Abstract
Malaria parasites express a broad repertoire of proteins whose expression is tightly regulated depending on the life-cycle stage of the parasite and the environment of target organs in the respective host. Transmission of malaria parasites from the human to the anopheline mosquito is mediated by intraerythrocytic sexual stages, termed gametocytes, which circulate in the peripheral blood and are essential for the spread of the tropical disease. In Plasmodium falciparum, gametocytes express numerous extracellular proteins with adhesive motifs, which might mediate important interactions during transmission. Among these is a family of six secreted proteins with adhesive modules, termed PfCCp proteins, which are highly conserved throughout the apicomplexan clade. In P. falciparum, the proteins are expressed in the parasitophorous vacuole of gametocytes and are subsequently exposed on the surface of macrogametes during parasite reproduction in the mosquito midgut. One characteristic of the family is a co-dependent expression, such that loss of all six proteins occurs if expression of one member is disrupted via gene knockout. The six PfCCp proteins interact by adhesion domain-mediated binding and thus form complexes on the sexual stage surface having adhesive properties. To date, the PfCCp proteins represent the only protein family of the malaria parasite sexual stages that assembles to multimeric complexes, and only a small number of such protein complexes have so far been identified in other life-cycle stages of the parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an estimated 250 million cases every year and an annual death toll of one million people, the tropical disease malaria remains a major global health threat and a serious economic burden (reviewed in [1, 2]). Approximately 50% of the world’s population live in affected areas within wide regions of Africa, South-East Asia, Central America, and the Eastern Mediterranean [3]. In most endemic countries, the treatment and control of malaria is undermined by the spread of drug resistant parasites, extreme poverty, and the impact of HIV infections.
Malaria is a vector-borne disease that is caused by protozoan parasites of the genus Plasmodium and transmitted by blood-feeding anopheline mosquitoes. There are four human malaria species, among them P. falciparum, the causative agent of the dangerous malaria tropica. When infecting a human host, malaria parasites initially target liver hepatocytes, causing a primary but asymptomatic infection. The parasites subsequently infect erythrocytes, and the recurrent intraerythrocytic replication cycles cause the typical symptoms of the disease, such as fever and anemia, which in severe cases are followed by respiratory distress and organ failure.
Besides three different periods of asexual replication, which take place in the human hepatocytes and erythrocytes as well as inside the mosquito vector, the malaria life cycle also includes a phase of sexual reproduction that mediates parasite transition from the human to the mosquito and thus plays a crucial part in disease transmission. In P. falciparum, the malaria sexual phase begins with the differentiation of intraerythrocytic parasites to the non-replicative gametocyte lineage. Following maturation to crescent-shaped intraerythrocytic forms, the gametocytes freely circulate in the peripheral circulation in anticipation of being taken up by the mosquito during the blood meal. Environmental stimuli in the mosquito midgut during blood feeding rapidly activate the gametocytes, which within minutes egress from the host erythrocyte and undergo gametogenesis, resulting in the development of female macro- and male microgametes. The motile microgamete fertilizes the sessile macrogamete, and within 20 h, the resulting zygote transforms into a motile form, termed the ookinete. The ookinete exits the midgut lumen and crosses the gut epithelium, which marks the end of the malaria sexual phase (reviewed in [4, 5]).
It was only in the past two decades, when research focused on the sexual phase of the parasite life cycle. This interest arose upon the observation that the host’s immune response to sexual stage antigens can completely inhibit the development of Plasmodium in the insect vector, and such antigens would therefore represent ideal candidates for the development of transmission-blocking vaccines (TBVs) (reviewed in [6]). This type of vaccine is based on human antibodies directed against select sexual stage antigens, which are taken up by the mosquito with the blood meal and which eliminate the parasite sexual stages inside the mosquito midgut, as soon as these lose protection by the enveloping erythrocyte. Hence, TBVs represent a powerful tool in malaria control efforts, particularly together with new combination-therapy drugs, and vaccines targeting morbidity and mortality due to the asexual stages (reviewed in [7, 8]).
Among the first vaccine candidates discovered in the 1980s were Pfs230 and Pfs48/45, which are expressed at the surface of the developing gametocytes and later of male and female gametes (reviewed in [4]). Both proteins share a primary structure composed of several cysteine-rich domains assigning them to the cysteine-rich motif superfamily that is comprised of ten members (Fig. 1a) [9–11]. Two additional antigens, found on the surface of zygotes and ookinetes, are Pfs25 and its paralog Pfs28, both possessing four epidermal growth factor (EGF) domains and glycosylphosphatidylinositol (GPI) anchors. The transmission-blocking potential of antisera targeting Pfs25 and its vivax malaria ortholog Pvs25 in humans was subject of clinical phase I trials [12, 13], further confirming the suitability of this protein for the generation of TBVs.
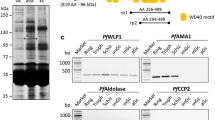
a Schematic of the domain structure of select Plasmodium falciparum sexual stage proteins containing adhesive motifs, i.e., the PfCCp multi-domain adhesion protein family, the EGF domain-containing protein Pfs25, and the cysteine-rich motif proteins Pfs230 and Pfs48/45. Encircled black-dotted domains indicate newly identified motifs. b Schematic demonstrating the proposed network of interactions between sexual stage adhesion proteins. The multimeric protein complex most likely is retained at the plasma membrane of gametocytes via binding to the GPI-anchored Pfs48/45. After gametocyte activation, Pfs25 also locates to the cell surface of the forming macrogamete, covalently linked by a GPI anchor, thereby providing an additional retention site for the multi-protein complex. PPM parasite plasma membrane
Additional molecules were identified in the following years, including the here discussed PfCCp multi-domain adhesion protein family [14]. Noteworthy is that the majority of sexual stage surface proteins have adhesive properties and can be divided into two classes. One class of sexual stage proteins, including Pfs230, Pfs48/45 and the six PfCCp proteins, is expressed within the parasitophorous vacuole (PV) of the developing gametocyte and subsequently present on the gamete surface, but expression of these proteins usually ceases during fertilization. The expression of the second class of surface proteins, among them Pfs25 and Pfs28, is initiated after the parasites have entered the mosquito midgut and persists until the ookinete has formed (reviewed in [4, 5]).
The reasons for the high number of adhesive proteins in the malaria parasite sexual stages and the coordinated expression pattern of these proteins during transmission remain elusive. A recent study from our laboratory indicated that a subset of these proteins assemble to multimeric complexes on the parasite surface. In this review, we highlight our previous findings on the PfCCp protein family and provide evidence for complex formation of multiple surface-associated sexual stage proteins. We will compare these data with findings on other protein complexes of malaria parasites and speculate on the possible function of the sexual stage multimeric complexes.
Domain structure of the PfCCp protein family
The PfCCp protein family was identified via annotation of the P. falciparum genome sequence for proteins encoding multi-domain “animal-like” architectures. Six members were described exhibiting multiple adhesion domains conserved among animals and bacteria (Fig. 1a) [14], five of which share a common LCCL (Limulus coagulation factor C, cochlear protein Coch-5b2, and late gestation lung protein Lgl1) domain, which was eponymous for the proteins thenceforward named PfCCp1 to PfCCp5 (LCCL domain-containing protein). Despite the lack of a LCCL domain, a sixth protein referred to as PfFNPA was assigned to the PfCCp protein family due to its structural similarity to PfCCp5. The predicted signal peptide sequence common to all PfCCp proteins indicated an extracellular location, yet GPI-anchor signal sequences or transmembrane regions were not detected [14]. Significantly, all members of the PfCCp protein family possess orthologs in other organisms of the apicomplexan clade.
PfCCp1 and PfCCp2 have similar architectures and are paralogs arising from gene duplication after domain accretion. PfCCp1 and PfCCp2 possess binding domains, corresponding to ricin, discoidin, and levanase-type lectin domains [15, 16]. C-terminal to these modules is two copies of an apicomplexan-specific cysteine-rich module, termed ApicA. Between the discoidin and levanase lectin domains are two LCCL domains [17] and a NEC module (termed after neurexins [18] and collagens).
PfCCp3 contains in total four LCCL domains, a tandem repeat of a scavenger receptor (SR) domain, a lipoxygenase LH2 domain, and a region similar to pentraxin. PfCCp4 possesses two center LCCL domains, followed by tandem levanase domains. PfCCp5 encodes a region at the amino terminus that is similar to the type 2 domain of fibronectin (FN2), fused to a domain similar to the N-terminal region of anthrax protective antigen (PA) and a single LCCL domain near the C-terminus. As described above, PfFNPA is similar to PfCCp5 in overall architecture but lacks an LCCL domain. Given that PfFNPA has an apparent ortholog in the distantly related apicomplexan, Cryptosporidium parvum, the two genes are likely to have diverged in P. falciparum from a common ancestor that was similar in architecture to PfFNPA, followed by accretion of an LCCL domain in PfCCp5.
Recent re-annotation of the PfCCp protein sequences [I] revealed several additional adhesion domains, i.e., a second discoidin domain in PfCCp1 and PfCCp2, which is located at the N-terminus of the respective protein, and a discoidin domain in PfCCp5, which is located between the anthrax PA N-terminal domain and the LCCL domain (Fig. 1a). Furthermore, two novel N-terminal adhesion domains were identified in PfCCp4, which correspond to ricin domain and anthrax PA N-terminal domain. The various adhesion domains and their potential ligands are discussed as follows.
Expression of PfCCp proteins in sexual stage parasites
Expression profile studies on the PfCCp proteins revealed transcript and protein expression specific to gametocytes within the five developmental stages during maturation (termed stages I–V) [19, 20]. As predicted from the signal peptide sequences, the proteins are secreted and localized to the parasite plasma membrane within the PV. PfCCp1, PfCCp2, and PfCCp3 co-localize in a punctate pattern at the parasite surface, while the surface-associated expression of PfCCp4 is rather homogenous and is thus similar to the expression pattern of Pfs230 and Pfs48/45. PfCCp5 and PfFNPA are less abundant in gametocytes than the other four PfCCp proteins and in mature gametocytes often localize to the cell poles [20].
After gametogenesis in the mosquito midgut, which involves parasite egress from the host erythrocyte, the PfCCp proteins are present on the cell surface of macrogametes. For PfCCp1, PfCCp2, and PfCCp3, partial release of the proteins was postulated [14]. Expression of the six proteins ceases within a few hours after egress; hence, the PfCCp proteins belong to class I of sexual stage proteins.
To gain information on possible protein functions, we constructed gene-disruptant parasite lines for PfCCp2, PfCCp3, PfCCp4, and PfFNPA, resulting from single-crossover recombination, as well as a double-crossover knock-out mutant for PfCCp1 [14, 20, 21]. Expression profile studies in the mutants (herein referred to as PfCCp-KOs) led to the remarkable observation that the six PfCCp proteins are expressed in a co-dependent manner and that the absence of one protein results in the complete or partial loss of all other protein family members. While the transcript abundances of intact PfCCp genes in the knock-out mutants are comparable to those in wild-type parasites, the proteins are not detectable via Western blot analysis and immunofluorescence assay [21, 22]. Thus, the co-dependent expression manifests at the translational or post-translational level rather than at the transcriptional level of gene expression. The observed co-dependent expression of the PfCCp family led to the assumption that the proteins interact in a way, which ensures localization to and/or retention at the correct expression site and prevents a likely degradation and loss of protein.
Intermolecular binding between PfCCp proteins
Based on the co-dependent PfCCp protein expression, we hypothesized that the six proteins interact in a complex and that instability in the complex due to gene ablation results in co-dependent degradation. By means of co-immunoprecipitation on wild-type gametocyte lysates followed by Western blot analysis, numerous protein–protein interactions between the distinct PfCCp proteins were revealed and interplays on the molecular level were observed between all PfCCp proteins (Fig. 1b) [21]. While no interactions were detectable between PfCCp4 and PfCCp5, the lack of signal for the respective precipitated protein by Western blot analysis is possibly due to the low efficiency of the available antisera against PfCCp4 and PfCCp5.
The molecular interactions between the PfCCp proteins were subsequently studied via affinity chromatography co-elution binding assays, using recombinant proteins comprising selected adhesion domains. The eluted protein complexes were screened by Western blot analyses. Out of 33 combinations investigated, 18 showed adhesive interaction, while 15 recombinant protein pairs did not interact. Adhesion domains that were predominant in protein–protein binding include the LCCL domains of all PfCCp proteins and the SR domains of PfCCp3 (with an involvement of 32% (LCCL) and 19% (SR) of all binding events). Moreover, the ApicA, NEC, and discoidin domains of PfCCp1 and PfCCp2 showed involvement in interactions between PfCCp proteins [21]. Subsequent latex beads-mediated cell binding assays confirmed the interaction of recombinant PfCCp proteins with endogenous proteins on the macrogamete surface. Here, fluorescent latex beads, coated with recombinant proteins corresponding to PfCCp1, PfCCp2, or PfCCp3 domains, bound significantly more often to newly emerged (PfCCp protein-positive) macrogametes than to intraerythrocytic gametocytes, older (PfCCp-negative) macrogametes, and PfCCp3-KO (PfCCp-negative) macrogametes [21].
An interaction of sexual stage adhesion proteins at the gamete plasma membrane leading to a stable protein complex has been shown before for the two TBV candidates Pfs48/45 and Pfs230 [23, 24]. Here, the GPI-anchored Pfs48/45 ensures the retention of Pfs230 to the gamete surface. While in the Pfs48/45-deficient gametocytes, Pfs230 is expressed as in wild-type parasites, it is not present on the surface of gametes after egression from the host cell [25].
New data from our laboratory indicate that the molecular interactions of sexual stage adhesion proteins are more complex than originally expected. We recently detected interactions of select PfCCp proteins with Pfs230 and of PfCCp4 with Pfs48/45 by co-immunoprecipitation assays on gametocyte lysates, and we further revealed the binding of these proteins with Pfs25 following gametocyte activation (Fig. 1b) [20] (N. Simon, A. Kuehn, S.M. Scholz, and G. Pradel, unpublished observation). Pfs25 belongs to class II of sexual stage proteins that are translationally repressed in gametocytes, as was shown for Plasmodium berghei Pbs25 [26, 27]. However, small amounts of protein can be found in vesicular structures in the maturing gametocytes, and the protein is relocated to the surface of macrogametes within minutes after activation [20].
CCp orthologous proteins
Select LCCL domain proteins, i.e., CCp1, CCp2, and CCp3, are highly conserved throughout the apicomplexan clade, including Cryptosporidium spp., Toxoplasma gondii, Babesia bovis, and Theileria annulata. FNPA was further described for C. parvum [14, 28] (reviewed in [29, 30]), and CCp5 was identified in T. gondii [II]. A recent genome survey study also identified the CCp family members in the early branching apicomplexan class of gregarines, Ascogregarina taiwanensis [31], and thus, the family is likely widely conserved in apicomplexans. The LCCL domain proteins are absent in Tetrahymena, for which complete genome sequence is available, and thus, it might be concluded that the acquisition and accretion of the domain architectures was relatively ancient and predates the divergence of the apicomplexan clade, but that the domains entered the apicomplexan lineage after the split of the ciliates [30].
For most of the CCp orthologs, sites of expression in the respective parasite species are yet unknown. New studies in Babesia divergens indicate an expression of BdCCp2 in gametocytes within the tick midgut (C. Becker, S. Bonnet, UMR INRA ENVN Nantes, personal communication). In contrast, expression of CpCCp1 of C. parvum (also referred to as Cpa135) starts in oocyst-sporozoites and increases rapidly after excystation [32]. In sporozoites, CpCCp1/Cpa135 is seemingly stored in the micronemes and is excreted upon host cell invasion. Subsequently, the protein localizes to the PV membrane. With the formation of merozoites, new transcript of CpCCp1/Cpa135 is detectable [33]. It is not yet known if the protein is expressed in Cryptosporidium gametocytes. Notably, both Cryptosporidium sporozoites and Plasmodium gametocytes have to resist the gut environment of either mammalian host or mosquito vector.
CCp orthologs are further present in the rodent malaria parasite P. berghei, where they are also termed LAP proteins [34, 35]. Transcript analysis revealed a predominant expression in P. berghei gametocytes for the six proteins, which was confirmed by transgenic parasites expressing GFP driven by select PbCCp/LAP promoters [36]. Genetic cross studies with female-deficient or male-deficient parasites reported that the functional genes are inherited from female gametocytes, indicating that the PbCCp/LAP proteins are female specific [35]. Recent studies on GFP fusions of PbCCp3/LAP1 (also termed PbSR), PbCCp1/LAP2, and PbCCp5/LAP3 showed protein expression in macrogametocytes and accumulation of these proteins in the crystalloids, organelles that are formed in the ookinete and persist until the early oocyst stage [37–39].
Adhesive modules of CCp proteins
A striking feature of all CCp proteins is the presence of numerous modules, most of which were described to possess adhesive properties in other organisms and were likely acquired by the parasite via lateral transfer and converted into multi-domain adhesive molecules. With the exception of the ApicA and the levanase domains, the adhesion domains of the CCp protein family were previously identified in animal cells and bacteria and assigned to diverse adhesive functions, which are described below.
The eponymous LCCL domain was first described in the horseshoe crab Limulus, where it is found in the coagulation factor C, as well as identified in the vertebrate cochlear protein Coch-5b2 and in the mammalian late gestation lung protein Lgl1 [17]. For Limulus factor C and Lgl1, a role in antimicrobial defense via recognition of cell-surface carbohydrates of pathogens has been proposed [17]. The LCCL domain has been found in association with a number of other adhesion domains, e.g., complement B-type domains, C-type lectin domains, von-Willebrand factor A domains, or discoidin domains [17].
The prototypic discoidin domain is derived from the cell adhesion protein discoidin of the slime mold Dictyostelium discoideum. The domain is found in the coagulation factors V and VIII, where it promotes phospholipid binding on the surface of platelets and endothelial cells [15, 40]. Pentraxins, on the other hand, are a family of evolutionarily conserved pattern-recognition proteins that are made up of five identical subunits. Proteins of the pentraxin family are involved in acute immunological responses and often represent serum proteins. They play a role in the binding of lipoproteins and carbohydrates [41–44] and are reported to interact with other pentraxin domains as well as with complement control protein modules (also called sushi domains) [45]. Interestingly, pentraxin 3 is essential for female fertility, participating in the assembly of the cumulus oophorus extracellular matrix [46].
The LH2 lipoxygenase domain is found in a variety of membrane- or lipid-associated proteins [47], and it is suggested that the domain mediates membrane attachment via other protein binding partners. Interactions with lectin domains and other lipoxygenase domains were predicted [III]. Fibronectin, on the other hand, is a multi-domain glycoprotein found in the plasma as well as in basement membranes, where it exhibits functions in wound healing and blood coagulation, as well as in cell adhesion and migration [48]. The FN2 domain is predicted to interact with EGF domains, lectin C, and ricin domains [IV].
Ricin is a legume lectin from seeds of the castor bean plant, Ricinus communis. The ricin B lectin domain, present in CCp1 and CCp2, binds to carbohydrates [49]. Interactions with FN2 domains, lectins, and other ricin domains were predicted [V]. The proteins CCp1, CCp2, and CCp4 also contain a pair of levanase-type lectin domains, which are found in secreted glucosidases and levanases of Bacillus subtilis and proposed to be involved in sugar binding [28].
CCp4, CCp5, and FNPA share a domain termed PA (protective antigen) that is proposed to have affinity with a domain described in Bacillus anthracis. This bacterial pathogen produces a toxin composed of three distinct proteins, i.e., protective antigen, edema factor, and lethal factor. PA is secreted in a precursor form, which can heptamerize and form a channel in membranes, allowing the other two factors to enter the target cell. The anthrax PA N-terminal domain is reported to be involved in carbohydrate binding [50]. Perhaps the best described domain of the CCp family is the scavenger receptor (SR) domain of CCp3. SR is found in several extracellular receptors, like the macrophage scavenger receptor type I. The domain is involved in protein–protein interactions and also interacts with other SR domains [45, 51, 52].
As mentioned above, the ApicA domain is an apicomplexan-specific cysteine-rich module. The NEC domain, on the other hand, appears in a wide range of animal proteins in combination with other cysteine-rich segments [53, 54], such as neurexins [18], fibrillar collagen α globular domain [55] from vertebrates and sponges and the fibrinogen family of proteins [53]. The NEC domain is also known as fibrinogen C domain, which represents the C-termini of fibrinogen ß and γ chains and which is predicted to interact, among others, with fibronectin type 3, laminin, discoidin, and other fibrinogen C domains [VI]. Fibrinogen is a dimeric protein involved in blood clotting and platelet aggregation [54, 56]. When the PfCCp protein family was described in 2004 [14], it was the first instance where the NEC domain was reported as a stand-alone form. Several animal proteins functioning as lectins contain the NEC domain, such as ficolin and intelectin [57, 58], suggesting that it is involved in sugar binding.
Multi-protein complexes of malaria parasites
Multi-protein complexes play important roles in regulating the activity and stability of polypeptides. Moreover, protein complexes function in establishing cell–cell contacts and thus are of great importance for interactions between pathogens and host cells. For intracellular pathogens, such as the majority of apicomplexan parasites, recognition, adhesion, and invasion of host cells are essential steps during infection by zoite stages (sporozoites, merozoites, bradyzoites, tachyzoites) and are often mediated by protein–protein interactions. Examples of apicomplexan multi-protein complexes are summarized in Table 1.
One well-studied example of an apicomplexan multi-protein complex is represented by protein interactions comprising the so-called glideosome that has been described in T. gondii and several Plasmodium species (e.g., [59–63], reviewed in [64, 65]. This striking protein network mediates gliding motility, which is required for the movement of the parasite along host tissue barriers and for host cell invasion. A group of transmembrane proteins belonging to the TRAP family plays a key role within the glideosome due to their transmembrane linkage of the intracellular actin-myosin motor of the infective parasite stages with the host cell surface. The P. falciparum TRAP family includes five members, namely the sporozoite micronemal proteins PfTRAP (thrombospondin-related anonymous protein; [66]), PfTLP (TRAP-like protein; [62, 67]), and PfTREP (TRAP-related protein; [68]) as well as the ookinete-specific micronemal protein PfCTRP (CSP and TRAP-related protein; [69]) and the merozoite-specific protein PfMTRAP [60]). Unifying features of P. falciparum TRAP proteins include a transmembrane domain, a short acidic cytoplasmic tail with one or more aromatic residues near the C-terminus, an extracellularly exposed array of adhesive domains comprised of type-1 thrombospondin-like domains and, with the exception of TLP, von-Willebrand factor A domains. When secreted by the zoite micronemes, the proteins become integrated into the apical surface of the parasite and bind to host cell receptors. For PfTRAP, PfMTRAP, and PfTLP, it was shown that they are connected with the intracellular actin-myosin motor complex of the parasite, which is located between the parasite outer and inner membrane, by interacting with the adapter protein aldolase [59–62] (reviewed in [65]). The motor complex consists of actin and the class XIV myosin MyoA and features several additional proteins; for instance, the glideosome-associated proteins of merozoites, GAP45, and GAP50 that in concert with PfMTIP (myosin tail-interacting protein) link MyoA to the inner membrane complex [59, 60, 70] (reviewed in [65]).
Similar glideosome structures were described in T. gondii, where the MIC (micronemal) proteins represent major key players and, indeed, much of the mechanistics were worked out using the experimental system of this pathogen. The TRAP family protein TgMIC2 is responsible for linking receptors of the host cell surface with the intracellular motor complex of tachyzoites via aldolase binding [71]. TgMIC2 forms a hexameric complex with TgM2AP, which might be necessary for TgMIC2 transport and is considered prototypical of other TRAP family members [72]. Further members of the T. gondii MIC family are the soluble TgMIC1 and TgMIC4. The two proteins interact with TgMIC6 that acts as an escorter to ensure correct targeting of both proteins to the micronemes [73]. TgMIC8 appears to escort the soluble adhesin TgMIC3 [74]. Furthermore, TgMIC6 interacts with aldolase, pointing at an involvement in host cell invasion [75]. Noteworthy, orthologs for most glideosome components were recently also identified in most other apicomplexan parasites (reviewed in [76]), including their recent discovery in Eimeria tenella [77]. The MIC orthologs EtMIC4 and EtMIC5 also aggregate to a multimeric protein complex [78].
Another extensive complex involved in merozoite attachment is constituted by the P. falciparum MSP (merozoite surface protein) group. The majority of these peripheral proteins are secreted into the PV of schizonts and subsequently binds to the surface of developing merozoites via interaction with GPI-anchored proteins such as PfMSP1, which exhibits two EGF-like domains [79]. Surface-associated proteins include the MSP3/6 group, the MSP7 family as well as Pf41, a member of the cysteine-rich motif superfamily (reviewed in [76]). This adhesive MSP-based multi-protein complex, which consists of multiple secreted proteins assembling around a GPI-anchored EGF-domain protein, shows superficial similarities with the here discussed cell surface-associated PfCCp multi-protein complex.
During invasion, a focal adhesion to the host cell, referred to as tight junction or moving junction, is established in order to enable the movement of the apicomplexan parasite toward the interior of the host cell [80]. In T. gondii, this attachment is mediated by a protein complex comprised of the highly conserved micronemal apical membrane antigen 1 (AMA1) [81], which is inserted into the parasite plasma membrane, and of the four rhoptry neck proteins RON2/4/5/8 [82, 83]. Recent data led to the provocative hypothesis that the receptor for TgAMA1 located at the host cell surface is provided by TgRON2, which upon secretion becomes inserted into the host cell plasma membrane, whereas TgRON4, TgRON5, and TgRON8 locate to the cytosolic face of the host cell [83, 84]. Although the intracellular motor complex had been proposed to associate with the site of the tight junction, direct interaction partners have not been identified [85]. An additional role of the moving junction complex might be the exclusion of certain host cell plasma membrane proteins from the newly formed PV [86, 87]. AMA1 and RON orthologs are also present in other apicomplexan parasites [77, 84, VII]. In P. falciparum, the PfRON2, PfRON4, PfRON5, and PfAMA1 have been proposed to form a multimeric complex [88, 89]. PfAMA1 is translocated to the merozoite surface before invasion (reviewed in [76]). A recent study suggests a role after reorientation and establishment of the tight junction, which succeed the initial weak contact between merozoite and red blood cell (RBC). Therefore, PfAMA1 might be involved in the secretion of rhoptry contents which presumably form the nascent PV [89].
Another rhoptry-derived protein complex crucial for parasite survival is the Rhop complex of P. falciparum, which features two subgroups of protein associations, referred to as PfRhopL and PfRhopH complexes (low and high molecular weight rhoptry protein complex, respectively, [90]). PfRhopH complex is formed by the non-covalent-associated polypeptides PfRhopH1 (155 kDa), PfRhopH2 (140 kDa), and PfRhopH3 (110 kDa) [91, 92]. PfRhopH1 is encoded by one gene of the rhoph1/clag gene family (named after the cytoadherence-linked asexual gene, clag9, [93]) comprised of five polymorphic paralogous genes, while PfRhopH2 and PfRhopH3 are encoded by a single gene [94, 95]. The binding of the PfRhopH complex to the erythrocyte membrane is essential for merozoite invasion as in vitro studies showed, employing inhibitory antibodies raised against PfRhopH3 as well as PfRhopH3 peptides masking the putative receptor at the RBC surface [90, 96, 97]. In addition to cytoadherence, the PfRhopH complex is also proposed to participate in PV formation (reviewed in [98]). The low molecular weight PfRhopL complex comprises rhoptry-associated proteins such as PfRAP1 (83 kDa), PfRAP2 (40 kDa), and PfRAP3 (37 kDa) [99], and likewise PfRhopH complex binds to the erythrocyte cell surface [100]. Both PfRhopH and PfRhopL complexes bind non-covalently to the rhoptry-associated membrane antigen (RAMA), which is anchored to the inner face of the rhoptry membrane via a GPI tail, thereby mediating the retention of the protein complexes prior to rhoptry discharge [90, 101]. Both RhopH and RhopL complex constituents have been shown to possess homologs in several Plasmodium species [102–104], but not in other apicomplexan parasites [88]. However, RhopH1/Clag exhibits a region homologous to RON2, suggesting a common function of this region within the respective protein complex [88].
A prominent multimeric protein association modifying a host cell membrane is responsible for the formation of knob-like protuberances of the erythrocyte membrane after infection of malaria parasites (reviewed in [105, 106]). Knob formation in erythrocytes is limited to infections by P. falciparum and underpins virulence mechanisms causing the symptoms of severe malaria. Erythrocyte knobs mediate cytoadherence of these cells to a variety of host cell receptors, among them CD36 and ICAM-1 of endothelial cells, leading to clotting and capillary damage and subsequently resulting in organ failure (reviewed in [107]). Fundamental for knob formation is the knob-associated histidine-rich protein KAHRP, which is deposited at the erythrocyte plasma membrane facing the cytoplasm [108, 109]. KAHRP seems to assist the presentation of PfEMP1 (erythrocyte membrane protein 1) via binding to the PfEMP1 cytosolic region [110]. PfEMP1 is an antigenically highly diverse transmembrane protein comprised of multiple adhesion modules that enable the protein to bind to the different types of host cell receptors (reviewed in [111, 112]). KAHRP further interacts with actin and spectrin, thereby establishing a linkage of the knobs with the erythrocyte cytoskeleton [113, 114]. Components of the erythrocyte cytoskeleton in turn interact with further parasite-derived proteins such as PfEMP3, mature-parasite-infected erythrocyte surface antigen (MESA), and Pf322, which presumably alter the deformability of the cytoskeleton (reviewed in [106]).
Multimeric complexes of sexual stage parasites: which role do they play?
Initially phenotypic studies on PfCCp-KO lines were performed in order to reveal protein function. It was anticipated that a knock-out phenotype would manifest at the gametocyte stages, thus mirroring the stage specificity of PfCCp transcript and protein expression. Surprisingly, despite the loss of all PfCCp proteins in the respective PfCCp-KO lines, all lines exflagellated upon gametocyte activation and apparently normal ookinete and oocyst formation was observed [14, 20, 21] (A. Kuehn, G. Pradel, unpublished observations). In PfCCp2- and PfCCp3-KO mutants, however, no sporozoites were detected in the salivary glands. PfCCp4-KO parasites, on the other hand, did not reveal any differences in the mosquito-specific development compared to wild-type parasite [20], and no phenotype analyses on mosquito-specific parasites stages have so far been performed for PfCCp1-KO and PfFNPA-KO. Hence, PfCCp2-KO and PfCCp3-KO parasites appeared to be blocked in the transition of midgut sporozoites to the salivary glands [14], and thus, the respective knock-out phenotype for these mutants manifested in the blockage of sporogonic mosquito-specific parasite stages, in which PfCCp proteins were not present.
Similar phenotypes were observed, when the orthologous genes were knocked out in P. berghei. Loss of function mutants were generated for PbCCp1/LAP2, PbCCp2/LAP4, PbCCp3/LAP1, and PbCCp4/LAP6 [35] as well as for two double knock-out mutants, PbCCp1/CCp3 and PbCCp1/CCp4 [36]. For all mutants, the formation of sporozoites in the midgut oocysts of parasite-infected mosquitoes was aborted [35, 36]. Interestingly, sporulation of PbCCp3/LAP1 (termed PbSR in this study) appeared to be normal in in vitro assays, pointing to the involvement of mosquito factors in the loss-of-function phenotype of this protein [38].
Up to date, there is no explanation for the obvious difference in the stage of expression and stage of knock-out phenotype for the PfCCp proteins. It might represent a delayed phenotypic effect, in which reduced fertilization events result in a lower number of ookinetes and subsequently oocysts, in turn leading to a diminishing number of salivary gland sporozoites. An alternative explanation is offered by the observation that select PbCCp/LAP proteins of P. berghei associate with the crystalloid, a cytoplasmic aggregation in the developing ookinete [37–39, 115], which has been suggested to serve as storage for protein required during oocyst maturation [116, 117]. PbCCp3/LAP1 (PbSR) seems to play a role in crystalloid formation leading to the assumption that the impaired sporulation of PbCCp-KO mutants results from the absence of crystalloids [38]. Notably in this context, both the LCCL domain protein family and the ookinete crystalloid are inventions specific to the apicomplexan clade.
In view of our current data and despite the observed delayed knock-out phenotypic effect, we presume that the PfCCp functions lie in mediating molecular interplay during sexual reproduction. We hypothesize that the PfCCp proteins assemble to surface-associated multimeric protein complexes via intermolecular binding between select adhesion motifs. The secreted PfCCp proteins, together with Pfs230, are kept to the gametocyte surface by interacting with the GPI-anchored Pfs48/45 and, after activation, with Pfs25 to the macrogamete surface (Fig. 1b). Such assembly of secreted adhesion proteins around a GPI-anchored EGF-domain protein would be analog to the MSP-based multimeric complexes on the surface of merozoites.
The PfCCp complexes might be involved in important adhesive processes of macrogametes during sexual reproduction and transmission. On the one hand, the complexes might play a role in promoting contact between the emerging gametes within the blood meal. The physical contact between mating gametes is a requirement to initiate fertilization but can represent a major challenge for midgut parasites under natural conditions due to the low number of gametocytes taken up by the mosquito [118]. For example, the complexes might support the binding of macrogametes to microgametes due to interaction of select PfCCp proteins with Pfs230 or Pfs48/45 on the microgamete surface. In this context, recent studies described the identification of the microgamete protein GCS1/HAP2 in P. berghei that enables gamete fusion, but not initial binding between the two mating partners, which appears to involve other adhesion proteins [119, 120].
On the other hand, the complexes might mediate the protection of the exposed macrogametes from the aggressive environment of the mosquito midgut by forming a barrier between parasite and midgut content. During egress from the host erythrocyte, the emerging gametes become vulnerable to the factors of the mosquito midgut, resulting in an approximately 300-fold loss in parasite number between gametocytes and ookinetes [121]. Harmful factors of the midgut content include digestive enzymes, midgut bacteria as well as potential immune defense molecules of the mosquito. It was recently shown that a subset of immune genes become upregulated by the mosquito microbial flora, which then reduce the infection level of malaria parasites [122]. Of particular interest is an immune gene family of fibrinogen-related proteins (termed FREP), which is up-regulated after challenge with bacteria or malaria parasites. One family member, FBN 9, interacts with gut bacteria as well as with rodent and human malaria parasites in the mosquito midgut epithelium [123]. Further harmful components of the blood meal include human antibodies and complement proteins. Noteworthy, complement of the alternative pathway can be active for several hours post-feeding, as was shown in rodent and avian malaria models [124–126]. The predicted binding of selected sexual stage protein-specific adhesion domains with complement control protein modules might provide a key to identifying potential protection mechanisms of the sexual stage parasite.
Concluding remarks
Apicomplexan parasites exhibit a variety of life-cycle stage-dependent protein complexes, all of which are inventions specific to the clade. Proteins of these complexes either show lineage specificity, like the Rhop proteins of the malaria parasite erythrocytic stages, or possess adhesion motifs of wider conservation, as found in the TRAP and the CCp families, which were acquired by the pathogen via lateral gene transfer. The multi-protein complexes are utilized by the parasite for the different types of intercellular contact, ranging from attachment to and gliding on target cells to host cell invasion and cytoadherence of infected erythrocytes. While the majority of multimeric complexes are so far described for the infective parasite stages, a new type of complex involving the PfCCp protein family was recently identified in the sexual stages of the malaria parasite P. falciparum. The complex-forming proteins support the adhesiveness of macrogametes, but their detailed functions remain elusive. Follow-up studies will reveal if these complexes mediate intercellular contact, the way it was described for the complexes of other life-cycle stages, and which receptors they would use for these binding events. Another aspect worth investigating is the possible connection of these adhesion proteins to intracellular complexes by yet unknown linker proteins, thereby either participating in motility or in protein trafficking and/or re-cycling. Whichever role the complex-forming PfCCp proteins play for the malaria parasite sexual stages, the evolutionary conservation of the numerous adhesion modules and architectures of those proteins strongly argues that the proteins exhibit similar functions in other apicomplexan species.
References
Publications originating from the SFB479 funding period are marked with an asterisk (*)
Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415(6872):680–685. 10.1038/415680a
Kokwaro G (2009) Ongoing challenges in the management of malaria. Malar J 8(Suppl 1):S2. doi:10.1186/1475-2875-8-S1-S2
World Health Organisation (2009) World Malaria Report 2009, Geneva
*Pradel G (2007) Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology 134(Pt. 14):1911–1929. doi:10.1017/S0031182007003381
*Kuehn A, Pradel G (2010) The coming-out of malaria gametocytes. J Biomed Biotechnol 2010:976827. doi:10.1155/2010/976827
Carter R (2001) Transmission blocking malaria vaccines. Vaccine 19(17–19):2309–2314
Carter R, Mendis KN, Miller LH, Molineaux L, Saul A (2000) Malaria transmission-blocking vaccines—how can their development be supported? Nat Med 6(3):241–244. doi:10.1038/73062
Sauerwein RW (2007) Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect 9(6):792–795. doi:10.1016/j.micinf.2007.02.011
Carter R, Coulson A, Bhatti S, Taylor BJ, Elliott JF (1995) Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol Biochem Parasitol 71(2):203–210
Templeton TJ, Kaslow DC (1999) Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol Biochem Parasitol 101(1–2):223–227
Gerloff DL, Creasey A, Maslau S, Carter R (2005) Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci USA 102(38):13598–13603. doi:10.1073/pnas.0502378102
Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, Stowers A, Miller LH, Saul A (2005) Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23(24):3131–3138. doi:10.1016/j.vaccine.2004.12.019
Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP (2008) Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3(7):e2636. doi:10.1371/journal.pone.0002636
Pradel G, Hayton K, Aravind L, Iyer LM, Abrahamsen MS, Bonawitz A, Mejia C, Templeton TJ (2004) A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J Exp Med 199(11):1533–1544
Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P (1998) The discoidin domain family revisited: new members from prokaryotes and a homology-based fold prediction. Protein Sci 7(7):1626–1631. doi:10.1002/pro.5560070717
Ponting CP, Russell RB (2000) Identification of distant homologues of fibroblast growth factors suggests a common ancestor for all beta-trefoil proteins. J Mol Biol 302(5):1041–1047. doi:10.1006/jmbi.2000.4087
Trexler M, Banyai L, Patthy L (2000) The LCCL module. Eur J Biochem 267(18):5751–5757
Missler M, Fernandez-Chacon R, Sudhof TC (1998) The making of neurexins. J Neurochem 71(4):1339–1347
Hawking F, Wilson ME, Gammage K (1971) Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg 65(5):549–559
Scholz SM, Simon N, Lavazec C, Dude MA, Templeton TJ, Pradel G (2008) PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int J Parasitol 38(3–4):327–340. doi:10.1016/j.ijpara.2007.08.009
Simon N, Scholz SM, Moreira CK, Templeton TJ, Kuehn A, Dude MA, Pradel G (2009) Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. J Biol Chem 284(21):14537–14546. doi:10.1074/jbc.M808472200
Pradel G, Wagner C, Mejia C, Templeton TJ (2006) Plasmodium falciparum: co-dependent expression and co-localization of the PfCCp multi-adhesion domain proteins. Exp Parasitol 112(4):263–268. doi:10.1016/j.exppara.2005.11.010
Kumar N (1987) Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol 9(3):321–335
Kumar N, Wizel B (1992) Further characterization of interactions between gamete surface antigens of Plasmodium falciparum. Mol Biochem Parasitol 53(1–2):113–120
Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC (2006) Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 61(4):991–998. doi:10.1111/j.1365-2958.2006.05284.x
Paton MG, Barker GC, Matsuoka H, Ramesar J, Janse CJ, Waters AP, Sinden RE (1993) Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biochem Parasitol 59(2):263–275
Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP (2006) Regulation of sexual development of Plasmodium by translational repression. Science 313(5787):667–669. doi:10.1126/science.1125129
Templeton TJ, Iyer LM, Anantharaman V, Enomoto S, Abrahante JE, Subramanian GM, Hoffman SL, Abrahamsen MS, Aravind L (2004) Comparative analysis of apicomplexa and genomic diversity in eukaryotes. Genome Res 14(9):1686–1695
Pradel G, Templeton TJ (2006) Genomics of pathogenic parasites. In: Dobrindt U, Hacker JH (eds) Pathogenomics: genome analysis of pathogenic microbes. WILEY-VCH, Weinheim, pp 417–444
Templeton TJ (2007) Whole-genome natural histories of apicomplexan surface proteins. Trends Parasitol 23(5):205–212. doi:10.1016/j.pt.2007.03.001
Templeton TJ, Enomoto S, Chen WJ, Huang CG, Lancto CA, Abrahamsen MS, Zhu G (2010) A genome-sequence survey for Ascogregarina taiwanensis supports evolutionary affiliation but metabolic diversity between a Gregarine and Cryptosporidium. Mol Biol Evol 27(2):235–248. doi:10.1093/molbev/msp226
Snelling WJ, Lin Q, Moore JE, Millar BC, Tosini F, Pozio E, Dooley JS, Lowery CJ (2007) Proteomics analysis and protein expression during sporozoite excystation of Cryptosporidium parvum (Coccidia, Apicomplexa). Mol Cell Proteomics 6(2):346–355. doi:10.1074/mcp.M600372-MCP200
Tosini F, Agnoli A, Mele R, Gomez Morales MA, Pozio E (2004) A new modular protein of Cryptosporidium parvum, with ricin B and LCCL domains, expressed in the sporozoite invasive stage. Mol Biochem Parasitol 134(1):137–147
Trueman HE, Raine JD, Florens L, Dessens JT, Mendoza J, Johnson J, Waller CC, Delrieu I, Holders AA, Langhorne J, Carucci DJ, Yates JR 3rd, Sinden RE (2004) Functional characterization of an LCCL-lectin domain containing protein family in Plasmodium berghei. J Parasitol 90(5):1062–1071
Raine JD, Ecker A, Mendoza J, Tewari R, Stanway RR, Sinden RE (2007) Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog 3(3):e30. doi:10.1371/journal.ppat.0030030
Lavazec C, Moreira CK, Mair GR, Waters AP, Janse CJ, Templeton TJ (2009) Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol Biochem Parasitol 163(1):1–7. doi:10.1016/j.molbiopara.2008.09.002
Garnham PC, Bird RG, Baker JR, Desser SS, el-Nahal HM (1969) Electron microscope studies on motile stages of malaria parasites. VI. The ookinete of Plasmodium berghei yoelii and its transformation into the early oocyst. Trans R Soc Trop Med Hyg 63(2):187–194
Carter V, Shimizu S, Arai M, Dessens JT (2008) PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids. Mol Microbiol 68(6):1560–1569. doi:10.1111/j.1365-2958.2008.06254.x
Saeed S, Carter V, Tremp AZ, Dessens JT (2010) Plasmodium berghei crystalloids contain multiple LCCL proteins. Mol Biochem Parasitol 170(1):49–53. doi:10.1016/j.molbiopara.2009.11.008
Kane WH, Davie EW (1988) Blood coagulation factors V and VIII: structural and functional similarities and their relationship to hemorrhagic and thrombotic disorders. Blood 71(3):539–555
Srinivasan N, White HE, Emsley J, Wood SP, Pepys MB, Blundell TL (1994) Comparative analyses of pentraxins: implications for protomer assembly and ligand binding. Structure 2(11):1017–1027
Gewurz H, Zhang XH, Lint TF (1995) Structure and function of the pentraxins. Curr Opin Immunol 7(1):54–64
Romero IR, Morris C, Rodriguez M, Du Clos TW, Mold C (1998) Inflammatory potential of C-reactive protein complexes compared to immune complexes. Clin Immunol Immunopathol 87(2):155–162
Dormitzer PR, Sun ZY, Wagner G, Harrison SC (2002) The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J 21(5):885–897. doi:10.1093/emboj/21.5.885
Finn RD, Marshall M, Bateman A (2005) iPfam: visualization of protein-protein interactions in PDB at domain and amino acid resolutions. Bioinformatics 21(3):410–412
Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L, Mantovani A (2004) PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131(7):1577–1586
Bateman A, Sandford R (1999) The PLAT domain: a new piece in the PKD1 puzzle. Curr Biol 9(16):R588–R590
Dean DC, Bowlus CL, Bourgeois S (1987) Cloning and analysis of the promotor region of the human fibronectin gene. Proc Natl Acad Sci USA 84(7):1876–1880
Rutenber E, Ready M, Robertus JD (1987) Structure and evolution of ricin B chain. Nature 326(6113):624–626. doi:10.1038/326624a0
Rigden DJ, Mello LV, Galperin MY (2004) The PA14 domain, a conserved all-beta domain in bacterial toxins, enzymes, adhesins and signaling molecules. Trends Biochem Sci 29(7):335–339
Resnick D, Pearson A, Krieger M (1994) The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci 19(1):5–8
Hohenester E, Sasaki T, Timpl R (1999) Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nat Struct Biol 6(3):228–232. doi:10.1038/6669
Doolittle RF (1992) A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci 1(12):1563–1577. doi:10.1002/pro.5560011204
Spraggon G, Everse SJ, Doolittle RF (1997) Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature 389(6650):455–462. doi:10.1038/38947
Bork P (1992) The modular architecture of vertebrate collagens. FEBS Lett 307(1):49–54
Podolnikova NP, Yakubenko VP, Volkov GL, Plow EF, Ugarova TP (2003) Identification of a novel binding site for platelet integrins alpha IIb beta 3 (GPIIbIIIa) and alpha 5 beta 1 in the gamma C-domain of fibrinogen. J Biol Chem 278(34):32251–32258
Lu J, Le Y (1998) Ficolins and the fibrinogen-like domain. Immunobiology 199(2):190–199
Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T (2001) Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem 276(26):23456–23463
Bergman LW, Kaiser K, Fujioka H, Coppens I, Daly TM, Fox S, Matuschewski K, Nussenzweig V, Kappe SH (2003) Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J Cell Sci 116(Pt 1):39–49
Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF (2006) A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem 281(8):5197–5208. doi:10.1074/jbc.M509807200
Bosch J, Buscaglia CA, Krumm B, Ingason BP, Lucas R, Roach C, Cardozo T, Nussenzweig V, Hol WG (2007) Aldolase provides an unusual binding site for thrombospondin-related anonymous protein in the invasion machinery of the malaria parasite. Proc Natl Acad Sci USA 104(17):7015–7020. doi:10.1073/pnas.0605301104
Heiss K, Nie H, Kumar S, Daly TM, Bergman LW, Matuschewski K (2008) Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test. Eukaryot Cell 7(6):1062–1070. doi:10.1128/EC.00089-08
Bullen HE, Tonkin CJ, O’Donnell RA, Tham WH, Papenfuss AT, Gould S, Cowman AF, Crabb BS, Gilson PR (2009) A novel family of Apicomplexan glideosome-associated proteins with an inner membrane-anchoring role. J Biol Chem 284(37):25353–25363. doi:10.1074/jbc.M109.036772
Keeley A, Soldati D (2004) The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol 14(10):528–532
Baum J, Gilberger TW, Frischknecht F, Meissner M (2008) Host-cell invasion by malaria parasites: insights from Plasmodium and Toxoplasma. Trends Parasitol 24(12):557–563. doi:10.1016/j.pt.2008.08.006
Robson KJ, Frevert U, Reckmann I, Cowan G, Beier J, Scragg IG, Takehara K, Bishop DH, Pradel G, Sinden R et al (1995) Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. EMBO J 14(16):3883–3894
Moreira CK, Templeton TJ, Lavazec C, Hayward RE, Hobbs CV, Kroeze H, Janse CJ, Waters AP, Sinnis P, Coppi A (2008) The Plasmodium TRAP/MIC2 family member, TRAP-Like Protein (TLP), is involved in tissue traversal by sporozoites. Cell Microbiol 10(7):1505–1516. doi:10.1111/j.1462-5822.2008.01143.x
Combe A, Moreira C, Ackerman S, Thiberge S, Templeton TJ, Menard R (2009) TREP, a novel protein necessary for gliding motility of the malaria sporozoite. Int J Parasitol 39(4):489–496. doi:10.1016/j.ijpara.2008.10.004
Templeton TJ, Kaslow DC, Fidock DA (2000) Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol Microbiol 36(1):1–9
Green JL, Martin SR, Fielden J, Ksagoni A, Grainger M, Yim Lim BY, Molloy JE, Holder AA (2006) The MTIP-myosin A complex in blood stage malaria parasites. J Mol Biol 355(5):933–941. doi:10.1016/j.jmb.2005.11.027
Jewett TJ, Sibley LD (2003) Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol Cell 11(4):885–894
Jewett TJ, Sibley LD (2004) The toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J Biol Chem 279(10):9362–9369
Reiss M, Viebig N, Brecht S, Fourmaux MN, Soete M, Di Cristina M, Dubremetz JF, Soldati D (2001) Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. J Cell Biol 152(3):563–578
Meissner M, Reiss M, Viebig N, Carruthers VB, Toursel C, Tomavo S, Ajioka JW, Soldati D (2002) A family of transmembrane microneme proteins of Toxoplasma gondii contain EGF-like domains and function as escorters. J Cell Sci 115(Pt 3):563–574
Zheng B, He A, Gan M, Li Z, He H, Zhan X (2009) MIC6 associates with aldolase in host cell invasion by Toxoplasma gondii. Parasitol Res 105(2):441–445. doi:10.1007/s00436-009-1401-5
Cowman AF, Crabb BS (2006) Invasion of red blood cells by malaria parasites. Cell 124(4):755–766. doi:10.1016/j.cell.2006.02.006
Lal K, Bromley E, Oakes R, Prieto JH, Sanderson SJ, Kurian D, Hunt L, Yates JR 3rd, Wastling JM, Sinden RE, Tomley FM (2009) Proteomic comparison of four Eimeria tenella life-cycle stages: unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite. Proteomics 9(19):4566–4576. doi:10.1002/pmic.200900305
Periz J, Gill AC, Hunt L, Brown P, Tomley FM (2007) The microneme proteins EtMIC4 and EtMIC5 of Eimeria tenella form a novel, ultra-high molecular mass protein complex that binds target host cells. J Biol Chem 282(23):16891–16898. doi:10.1074/jbc.M702407200
Blackman MJ, Ling IT, Nicholls SC, Holder AA (1991) Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol 49(1):29–33
Aikawa M, Miller LH, Johnson J, Rabbege J (1978) Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 77(1):72–82
Donahue CG, Carruthers VB, Gilk SD, Ward GE (2000) The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol Biochem Parasitol 111(1):15–30
Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC (2005) Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog 1(2):e17. doi:10.1371/journal.ppat.0010017
Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M (2009) Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5(2):e1000309. doi:10.1371/journal.ppat.1000309
Straub KW, Cheng SJ, Sohn CS, Bradley PJ (2009) Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell Microbiol 11(4):590–603. doi:10.1111/j.1462-5822.2008.01276.x
Baum J, Tonkin CJ, Paul AS, Rug M, Smith BJ, Gould SB, Richard D, Pollard TD, Cowman AF (2008) A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe 3(3):188–198. doi:10.1016/j.chom.2008.02.006
Mordue DG, Desai N, Dustin M, Sibley LD (1999) Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med 190(12):1783–1792
Charron AJ, Sibley LD (2004) Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic 5(11):855–867. doi:10.1111/j.1600-0854.2004.00228.x
Cao J, Kaneko O, Thongkukiatkul A, Tachibana M, Otsuki H, Gao Q, Tsuboi T, Torii M (2009) Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol Int 58(1):29–35. doi:10.1016/j.parint.2008.09.005
Richard D, Macraild CA, Riglar DT, Chan JA, Foley M, Baum J, Ralph SA, Norton RS, Cowman AF (2010) Interaction between plasmodium falciparum apical membrane antigen 1 and the Rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem. doi:10.1074/jbc.M109.080770
Pinzon CG, Curtidor H, Reyes C, Mendez D, Patarroyo ME (2008) Identification of Plasmodium falciparum RhopH3 protein peptides that specifically bind to erythrocytes and inhibit merozoite invasion. Protein Sci 17(10):1719–1730. doi:10.1110/ps.035923.108
Cooper JA, Ingram LT, Bushell GR, Fardoulys CA, Stenzel D, Schofield L, Saul AJ (1988) The 140/130/105 kilodalton protein complex in the rhoptries of Plasmodium falciparum consists of discrete polypeptides. Mol Biochem Parasitol 29(2–3):251–260
Vincensini L, Fall G, Berry L, Blisnick T, Braun Breton C (2008) The RhopH complex is transferred to the host cell cytoplasm following red blood cell invasion by Plasmodium falciparum. Mol Biochem Parasitol 160(2):81–89. doi:10.1016/j.molbiopara.2008.04.002
Holt DC, Gardiner DL, Thomas EA, Mayo M, Bourke PF, Sutherland CJ, Carter R, Myers G, Kemp DJ, Trenholme KR (1999) The cytoadherence linked asexual gene family of Plasmodium falciparum: are there roles other than cytoadherence? Int J Parasitol 29(6):939–944
Kaneko O, Yim Lim BY, Iriko H, Ling IT, Otsuki H, Grainger M, Tsuboi T, Adams JH, Mattei D, Holder AA, Torii M (2005) Apical expression of three RhopH1/Clag proteins as components of the Plasmodium falciparum RhopH complex. Mol Biochem Parasitol 143(1):20–28. doi:10.1016/j.molbiopara.2005.05.003
Iriko H, Kaneko O, Otsuki H, Tsuboi T, Su XZ, Tanabe K, Torii M (2008) Diversity and evolution of the rhoph1/clag multigene family of Plasmodium falciparum. Mol Biochem Parasitol 158(1):11–21. doi:10.1016/j.molbiopara.2007.11.004
Campbell GH, Miller LH, Hudson D, Franco EL, Andrysiak PM (1984) Monoclonal antibody characterization of Plasmodium falciparum antigens. Am J Trop Med Hyg 33(6):1051–1054
Yang JC, Blanton RE, King CL, Fujioka H, Aikawa M, Sam-Yellowe TY (1996) Seroprevalence and specificity of human responses to the Plasmodium falciparum rhoptry protein Rhop-3 determined by using a C-terminal recombinant protein. Infect Immunol 64(9):3584–3591
Kaneko O (2007) Erythrocyte invasion: vocabulary and grammar of the Plasmodium rhoptry. Parasitol Int 56(4):255–262. doi:10.1016/j.parint.2007.05.003
Howard RF, Reese RT (1990) Plasmodium falciparum: hetero-oligomeric complexes of rhoptry polypeptides. Exp Parasitol 71(3):330–342
Sterkers Y, Scheidig C, da Rocha M, Lepolard C, Gysin J, Scherf A (2007) Members of the low-molecular-mass rhoptry protein complex of Plasmodium falciparum bind to the surface of normal erythrocytes. J Infect Dis 196(4):617–621. doi:10.1086/519685
Topolska AE, Black CG, Coppel RL (2004) Identification and characterisation of RAMA homologues in rodent, simian and human malaria species. Mol Biochem Parasitol 138(2):237–241. doi:10.1016/j.molbiopara.2004.05.018
Patarroyo MA, Perez-Leal O, Lopez Y, Cortes J, Rojas-Caraballo J, Gomez A, Moncada C, Rosas J, Patarroyo ME (2005) Identification and characterisation of the Plasmodium vivax rhoptry-associated protein 2. Biochem Biophys Res Commun 337(3):853–859. doi:10.1016/j.bbrc.2005.09.120
Perez-Leal O, Mongui A, Cortes J, Yepes G, Leiton J, Patarroyo MA (2006) The Plasmodium vivax rhoptry-associated protein 1. Biochem Biophys Res Commun 341(4):1053–1058. doi:10.1016/j.bbrc.2006.01.061
Mongui A, Perez-Leal O, Rojas-Caraballo J, Angel DI, Cortes J, Patarroyo MA (2007) Identifying and characterising the Plasmodium falciparum RhopH3 Plasmodium vivax homologue. Biochem Biophys Res Commun 358(3):861–866. doi:10.1016/j.bbrc.2007.05.015
Cooke B, Coppel R, Wahlgren M (2000) Falciparum malaria: sticking up, standing out and out-standing. Parasitol Today 16(10):416–420
Maier AG, Cooke BM, Cowman AF, Tilley L (2009) Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol 7(5):341–354. doi:10.1038/nrmicro2110
Sherman IW, Eda S, Winograd E (2003) Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect 5(10):897–909
Taylor DW, Parra M, Chapman GB, Stearns ME, Rener J, Aikawa M, Uni S, Aley SB, Panton LJ, Howard RJ (1987) Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol 25(2):165–174
Crabb BS, Cooke BM, Reeder JC, Waller RF, Caruana SR, Davern KM, Wickham ME, Brown GV, Coppel RL, Cowman AF (1997) Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89(2):287–296
Waller KL, Cooke BM, Nunomura W, Mohandas N, Coppel RL (1999) Mapping the binding domains involved in the interaction between the Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and the cytoadherence ligand P. falciparum erythrocyte membrane protein 1 (PfEMP1). J Biol Chem 274(34):23808–23813
Scherf A, Lopez-Rubio JJ, Riviere L (2008) Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 62:445–470. doi:10.1146/annurev.micro.61.080706.093134
Pasternak ND, Dzikowski R (2009) PfEMP1: an antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum. Int J Biochem Cell Biol 41(7):1463–1466. doi:10.1016/j.biocel.2008.12.012
Kilejian A, Rashid MA, Aikawa M, Aji T, Yang YF (1991) Selective association of a fragment of the knob protein with spectrin, actin and the red cell membrane. Mol Biochem Parasitol 44(2):175–181
Oh SS, Voigt S, Fisher D, Yi SJ, LeRoy PJ, Derick LH, Liu S, Chishti AH (2000) Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin-spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton. Mol Biochem Parasitol 108(2):237–247
Garnham PC, Bird RG, Baker JR (1962) Electron microscope studies of motile stages of malaria parasites. III. The ookinetes of Haemamoeba and Plasmodium. Trans R Soc Trop Med Hyg 56:116–120
Garnham P (1966) Malaria parasites and other haemosporidia. Blackwell, Oxford
Trefiak WD, Desser SS (1973) Crystalloid inclusions in species of leucocytozoon, parahaemoproteus, and plasmodium. J Protozool 20(1):73–80
Pichon G, Awono-Ambene HP, Robert V (2000) High heterogeneity in the number of Plasmodium falciparum gametocytes in the bloodmeal of mosquitoes fed on the same host. Parasitology 121(Pt 2):115–120
Hirai M, Arai M, Mori T, Miyagishima SY, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H (2008) Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr Biol 18(8):607–613. doi:10.1016/j.cub.2008.03.045
Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O (2008) The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22(8):1051–1068. doi:10.1101/gad.1656508
Vaughan JA, Noden BH, Beier JC (1994) Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. Am J Trop Med Hyg 51(2):233–243
Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5(5):e1000423. doi:10.1371/journal.ppat.1000423
Dong Y, Dimopoulos G (2009) Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem 284(15):9835–9844. doi:10.1074/jbc.M807084200
Grotendorst CA, Carter R, Rosenberg R, Koontz LC (1986) Complement effects on the infectivity of Plasmodium gallinaceum to Aedes aegypti mosquitoes. I. Resistance of zygotes to the alternative pathway of complement. J Immunol 136(11):4270–4274
Grotendorst CA, Carter R (1987) Complement effects of the infectivity of Plasmodium gallinaceum to Aedes aegypti mosquitoes. II. Changes in sensitivity to complement-like factors during zygote development. J Parasitol 73(5):980–984
Margos G, Navarette S, Butcher G, Davies A, Willers C, Sinden RE, Lachmann PJ (2001) Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect Immun 69(8):5064–5071. doi:10.1128/IAI.69.8.5064-5071.2001
Tomley FM, Soldati DS (2001) Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol 17(2):81–88
Pasamontes L, Hug D, Humbelin M, Weber G (1993) Sequence of a major Eimeria maxima antigen homologous to the Eimeria tenella microneme protein Etp100. Mol Biochem Parasitol 57(1):171–174
Sinnis P, Nussenzweig V (1996) Preventing sporozoite invasion of hepatocytes. In: Hoffman SL (ed) Malaria vaccine development: a multi-immune response approach. ASM, Washington, DC, pp 15–33
Sidjanski SP, Vanderberg JP, Sinnis P (1997) Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol 90(1):33–41
Wan KL, Carruthers VB, Sibley LD, Ajioka JW (1997) Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol Biochem Parasitol 84(2):203–214
Carruthers VB, Sibley LD (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73(2):114–123
Spano F, Putignani L, Naitza S, Puri C, Wright S, Crisanti A (1998) Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol Biochem Parasitol 92(1):147–162
Lovett JL, Howe DK, Sibley LD (2000) Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol Biochem Parasitol 107(1):33–43
Knapp B, Hundt E, Kupper HA (1990) Plasmodium falciparum aldolase: gene structure and localization. Mol Biochem Parasitol 40(1):1–12
Kim H, Certa U, Dobeli H, Jakob P, Hol WG (1998) Crystal structure of fructose-1, 6-bisphosphate aldolase from the human malaria parasite Plasmodium falciparum. Biochemistry 37(13):4388–4396
Buscaglia CA, Coppens I, Hol WG, Nussenzweig V (2003) Sites of interaction between aldolase and thrombospondin-related anonymous protein in plasmodium. Mol Biol Cell 14(12):4947–4957
Wetzel DM, Hakansson S, Hu K, Roos D, Sibley LD (2003) Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol Biol Cell 14(2):396–406. doi:10.1091/mbc.E02-08-0458
Kursula I, Kursula P, Ganter M, Panjikar S, Matuschewski K, Schuler H (2008) Structural basis for parasite-specific functions of the divergent profilin of Plasmodium falciparum. Structure 16(11):1638–1648. doi:10.1016/j.str.2008.09.008
Daher W, Soldati-Favre D (2009) Mechanisms controlling glideosome function in apicomplexans. Curr Opin Microbiol 12(4):408–414. doi:10.1016/j.mib.2009.06.008
Pinder JC, Fowler RE, Dluzewski AR, Bannister LH, Lavin FM, Mitchell GH, Wilson RJ, Gratzer WB (1998) Actomyosin motor in the merozoite of the malaria parasite, Plasmodium falciparum: implications for red cell invasion. J Cell Sci 111(Pt 13):1831–1839
Margos G, Siden-Kiamos I, Fowler RE, Gillman TR, Spaccapelo R, Lycett G, Vlachou D, Papagiannakis G, Eling WM, Mitchell GH, Louis C (2000) Myosin A expressions in sporogonic stages of Plasmodium. Mol Biochem Parasitol 111(2):465–469
Matuschewski K, Mota MM, Pinder JC, Nussenzweig V, Kappe SH (2001) Identification of the class XIV myosins Pb-MyoA and Py-MyoA and expression in Plasmodium sporozoites. Mol Biochem Parasitol 112(1):157–161
Meissner M, Schluter D, Soldati D (2002) Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298(5594):837–840
Heintzelman MB, Schwartzman JD (2001) Myosin diversity in Apicomplexa. J Parasitol 87(2):429–432
Gaskins E, Gilk S, DeVore N, Mann T, Ward G, Beckers C (2004) Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J Cell Biol 165(3):383–393
Holder AA, Blackman MJ, Burghaus PA, Chappel JA, Ling IT, McCallum-Deighton N, Shai S (1992) A malaria merozoite surface protein (MSP1)-structure, processing and function. Mem Inst Oswaldo Cruz 87(Suppl 3):37–42
Holder AA, Guevara Patino JA, Uthaipibull C, Syed SE, Ling IT, Scott-Finnigan T, Blackman MJ (1999) Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41(1–3):409–414
Oeuvray C, Bouharoun-Tayoun H, Grass-Masse H, Lepers JP, Ralamboranto L, Tartar A, Druilhe P (1994) A novel merozoite surface antigen of Plasmodium falciparum (MSP-3) identified by cellular-antibody cooperative mechanism antigenicity and biological activity of antibodies. Mem Inst Oswaldo Cruz 89(Suppl 2):77–80
McColl DJ, Silva A, Foley M, Kun JF, Favaloro JM, Thompson JK, Marshall VM, Coppel RL, Kemp DJ, Anders RF (1994) Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol Biochem Parasitol 68(1):53–67
Trucco C, Fernandez-Reyes D, Howell S, Stafford WH, Scott-Finnigan TJ, Grainger M, Ogun SA, Taylor WR, Holder AA (2001) The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasitol 112(1):91–101
Pachebat JA, Ling IT, Grainger M, Trucco C, Howell S, Fernandez-Reyes D, Gunaratne R, Holder AA (2001) The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol Biochem Parasitol 117(1):83–89
Sanders PR, Gilson PR, Cantin GT, Greenbaum DC, Nebl T, Carucci DJ, McConville MJ, Schofield L, Hodder AN, Yates JR 3rd, Crabb BS (2005) Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem 280(48):40169–40176. doi:10.1074/jbc.M509631200
Mitchell GH, Thomas AW, Margos G, Dluzewski AR, Bannister LH (2004) Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immunol 72(1):154–158
Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, Rubinstein E, Hannoun L, Charoenvit Y, Kocken CH, Thomas AW, Van Gemert GJ, Sauerwein RW, Blackman MJ, Anders RF, Pluschke G, Mazier D (2004) A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem 279(10):9490–9496
Zhang H, Compaore MK, Lee EG, Liao M, Zhang G, Sugimoto C, Fujisaki K, Nishikawa Y, Xuan X (2007) Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol Biochem Parasitol 151(2):205–212. doi:10.1016/j.molbiopara.2006.11.005
Kaneko O, Tsuboi T, Ling IT, Howell S, Shirano M, Tachibana M, Cao YM, Holder AA, Torii M (2001) The high molecular mass rhoptry protein, RhopH1, is encoded by members of the clag multigene family in Plasmodium falciparum and Plasmodium yoelii. Mol Biochem Parasitol 118(2):223–231
Sam-Yellowe TY, Perkins ME (1991) Interaction of the 140/130/110 kDa rhoptry protein complex of Plasmodium falciparum with the erythrocyte membrane and liposomes. Exp Parasitol 73(2):161–171
Ling IT, Kaneko O, Narum DL, Tsuboi T, Howell S, Taylor HM, Scott-Finnigan TJ, Torii M, Holder AA (2003) Characterisation of the rhoph2 gene of Plasmodium falciparum and Plasmodium yoelii. Mol Biochem Parasitol 127(1):47–57
Topolska AE, Lidgett A, Truman D, Fujioka H, Coppel RL (2004) Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J Biol Chem 279(6):4648–4656
Garzon-Ospina D, Romero-Murillo L, Patarroyo MA (2009) Limited genetic polymorphism of the Plasmodium vivax low molecular weight rhoptry protein complex in the Colombian population. Infect Genet Evol. doi:10.1016/j.meegid.2009.12.004
Baldi DL, Good R, Duraisingh MT, Crabb BS, Cowman AF (2002) Identification and disruption of the gene encoding the third member of the low-molecular-mass rhoptry complex in Plasmodium falciparum. Infect Immun 70(9):5236–5245
Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ (1995) Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82(1):77–87
Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH (1995) Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82(1):101–110
Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE (1995) The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82(1):89–100
Jeffares DC, Pain A, Berry A, Cox AV, Stalker J, Ingle CE, Thomas A, Quail MA, Siebenthall K, Uhlemann AC, Kyes S, Krishna S, Newbold C, Dermitzakis ET, Berriman M (2007) Genome variation and evolution of the malaria parasite Plasmodium falciparum. Nat Genet 39(1):120–125. doi:10.1038/ng1931
Magowan C, Coppel RL, Lau AO, Moronne MM, Tchernia G, Mohandas N (1995) Role of the Plasmodium falciparum mature-parasite-infected erythrocyte surface antigen (MESA/PfEMP-2) in malarial infection of erythrocytes. Blood 86(8):3196–3204
Pasloske BL, Baruch DI, van Schravendijk MR, Handunnetti SM, Aikawa M, Fujioka H, Taraschi TF, Gormley JA, Howard RJ (1993) Cloning and characterization of a Plasmodium falciparum gene encoding a novel high-molecular weight host membrane-associated protein, PfEMP3. Mol Biochem Parasitol 59(1):59–72
Waterkeyn JG, Wickham ME, Davern KM, Cooke BM, Coppel RL, Reeder JC, Culvenor JG, Waller RF, Cowman AF (2000) Targeted mutagenesis of Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) disrupts cytoadherence of malaria-infected red blood cells. EMBO J 19(12):2813–2823. doi:10.1093/emboj/19.12.2813
Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM (2002) Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 99(3):1060–1063
Pei X, Guo X, Coppel R, Mohandas N, An X (2007) Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) destabilizes erythrocyte membrane skeleton. J Biol Chem 282(37):26754–26758. doi:10.1074/jbc.M701612200
Bennett BJ, Mohandas N, Coppel RL (1997) Defining the minimal domain of the Plasmodium falciparum protein MESA involved in the interaction with the red cell membrane skeletal protein 4.1. J Biol Chem 272(24):15299–15306
Waller KL, Nunomura W, An X, Cooke BM, Mohandas N, Coppel RL (2003) Mature parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum binds to the 30-kDa domain of protein 4.1 in malaria-infected red blood cells. Blood 102(5):1911–1914
Mattei D, Scherf A (1992) The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene 110(1):71–79
Moll K, Chene A, Ribacke U, Kaneko O, Nilsson S, Winter G, Haeggstrom M, Pan W, Berzins K, Wahlgren M, Chen Q (2007) A novel DBL-domain of the P. falciparum 332 molecule possibly involved in erythrocyte adhesion. PLoS One 2(5):e477. doi:10.1371/journal.pone.0000477
Glenister FK, Fernandez KM, Kats LM, Hanssen E, Mohandas N, Coppel RL, Cooke BM (2009) Functional alteration of red blood cells by a megadalton protein of Plasmodium falciparum. Blood 113(4):919–928. doi:10.1182/blood-2008-05-157735
Hodder AN, Maier AG, Rug M, Brown M, Hommel M, Pantic I, Puig-de-Morales-Marinkovic M, Smith B, Triglia T, Beeson J, Cowman AF (2009) Analysis of structure and function of the giant protein Pf332 in Plasmodium falciparum. Mol Microbiol 71(1):48–65. doi:10.1111/j.1365-2958.2008.06508.x
Wagner C, Scholz SM, Abreu A, Frank R, Pradel G (2006) Molecular interactions between PfCCp multiadhesion proteins during gametogenesis in Plasmodium falciparum, 11th International Congress of Parasitology. ICOPA XI:631–635
van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W (2001) A central role for P48/45 in malaria parasite male gamete fertility. Cell 104(1):153–164
Vlachou D, Lycett G, Siden-Kiamos I, Blass C, Sinden RE, Louis C (2001) Anopheles gambiae laminin interacts with the P25 surface protein of Plasmodium berghei ookinetes. Mol Biochem Parasitol 112(2):229–237
Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, Sinha R, Lupetti P, Beetsma AL, Rodriguez MC, Karras M, Hager A, Mendoza J, Butcher GA, Kafatos F, Janse CJ, Waters AP, Sinden RE (2001) P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J 20(15):3975–3983. doi:10.1093/emboj/20.15.3975
Rodriguez Mdel C, Martinez-Barnetche J, Alvarado-Delgado A, Batista C, Argotte-Ramos RS, Hernandez-Martinez S, Gonzalez Ceron L, Torres JA, Margos G, Rodriguez MH (2007) The surface protein Pvs25 of Plasmodium vivax ookinetes interacts with calreticulin on the midgut apical surface of the malaria vector Anopheles albimanus. Mol Biochem Parasitol 153(2):167–177. doi:10.1016/j.molbiopara.2007.03.002
PlasmoDB, http://plasmodb.org/plasmo/
CDART: Conserved Domain Architecture Retrieval Tool, http://www.ncbi.nlm.nih.gov/Structure/lexington/lexington.cgi
Domine: Database of Protein Domain Interactions, http://domine.utdallas.edu/cgi-bin/Domine?page=pfam&pfamid=PF01477
Domine: Database of Protein Domain Interactions, http://domine.utdallas.edu/cgi-bin/Domine?page=pfam&pfamid=PF00040
Domine: Database of Protein Domain Interactions, (http://domine.utdallas.edu/cgi-bin/Domine?page=pfam&pfamid=PF00652
Domine: Database of Protein Domain Interactions, http://domine.utdallas.edu/cgi-bin/Domine?page=pfam&pfamid=PF00147
OrthoMCL Database, http://www.orthomcl.org/cgi-bin/OrthoMclWeb.cgi
Acknowledgments
We thank Tom Templeton for critical review of the manuscript and Claire Becker, Sarah Bonnet, and Fabio Tosini for helpful discussions. This work was funded by the Sonderforschungsbereich (Collaborative Research Centre) 479 and an Emmy-Noether grant of the Deutsche Forschungsgemeinschaft (to GP). AK is associated member of the BioMedTec International Graduate School of Science “Lead structures of cell function” of the Elite Network Bavaria. NS receives a fellowship from the program for female scientists of the University of Würzburg.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a Special Issue on Pathogen Variation and Host Response in Infectious Disease.
Rights and permissions
About this article
Cite this article
Kuehn, A., Simon, N. & Pradel, G. Family members stick together: multi-protein complexes of malaria parasites. Med Microbiol Immunol 199, 209–226 (2010). https://doi.org/10.1007/s00430-010-0157-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-010-0157-y