephalitis [83–88]. The acknowledged host defense value of necroptosis in mice [18, 19] prompted an evaluation of the importance of this pathway in human cells and the potential for HSV1 ICP6 and HSV2 ICP10 to block the pathway. Although these viral R1 proteins were shown to bind RIP1, the interaction was initially mapped outside of the N-terminal RHIM-like sequence homology [89]. It turns out that both ICP6 and ICP10 have an N-terminal RHIM that mediates binding to RIP1 and RIP3 (Fig. 4). In human cells, this interaction prevents RIP3 activation and formation of a necrosome downstream of TNFR1- and Fas-dependent necroptosis [29]. In mouse cells, similar interactions of ICP6 have revealed an ability to trigger necroptosis independent of death receptor, DAI or TRIF signaling [30, 31]. Thus, both HSV1 and HSV2 R1 function like M45 in human cells, but HSV1 R1 appears to contribute to virus-induced necroptosis in mouse cells and mice. The suppression of necroptosis in human cells comes with one important additional feature that distinguishes the protein from murine CMV M45/vIRA; the C-terminal RNR domain known to control apoptosis is also necessary for suppression of necroptosis by both R1 homologs. Neither M45 nor human CMV UL45 bind to Casp8 [29]. Both beta-herpesviruses encode the separate Casp8-binding protein, vICA. Thus, in addition to blocking Casp8-dependent apoptosis, the RNR domain of HSV1 and HSV2 R1 simultaneously opens the pro-necrotic trapdoor in human cells by blocking Casp8 activation, but closes this alternate outcome via RHIM-dependent disruption of RIP1–RIP3 interaction.
Viral initiation of necroptosis
Mouse cells infected with murine CMV M45 mutant virus die via DAI–RIP3-dependent necroptosis [27, 28]. Even though human cells infected with either WT or ICP6 mutant HSV1 do not undergo spontaneous death [29], HSV1-infected mouse cells die prematurely from necroptosis [30, 31]. HSV1-infected C57BL/6 mouse cells and mice show compromised titers compared to RIP3-deficient cells and mice [30, 31], a pattern reminiscent of vaccinia infection [23]. HSV1 ∆ICP6 deletion mutant or tetra-Ala substitution mutant (mutRHIM) viruses relieve this RIP3-dependent restriction of infection. Furthermore, transfection with HSV1 ICP6 into mouse, but not human cells, triggers the interaction with RIP1 and/or RIP3, resulting in RHIM-dependent initiation of necroptosis [30, 31]. The consequences of interacting with RIP1 and RIP3 result in an opposite outcome from HSV1-infected human cells where the R1 protein blocks Casp8-mediated apoptosis [71] as well as RIP3-mediated necroptosis [29]. These studies reveal the importance of apoptosis and necroptosis in human cell autonomous host defense against HSV1 and HSV2. The observations demonstrate how differently mouse cells respond to these human pathogens, raising serious questions about using WT HSV1 in mice for studies intended to model pathogenesis and latency in humans. In human cells, where both apoptosis and necroptosis are suppressed, ICP6 and ICP10 emerge as potent cell death suppressors in addition to enzymatic activity responsible for generating deoxyribonucleotides.
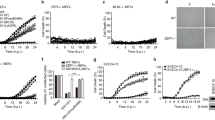
The direct role of HSV1 ICP6 in triggering necroptosis during infection in mouse cells [30, 31] suggests that necroptosis may provide a cross-species barrier to infection. Not much research has gone into defining the contribution of cell autonomous death to cross-species infection, although investigation of the block to murine CMV in human cells demonstrated that intrinsic apoptosis also contributes as a barrier [12]. Because recent studies investigating the initiation of necroptosis in HSV1-infected mouse cells and mice implicated RIP3 but left the question of a role for RIP1 incompletely resolved, we have explored the contribution of RIP1 and RIP3 kinase activity in the pro-necrotic potential of HSV1 infection for mouse cells. We first examined the sensitivity of control necroptosis-sensitive and RIP3 shRNA knockdown 3T3-SA cells to infection with two pairs of viruses, HSV1 KOS strain compared to ∆ICP6 mutant virus or F strain and mutRHIM virus used previously [29, 31]. As shown in Fig. 5a, dramatic levels of death were observed in 3T3-SA cells at 16 hpi with either KOS or F strain. Death was reduced when either ∆ICP6 or mutRHIM virus was employed. Consistent with previous published data [30, 31], knockdown of RIP3 reversed cell death induced by either WT or mutant HSV1 strains. Thus, HSV1 triggers RIP3- and MLKL-dependent necroptosis in mouse cells facilitated by the expression of ICP6. Somewhat surprisingly, by 24 hpi, HSV1 KOS and ∆ICP6 mutant virus induce similar levels of death in either 3T3-SA or L929 cells when assayed across a range of MOIs (Fig. 5b, c). These results suggested that HSV1-mediated necroptosis in mouse cells was facilitated by ICP6 at earlier times, but that death was independent of this gene product when cells were followed for longer times. In order to further understand the behavior, we infected cells carrying kinase inactive versions of RIP1 (K45A) or RIP3 (K51A). Cultured WT, RIP1 K45A, and RIP3 K51A mouse embryo fibroblasts (MEFs) were infected with HSV1 or ∆ICP6 mutant virus at an MOI of 5, and cell viability was assessed at 22 hpi. As shown in Fig. 5d, RIP1 K45A MEFs showed a modest decrease in necroptosis compared to WT MEFs, and RIP3 K51A MEFs were completely resistant to virus-induced necroptosis. Importantly, parental WT strains KOS and F did not show a difference from ICP6 mutant viruses, ∆ICP6 or mutRHIM. This result indicated that HSV1-induced necroptosis is RIP3 kinase-dependent. Similar to a recent report [31], these results suggest that RIP1 kinase partially influences the outcome. To more fully explore the differences in the time course of HSV1 KOS and ∆ICP6 virus-induced necroptosis, cell permeability was measured throughout infection using Sytox Green on an IncuCyte instrument [29]. In stark contrast to uninfected cells, which remained impermeable, MEFs infected with either KOS or ∆ICP6 became positive between 8 and 10 hpi and progressed to maximal levels by 20–24 hpi (Fig. 5e), with the ∆ICP6 mutant lagging the parental WT KOS strain. Differences in the extent of cell permeability became most dramatic around 12 hpi when the level of WT virus-induced necroptosis was approximately twice that in the ICP6 mutant virus infection and lasted until 20 hpi. Consistent with the viability assay shown in Fig. 5d, time course analysis of cell permeability showed that RIP1 kinase inactive mutant had very little impact on cell death when infected with HSV1 KOS or ∆ICP6, but that RIP3 kinase inactive cells completely resisted infection (Fig. 5f and data not shown).
HSV1 infection triggers RIP3-dependent necroptosis in mouse cells. a Cell viability of HSV1 KOS strain and HSV1 KOS-derived ∆ICP6, as well as HSV1 F strain and HSV1 F-derived ICP6 mutRHIM virus-infected 3T3-SA cells (MOI = 5) stably expressing either control scrambled (Sc) shRNA or RIP3 shRNA. Viability was determined by measuring ATP levels using a Cell Titer-Glo assay at 16 hpi. Immunoblot (IB) detection of RIP3 in transduced whole cell lysates is shown above. b Viability of indicated virus-infected 3T3-SA cells (MOI = 5, 10, 50, or 100), and viability was determined at 24 hpi as described in panel A. c Viability of indicated virus-infected L929 cells (MOI = 5, 10, 50, or 100), and viability was determined at 24 hpi as described in panel A. d Viability of indicated virus-infected WT, RIP1 kinase inactive mutant (RIP1 K45A), and RIP3 kinase inactive mutant (RIP3 K51A) MEFs (MOI = 5), and viability was determined at 22 hpi as described in panel A. e Time course (IncuCyte) cell death analysis of indicated virus-infected WT MEFs (MOI = 5) assessed by cell permeability using Sytox Green (50 nM) fluorescent dye stained cells per mm2. f Time course analysis showing cell death of HSV1 (KOS)-infected WT, RIP1 K45A, and RIP3 K51A MEFs assessed by cell permeability as described in panel E
Previous studies implicated HSV1 ICP6 as an inducer of necroptosis independent of infection by using transient overexpression in mouse cells [30, 31]. We were able to isolate necroptosis-sensitive 3T3-SA cells stably expressing either ICP6 or ICP10, relying on transduction methods we employed on human cells [29]. R1-expressing cells were readily isolated without any signs of spontaneous death reported when transient overexpression was used [30, 31]. We then evaluated the sensitivity of these HSV R1-expressing cells to necroptosis induced by TNF plus zVAD. HSV2 ICP10 modestly protected the cells and, as expected, RIP3 kinase inhibitor GSK’872 fully protected (Fig. 6a). In contrast, HSV1 ICP6 modestly increased sensitivity to death of transduced 3T3-SA cells, as we recently reported [29]. While both of these R1 proteins are potent cell death suppressors in human cells, they show inconsistent behavior in mouse cells. In addition to modest protection from TNFR1-induced necroptosis, ICP10 protected 3T3-SA cells from MCMVmutRHIM virus-induced, DAI-dependent necroptosis (Fig. 6b), but not from TLR3/TRIF-induced necroptosis. ICP10 sensitized 3T3-SA cells to poly(I:C)-induced death independent of the addition of caspase inhibitor zVAD (Fig. 6c). As expected, MCMV M45-encoded vIRA exhibited the broad capacity to block TNFR1–RIP1-induced, TLR3–TRIF-induced, and virus-induced DAI–RIP3-dependent necroptosis (Fig. 6d). In L929 cells, ICP10 modestly protected from TNF, zVAD, or TNF plus zVAD-induced necroptosis (Fig. 6e), although this R1 protein did not have any impact on MEFs (Fig. 6f). These results indicated that ICP10 exhibits anti-necroptosis function in a mouse cell line-dependent manner. Overall, HSV1 infection induces necroptosis in mouse cells but not in human cells. The UL39 gene product ICP6 alone is not necessary for the induction of necroptosis in mouse cells by HSV1 even though ICP6 alone induces necroptosis under some conditions when expressed in mouse cells. It is important to appreciate that other viral functions aside from ICP6 drive necroptosis in mouse cells as well as the fact that stress of transfection or other assay conditions may influence the potential of ICP6 to trigger necroptosis on its own.
Role of HSV2 ICP10 in necroptosis of mice cells. a Viability of 3T3-SA-EV or 3T3-SA-ICP10 cell lines treated for 18h with TNF (T, 25 ng/mL) and/or caspase inhibitor zVAD (V, 25 μM) in the absence or presence of RIP3 kinase inhibitor GSK’872 (5 μM). b Viability of 3T3-SA-EV or 3T3-SA-ICP10 cell lines infected with MCMV parental K181 or M45mutRHIM virus (MOI = 10) for 18 h. c Viability of IFNβ-primed 3T3-SA cells for 24 h following treated with poly(I:C) (25 μg/ml) in the absence or presence of zVAD for 18 h. d Viability of 3T3-SA-EV or 3T3-SA-M45 cells treated for 18 h with T+V or poly(I:C)+V, or infected with M45mutRHIM virus. e Viability of L929-EV or L929-ICP10 cells treated for 18 h with T and/or V. f Viability of MEF-EV or MEF-ICP10 cells treated for 18 h with T, V, IAP antagonist BV6 (S; 1 μM) or the indicated combinations. Viability was determined by measuring ATP levels as in Fig. 5
Given that MCMV M45-encoded vIRA disrupts the ability of RIP3 to interact with activator proteins RIP1, TRIF, and DAI [27, 28], and M45 is a homolog of HSV R1 [52], we were encouraged by the observation that HSV R1 blocked necroptosis in human cells. Neither ICP6 nor ICP10 functions consistently in cells derived from the non-natural mouse host. In contrast, MCMV M45 displays a broad capacity to prevent necroptosis in cells from either mice or humans (Fig. 6) [29, 31]. HSV1 infection triggers a dramatic level of necroptosis in mouse cells dependent upon RIP3 kinase activity, in line with two recent reports [30, 31]. In contrast to these reports, however, we observe that ICP6 mutant viruses retain pro-necrotic impact on mouse cells, albeit delayed, indicating that ICP6 is not the only viral gene product that is responsible for triggering necroptosis in the non-natural mouse host. Importantly, HSV1 ICP6 and its close relative HSV2 ICP10 are both necessary and sufficient to protect human cells from necroptosis as well as apoptosis whether assessed within the context of virus infection or independent of virus infection [29].
Synopsis and outlook
Necroptosis clearly provides cell autonomous host defense against viruses infecting mouse and human cells even though herpesviruses derail RIP3 activation by disrupting key signaling events. This pathway may be important in preventing cross-species infections where the viral suppressors are either inactive or contribute to the recruitment of RIP3. Future efforts will address how other viruses, particularly poxviruses, interface with this pathway. The relative importance of RIP1, TRIF, and DAI in triggering RIP3 kinase activity will also continue to receive deserved attention. Finally, it is widely presumed that necroptosis contributes to inflammatory disease pathology; however, more direct tools and interventions are needed to interrogate affected tissues.
References
Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G (2008) Viral control of mitochondrial apoptosis. PLoS Pathog 4(5):e1000018
Mocarski ES, Upton JW, Kaiser WJ (2011) Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol 12(2):79–88. doi:10.1038/nature09857
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219
McCormick AL, Mocarski ES (2013) Cell death pathways controlled by cytomegaloviruses. In: Reddehase MJ (ed) Cytomegaloviruses: from molecular pathogenesis to intervention, vol I. Caister Scientific Press, Norfolk, pp 263–276
Werden SJ, Rahman MM, McFadden G (2008) Poxvirus host range genes. Adv Virus Res 71:135–171. doi:10.1016/s0065-3527(08)00003-1
Jurak I, Schumacher U, Simic H, Voigt S, Brune W (2008) Murine cytomegalovirus m38.5 protein inhibits Bax-mediated cell death. J Virol 82(10):4812–4822
Manzur M, Fleming P, Huang DC, Degli-Esposti MA, Andoniou CE (2009) Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ 16(2):312–320. doi:10.1038/cdd.2008.152
Crosby LN, McCormick AL, Mocarski ES (2013) Gene products of the embedded m41/m41.1 locus of murine cytomegalovirus differentially influence replication and pathogenesis. Virology 346:274–283
Fleming P, Kvansakul M, Voigt V, Kile BT, Kluck RM, Huang DC, Degli-Esposti MA, Andoniou CE (2013) MCMV-mediated Inhibition of the pro-apoptotic Bak protein Is required for optimal in vivo replication. PLoS Pathog 9(2):e1003192. doi:10.1371/journal.ppat.1003192
Handke W, Luig C, Popovic B, Krmpotic A, Jonjic S, Brune W (2013) Viral inhibition of BAK promotes murine cytomegalovirus dissemination to salivary glands. J Virol 87(6):3592–3596. doi:10.1128/jvi.02657-12
E X, Hwang S, Oh S, Lee JS, Jeong JH, Gwack Y, Kowalik TF, Sun R, Jung JU, Liang C (2009) Viral Bcl-2-mediated evasion of autophagy aids chronic infection of gammaherpesvirus 68. PLoS Pathog 5(10):e1000609. doi:10.1371/journal.ppat.1000609
Jurak I, Brune W (2006) Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J 25(11):2634–2642
Micheau O, Tschopp J (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114(2):181–190
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, Macfarlane M, Hacker G, Leverkus M (2011) cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 43:449–463. doi:10.1016/j.molcel.2011.06.011
Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, Macfarlane M, Cain K, Meier P (2011) The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 43:432–448. doi:10.1016/j.molcel.2011.06.006
Feoktistova M, Leverkus M (2015) Programmed necrosis and necroptosis signalling. FEBS J 282(1):19–31. doi:10.1111/febs.13120
Upton JW, Chan FK (2014) Staying alive: cell death in antiviral immunity. Mol Cell 54(2):273–280. doi:10.1016/j.molcel.2014.01.027
Mocarski ES, Kaiser WJ, Livingston-Rosanoff D, Upton JW, Daley-Bauer LP (2014) True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. J Immunol 192(5):2019–2026. doi:10.4049/jimmunol.1302426
Chan FK, Luz NF, Moriwaki K (2014) Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol. doi:10.1146/annurev-immunol-032414-112248
Wallach D, Kang TB, Kovalenko A (2014) Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol 14(1):51–59. doi:10.1038/nri3561
Kaiser WJ, Upton JW, Mocarski ES (2013) Viral modulation of programmed necrosis. Curr Opin Virol 3:296–306. doi:10.1016/j.coviro.2013.05.019
Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ (2003) A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278(51):51613–51621
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123. doi:10.1016/j.cell.2009.05.037
Moquin D, Chan FK (2010) The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 35(8):434–441. doi:10.1016/j.tibs.2010.03.001
Berger AK, Danthi P (2013) Reovirus activates a caspase-independent cell death pathway. mBio 4(3):e00178-13. doi:10.1128/mBio.00178-13
Upton JW, Kaiser WJ, Mocarski ES (2008) Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem 283(25):16966–16970
Upton JW, Kaiser WJ, Mocarski ES (2010) Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7(4):302–313. doi:10.1016/j.chom.2010.03.006
Upton JW, Kaiser WJ, Mocarski ES (2012) DAI (ZBP1/DLM-1) complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11:290–297
Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES (2015) Herpes simplex virus suppression of necroptosis in human cells. Cell Host Microbe 17:243–251
Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S (2014) Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA 111:15438–15443. doi:10.1073/pnas.1412767111
Huang Z, Wu S-Q, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong C, Xia W, Zhou R, Zheng C, Han J (2015) Targeting HSV-1 protein ICP6 by RIP1 and RIP3 initiates necroptosis to restrict HSV-1 propagation in mice. Cell Host Microbe 17:229–242
Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S (2012) Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol 13:954–962. doi:10.1038/ni.2397
Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, Harris PA, Bertin J, Gough PJ, Shayakhmetov DM, Goguen JD, Fitzgerald KA, Silverman N, Lien E (2014) Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci USA 111:7391–7396. doi:10.1073/pnas.1403477111
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367. doi:10.1038/nature09852
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471(7338):368–372. doi:10.1038/nature09857
Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471(7338):373–376. doi:10.1038/nature09878
Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR (2012) Survival function of the FADD-caspase-8-cFLIP(L) complex. Cell Rep 1:401–407
Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES (2014) RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA 111:7753–7758. doi:10.1073/pnas.1401857111
Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J (2014) RIPK1 regulates RIPK3–MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157(5):1175–1188. doi:10.1016/j.cell.2014.04.019
Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR (2014) RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157(5):1189–1202. doi:10.1016/j.cell.2014.04.018
Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ (2014) RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56(4):481–495. doi:10.1016/j.molcel.2014.10.021
Newton K, Dugger DL, Wickliffe KE, Kapoor N, de-Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343:1357–1360. doi:10.1126/science.1249361
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137(6):1100–1111. doi:10.1016/j.cell.2009.05.021
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325:332–336. doi:10.1126/science.1172308
Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM (2011) Mechanisms of necroptosis in T cells. J Exp Med 208(4):633–641. doi:10.1084/jem.20110251
Thapa RJ, Chen P, Cheung M, Nogusa S, Pei J, Peri S, Testa JR, Balachandran S (2013) NF-kappaB inhibition by bortezomib permits IFN-gamma-activated RIP1 kinase-dependent necrosis in renal cell carcinoma. Mol Cancer Ther 12(8):1568–1578. doi:10.1158/1535-7163.mct-12-1010
McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S (2014) Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci USA 111(31):E3206–E3213. doi:10.1073/pnas.1407068111
He S, Liang Y, Shao F, Wang X (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA 108(50):20054–20059. doi:10.1073/pnas.1116302108
Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3 and MLKL. J Biol Chem 288:31268–31279. doi:10.1074/jbc.M113.462341
Brune W, Menard C, Heesemann J, Koszinowski UH (2001) A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291(5502):303–305. doi:10.1126/science.291.5502.303291/5502/303
Lembo D, Donalisio M, Hofer A, Cornaglia M, Brune W, Koszinowski U, Thelander L, Landolfo S (2004) The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J Virol 78(8):4278–4288
Lembo D, Brune W (2009) Tinkering with a viral ribonucleotide reductase. Trends Biochem Sci 34(1):25–32. doi:10.1016/j.tibs.2008.09.008
Galvan V, Roizman B (1998) Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA 95(7):3931–3936
Leopardi R, Van Sant C, Roizman B (1997) The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA 94(15):7891–7896
Peri P, Nuutila K, Vuorinen T, Saukko P, Hukkanen V (2011) Cathepsins are involved in virus-induced cell death in ICP4 and Us3 deletion mutant herpes simplex virus type 1-infected monocytic cells. J Gen Virol 92(Pt 1):173–180. doi:10.1099/vir.0.025080-0
Galvan V, Brandimarti R, Roizman B (1999) Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol 73(4):3219–3226
Zhou G, Roizman B (2000) Wild-type herpes simplex virus 1 blocks programmed cell death and release of cytochrome c but not the translocation of mitochondrial apoptosis-inducing factor to the nuclei of human embryonic lung fibroblasts. J Virol 74(19):9048–9053
Munger J, Chee AV, Roizman B (2001) The U(S)3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J Virol 75(12):5491–5497
Hagglund R, Munger J, Poon AP, Roizman B (2002) U(S)3 protein kinase of herpes simplex virus 1 blocks caspase 3 activation induced by the products of U(S)1.5 and U(L)13 genes and modulates expression of transduced U(S)1.5 open reading frame in a cell type-specific manner. J Virol 76(2):743–754
Langelier Y, Bergeron S, Chabaud S, Lippens J, Guilbault C, Sasseville AM, Denis S, Mosser DD, Massie B (2002) The R1 subunit of herpes simplex virus ribonucleotide reductase protects cells against apoptosis at, or upstream of, caspase-8 activation. J Gen Virol 83(Pt 11):2779–2789
Perkins D, Pereira EF, Aurelian L (2003) The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1. J Virol 77(2):1292–1305
Zhou G, Avitabile E, Campadelli-Fiume G, Roizman B (2003) The domains of glycoprotein D required to block apoptosis induced by herpes simplex virus 1 are largely distinct from those involved in cell-cell fusion and binding to nectin1. J Virol 77(6):3759–3767
Leopardi R, Roizman B (1996) The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA 93(18):9583–9587
Benetti L, Roizman B (2004) Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc Natl Acad Sci USA 101(25):9411–9416
Munger J, Roizman B (2001) The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci USA 98(18):10410–10415
Zhou G, Roizman B (2002) Truncated forms of glycoprotein D of herpes simplex virus 1 capable of blocking apoptosis and of low-efficiency entry into cells form a heterodimer dependent on the presence of a cysteine located in the shared transmembrane domains. J Virol 76(22):11469–11475
Benetti L, Munger J, Roizman B (2003) The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J Virol 77(11):6567–6573
Perkins D, Yu Y, Bambrick LL, Yarowsky PJ, Aurelian L (2002) Expression of herpes simplex virus type 2 protein ICP10 PK rescues neurons from apoptosis due to serum deprivation or genetic defects. Exp Neurol 174(1):118–122. doi:10.1006/exnr.2001.7849
Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L (2002) The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J Virol 76(3):1435–1449
Chabaud S, Sasseville AM, Elahi SM, Caron A, Dufour F, Massie B, Langelier Y (2007) The ribonucleotide reductase domain of the R1 subunit of herpes simplex virus type 2 ribonucleotide reductase is essential for R1 antiapoptotic function. J Gen Virol 88(Pt 2):384–394. doi:10.1099/vir.0.82383-0
Dufour F, Sasseville AM, Chabaud S, Massie B, Siegel RM, Langelier Y (2011) The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFalpha- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis Int J Progr Cell Death 16(3):256–271. doi:10.1007/s10495-010-0560-2
Han JY, Miller SA, Wolfe TM, Pourhassan H, Jerome KR (2009) Cell type-specific induction and inhibition of apoptosis by herpes simplex virus type 2 ICP10. J Virol 83(6):2765–2769. doi:10.1128/jvi.02088-08
Aurelian L (2005) HSV-induced apoptosis in herpes encephalitis. Curr Top Microbiol Immunol 289:79–111
Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina SM, Hofman FM, Ghiasi H, Nesburn AB, Wechsler SL (2000) Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287(5457):1500–1503
Du T, Zhou G, Roizman B (2012) Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci USA 109(36):14616–14621. doi:10.1073/pnas.1212661109
Gober MD, Laing JM, Thompson SM, Aurelian L (2006) The growth compromised HSV-2 mutant deltaRR prevents kainic acid-induced apoptosis and loss of function in organotypic hippocampal cultures. Brain Res 1119(1):26–39. doi:10.1016/j.brainres.2006.08.078
Bertin J, Armstrong RC, Ottilie S, Martin DA, Wang Y, Banks S, Wang GH, Senkevich TG, Alnemri ES, Moss B, Lenardo MJ, Tomaselli KJ, Cohen JI (1997) Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA 94(4):1172–1176
Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS (2001) A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci USA 98(14):7829–7834. doi:10.1073/pnas.141108798141108798
Lundberg P, Welander PV, Edwards CK 3rd, van Rooijen N, Cantin E (2007) Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J Virol 81(3):1451–1460. doi:10.1128/jvi.02243-06
Rossol-Voth R, Rossol S, Schutt KH, Corridori S, de Cian W, Falke D (1991) In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J Gen Virol 72(Pt 1):143–147
Sergerie Y, Rivest S, Boivin G (2007) Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis 196(6):853–860. doi:10.1086/520094
Sergerie Y, Boivin G, Gosselin D, Rivest S (2007) Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J Infect Dis 195(6):817–825. doi:10.1086/511987
Bousfiha A, Picard C, Boisson-Dupuis S, Zhang SY, Bustamante J, Puel A, Jouanguy E, Ailal F, El-Baghdadi J, Abel L, Casanova JL (2010) Primary immunodeficiencies of protective immunity to primary infections. Clin Immunol 135(2):204–209. doi:10.1016/j.clim.2010.02.001
Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, Herman M, Cardon A, Durandy A, Bustamante J, Vallabhapurapu S, Bravo J, Warnatz K, Chaix Y, Cascarrigny F, Lebon P, Rozenberg F, Karin M, Tardieu M, Al-Muhsen S, Jouanguy E, Zhang SY, Abel L, Casanova JL (2010) Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33(3):400–411. doi:10.1016/j.immuni.2010.08.014
Casanova JL, Abel L, Quintana-Murci L (2011) Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol 29:447–491. doi:10.1146/annurev-immunol-030409-101335
Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Perez de Diego R, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Valerie D, Jouanguy E, Chaussabel D, Geissmann F, Abel L, Casanova JL, Zhang SY (2011) Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med 208(10):2083–2098. doi:10.1084/jem.20101568
Sancho-Shimizu V, Perez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, Fabrega S, Cardon A, Maluenda J, Tatematsu M, Mahvelati F, Herman M, Ciancanelli M, Guo Y, AlSum Z, Alkhamis N, Al-Makadma AS, Ghadiri A, Boucherit S, Plancoulaine S, Picard C, Rozenberg F, Tardieu M, Lebon P, Jouanguy E, Rezaei N, Seya T, Matsumoto M, Chaussabel D, Puel A, Zhang SY, Abel L, Al-Muhsen S, Casanova JL (2011) Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J Clin Invest 121(12):4889–4902. doi:10.1172/jci59259
Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A, Ying SW, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova JL, Studer L, Notarangelo LD (2012) Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491(7426):769–773. doi:10.1038/nature11583
Dufour F, Bertrand L, Pearson A, Grandvaux N, Langelier Y (2011) The ribonucleotide reductase R1 subunits of herpes simplex virus 1 and 2 protect cells against poly(I:C)-induced apoptosis. J Virol 85(17):8689–8701. doi:10.1128/jvi.00362-11
Acknowledgments
Support was from NIH (PHS Grants R01 AI020211 and R01 GM112547 to E.S.M and DP1 OD012198 to W.J.K.), although the content is solely the responsibility of the authors and not the NIH or PHS.
Ethical standard
This manuscript represents the effort of the authors such that all data are original, and the ideas presented are the authors’ own. All co-authors have consented to submission and have contributed sufficiently to the scientific work and therefore share collective responsibility for the results.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Special Issue on Cytomegalovirus.
Rights and permissions
About this article
Cite this article
Guo, H., Kaiser, W.J. & Mocarski, E.S. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol 204, 439–448 (2015). https://doi.org/10.1007/s00430-015-0410-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-015-0410-5