Abstract
Domain III of Japanese encephalitis virus (JEV) envelope protein (E-DIII) was synthesized in E. coli as a fusion protein containing maltose-binding protein (MBP-E-DIII) or six contiguous histidine residues (His-E-DIII) at its N-terminus. MBP-E-DIII was found both in the soluble as well as the insoluble fraction of the bacterial lysate, while His-E-DIII was found exclusively in the inclusion bodies. These purified proteins were examined in mice for their immunogenicity in presence of an aluminium hydroxide based-adjuvant Alhydrogel and Freund’s adjuvant. While both proteins generated anti-JEV antibodies that neutralized JEV activity in vitro, His-E-DIII generated higher antibody titers than MBP-E-DIII. Mice immunized with His-E-DIII in presence of Alhydrogel generated antibody titers similar to those induced by the commercial vaccine and protected mice against lethal JEV challenge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese encephalitis (JE), the most important cause of epidemic encephalitis is caused by Japanese encephalitis virus (JEV), an arbovirus belonging to the family Flaviviridae that includes several medically important pathogens such as yellow fever, dengue and West Nile encephalitis viruses. JE is a zoonotic disease of public health importance, largely because of its epidemic potential and high case fatality rate. The disease is endemic in the Indian subcontinent, virtually all of Southeast Asia, China and parts of Oceania. It has been estimated that approximately 35,000–50,000 cases of JE occur annually, with 10,000–15,000 cases proving to be fatal [22]. The principal clinical manifestation of illness is encephalitis. The disease is characterized by seizures, poliomyelitis-like paralysis and Parkinsonian movement disorders. A large proportion of survivors develop neuropsychiatric sequelae that include impaired cognition, behavioral disturbances and convulsions [4]. There is no specific treatment for JE and hence vaccination of susceptible populations is the sole logical alternative. The only World Health Organization (WHO)-approved vaccine that is available, namely the mouse brain-derived formalin-inactivated JEV vaccine, is inherent with certain drawbacks. Besides being expensive and in short supply, it causes allergic reactions in some recipients [11]. Moreover, the immunity conferred by the vaccine is of short-term duration [12]. A formalin-inactivated, primary hamster kidney cell cultured vaccine based on the P3 strain of JEV is in use in China since the late 1960s. Besides, a live, attenuated vaccine using the SA14-14-2 strain of JEV is in use in China. However, both P3 and SA14-14-2 vaccines are not yet produced as per the internationally acceptable quality norms. Hence, there is an urgent need to develop alternate vaccine candidates that are effective, safe as well as affordable [1].
The single-stranded, positive-sense RNA genome of JEV is translated into a polyprotein of approximately 3,400 amino acids that is subsequently cleaved into three structural [capsid (C), membrane (M) and envelope (E)] and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) proteins [20]. The E protein (53–55 kDa) is a typical membrane glycoprotein, forming the outer structural protein component of the virus, and is responsible for a number of important processes such as viral attachment, fusion, penetration, cell tropism, virulence and attenuation [9]. The 500-amino acid E protein is also the major antigen responsible for eliciting neutralizing antibodies that confer protection to the host [6]. Structurally, the E protein exists as a flat, elongated dimer on the surface of the virion. The E protein consists of three domains: (a) domain I, consisting of a β-barrel, (b) domain II, consisting of an elongated dimerization region, and (c) domain III, located at the C-terminus and resembling an immunoglobulin constant domain [7, 14]. The JEV envelope domain III (E-DIII) possesses an integrin-binding motif characterized by the Arg-Gly-Asp (RGD) consensus sequence, indicating that domain III might be involved in cell surface interactions, similar to other flaviviruses [3]. Neutralizing epitopes have been identified on the lateral surface of JEV E-DIII, including residues 333 [2], 373–399 [17], 306, 331, 387 [23], and 331, 333 [8]. Moreover, E-DIII can be independently folded as an individual fragment requiring a disulphide bridge to maintain its conformation. These features of E-DIII suggest that it could be employed as an immunogen for developing a JEV subunit vaccine. In the present study we have synthesized JEV E-DIII in E. coli and have evaluated its immunogenicity and protective efficacy in mice.
Materials and methods
Virus and cells
The JaOArS982 strain of JEV, grown in suckling mice brain, was cultured in porcine stable kidney (PS) cells and titrated by plaque assay [21]. The PS cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum.
Synthesis of JEV E-DIII in E. coli
Viral RNA was isolated from the culture supernatant of JEV-infected PS cells using the RNeasy kit (Qiagen, Germany). The cDNA to E-DIII-encoding RNA was made using a synthetic oligonulceotide SV286 of the sequence AAGC TT ATTA TCCAGCTTTGTGCCAATGGTGGTTGAT. The underlined nucleotides here represent the sequence complementary to JEV RNA; bold nucleotides represent the sequence complementary to the stop codons added to the cDNA and those in italics represent the BamHI restriction site inserted for the cloning purpose. The cDNA was PCR-amplified using SV286 and another synthetic oligonulceotide SV285 of the sequence GGATCC ATGTGTACAGAAAAATTCTCGTTC where the underlined section represents the JEV RNA sequence and the nucleotides in italics indicate HindIII restriction site inserted for cloning of cDNA. The 307-base pair PCR product was digested with BamHI and HindIII and cloned into the respective sites in bacterial expression vectors pET28a (Novagen, Germany) and pMALC2x (New England Biolabs Inc., UK). The E-DIII protein synthesized with pET28a and pMALC2x would have additional N-terminal 6-histidine residues or the maltose-binding protein (MBP), respectively.
For the synthesis of E-DIII, the above plasmids were transferred to E. coli BL-21 (DE3) cells. The transformed bacteria were grown in Luria broth at 37°C until A600 of 0.5 was reached. At this stage, the cultures were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 4 h. The MBP-tagged protein (MBP-E-DIII) was purified from the culture supernatant using the pMAL purification kit (New England Biolabs Inc., UK) while the histidine-tagged protein (His-E-DIII) was purified from inclusion bodies in the cell pellet and active protein was obtained by refolding using pulsed dilution method [19]. The concentration of the purified proteins was estimated by using the BCATM Protein Assay kit (Pierce, Rockford, IL, USA). The purified proteins were further characterized by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting with anti-JEV, anti-Histidine and anti-MBP antibodies.
Mice immunizations and challenge with JEV
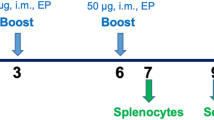
Groups (n = 8) of 4-week-old Balb/c mice were immunized intramuscular with 5.5 μg His-E-DIII or 25 μg MBP-E-DIII containing approximately 5 μg of JEV E-DIII peptide. The recombinant protein was administered with Freund’s adjuvant (Difco) or an aluminium hydroxide-based adjuvant Alhydrogel (Superphos Biosector, Denmark). Booster doses were given subsequently 3 and 5 weeks later with the same immunogen and the adjuvant. A control group of mice was immunized with 1/10th of recommended human dose of a mouse brain-derived JEV vaccine obtained from the Central Research Institute, Kasauli (India) and boosted as described above. Mice were challenged 6 weeks post-immunization with a highly lethal dose (100 LD50) of live JEV given intra-cerebrally (IC) as adult mice are refractory to peripheral JEV infection. Mice were bled a day prior to challenge and sera stored at −70°C.
Antibody assays and statistical analyses
End-point titers of anti-JEV antibodies were determined by an ELISA [6]. Briefly, 100 μl of JEV-infected C6/36 cell culture fluid (virus titer 7 × 107 PFU/ml) was coated in the wells of a microtiter plate as the antigen. The wells were blocked with 1% bovine serum albumin. Two-fold serial dilutions of serum samples, starting at 1:20, were then incubated in the wells. After washing the unbound antibody, the anti-JEV antibody was revealed with rabbit anti-mouse IgG conjugated to horseradish peroxidase. Orthophenylene diamide was used for the color development. The reciprocal of the highest serum dilution that gave an optical density at least twice of that given by the reagent blanks was considered to be the ELISA end-point. The titers of JEV neutralizing antibodies were determined by plaque reduction neutralization assay and the 50% neutralization titer (PRNT50) was determined using the Reed and Muench method [13]. The statistical significance of difference in antibody titers was established by deriving P-values by an unpaired Student’s t-test while protective efficacy of different proteins was compared using Fisher’s test. The p-value < 0.05 was considered statistically significant.
Results
Synthesis and purification of JEV E-DIII protein
The 98-amino acid E-DIII (amino acid 303–400 of JEV E protein) fused with the histidine tag (His-E-DIII) or MBP (MBP-E-DIII) was synthesized in E. coli transformed with the expression vectors. The predicted sizes of His-E-DIII and MBP-E-DIII are 15 and 53 kDa, respectively. SDS-PAGE of bacterial lysates showed over-expressed proteins of expected size in IPTG-induced E. coli cultures (Fig. 1). Fractionation of the bacterial lysate indicated that MBP-E-DIII was found both in the soluble as well as in the insoluble fraction (pellet), whereas His-E-DIII was present exclusively in the pellet. MBP-E-DIII, purified from the soluble fraction, and His-E-DIII, purified from the inclusion bodies, had apparent molecular sizes of 53 and 15 kDa and both these proteins could be blotted with anti-JEV antibodies. The presence of histidine tag or MBP in the fusion proteins was established by western blotting the proteins with anti-Histidine or anti-MBP antibodies, respectively (data not shown).
Synthesis of recombinant JEV E-DIII protein in E. coli. [A] E. coli transformed with the expression plasmids were induced with IPTG for the synthesis of the recombinant proteins. Four hour later, bacterial cells were harvested from 1 ml culture and lysates prepared that were separated into soluble and insoluble (pellet) fractions. Recombinant proteins were purified as described in methods and separated on SDS-PAGE. Shown above is coomassie-stained gel. Lane 0 protein size markers (indicated at the left in kDa); lane 1 untransformed E. coli lysate; lane 2 lysate containing His-E-DIII; lane 3 lysate containing MBP-E-DIII; lane 4 insoluble fraction of E. coli synthesizing His-E-DIII; lane 5 insoluble fraction of E. coli synthesizing MBP-E-DIII; lane 6, soluble fraction of E. coli synthesizing His-E-DIII; lane 7 soluble fraction of E. coli synthesizing MBP-E-DIII; lane 8 purified His-E-DIII (500 ng); lane 9 purified MBP-E-DIII (500 ng). [B] Purified His-E-DIII (lane 1) and MBP-E-DIII (lane 2) western blotted with anti-JEV antibodies
Anti-JEV antibody titers
In order to determine the immunogenicity of the purified fusion proteins, mice were immunized as described in the methods. Serum samples from individual mice obtained after the second booster were assayed for JEV antibody titers by ELISA (Fig. 2). All mice made anti-JEV antibodies following immunization with the recombinant proteins administered with Freund’s adjuvant (FA) or Alhydrogel (AG). Similar titers were induced in mice immunized with His-E-DIII with FA or AG. Similarly, difference was statistically not significant (P = 0.16) between the anti-JEV antibody titer induced by MBP-E-DIII with FA or AG. Geometric mean titers were higher in mice immunized with His-E-DIII protein than in those immunized with MBP-E-DIII. Thus in presence of FA, His-E-DIII induced titers that were around 7-folds higher than those induced by MBP-E-DIII (P = 0.038). In presence of AG, this difference was only about 2-folds although it was not statistically significant (P = 0.28). Compared to the commercial vaccine, the anti-JEV antibody titers induced by His-E-DIII were slightly higher when administered with FA (P = 0.085) or AG (P = 0.06) although the difference was statistically not significant.
Immunogenicity of recombinant E-DIII protein of JEV in mice. Mice were immunized with JE vaccine, or His-E-DIII and MBP-E-DIII along with Freund’s adjuvant (FA) or Alhydrogel (AG). Sera samples collected 1 week after the second booster were assayed for anti-JEV antibody titers and JEV-neutralizing antibodies. The upper panel shows geometric mean titers and standard error of anti-JEV antibody ELISA end-point titers while the bottom panel shows geometric mean PRNT50 titers and standard errors of estimations of various immunization groups as indicated at the top
Serum samples from individual mice obtained after the second booster were assayed for JEV neutralization activity (Fig. 2). These assays also indicated that His-E-DIII was more immunogenic than MBP-E-DIII. Thus, compared to MBP-E-DIII, His-E-DIII induced 1.7-folds higher (P = 0.11) JEV-neutralizing titers when FA was used and 4.4-folds higher (P = 0.0005) titers when AG was used as the adjuvant. Compared to His-E-DIII given with AG, the commercial vaccine induced 1.5-folds higher JEV neutralizing titers although the difference was statistically not significant (P = 0.14).
Mice challenge studies
The mice, having undergone the full course of immunization, were challenged by IC inoculation of a highly lethal dose (100 LD50) of JEV and observed for a period of 4 weeks post-challenge for mortality (Table 1). All the unimmunized mice died of the challenge whereas 68.8% of vaccine-immunized mice survived. Mice immunized with His-E-DIII in presence of both AG and FA showed 78.6% protection. Mice immunized with MBP-E-DIII showed lower level of protection than those immunized with His-E-DIII. Thus mice immunized with MBP-E-DIII plus AG showed 73.3% protection while those immunized with MBP-E-DIII plus FA showed only 56.3% protection.
Discussion
In the present study, domain III of JEV E protein was synthesized in E. coli fused to six contiguous histidine residues or MBP as tags and the recombinant proteins were administered in mice along with adjuvants to evaluate their vaccine potential against JEV. Previously, investigators have expressed the E-DIII protein of JEV in E. coli using fusion partners such as trpE [10] and thioredoxin [8] proteins. In the present study, MBP was selected as a fusion partner for the E-DIII peptide, mainly because MBP is known to promote proper folding of the attached peptide into its biologically active conformation, thereby functioning as a molecular chaperone [5]. Indeed, the MBP-E-DIII fusion protein (53 kDa) was localized predominantly in the soluble fraction of the bacterial lysate. Western blot analysis using anti-MBP and JEV-specific polyclonal serum confirmed the presence of the fusion protein along with the domain III fragment. The protein purified from the soluble fraction induced neutralizing antibodies that conferred protection in mice against a lethal IC challenge with live JEV. These findings are in accordance with a previous study where E. coli-synthesized, MBP-fused dengue-2 virus envelope fragment (amino acids 298–400) generated virus-neutralizing antibodies [18]. In the present study, in order to exclude the possibility of the MBP portion of the fusion protein contributing towards the elicitation of the anti-JEV immune response, E-DIII was also expressed with a tag of 6 histidine residues. The E. coli-expressed His-E-DIII protein also generated JEV-neutralizing antibodies that protected mice against lethal JEV challenge, thus providing evidence that the domain III of JEV E protein harbors the virus neutralization site.
In comparison to MBP-E-DIII, His-E-DIII induced higher anti-JEV antibody titers and JEV neutralization titers. Importantly, AG was found to be an efficient adjuvant in our studies where both His-E-DIII and MBP-E-DIII generated JEV-neutralizing antibodies. This is in contrast to a report where amino acids 303–396 of JEV E protein fused to trpE protein of E. coli did not elicit any virus-neutralizing antibodies in mice [10]. However, present findings corroborate previous observations where amino acid 373–399 of JEV E protein representing a part of JEV E-DIII, expressed as fusions with the coat protein of Johnson Grass Mosaic Virus [15] or GST [16] elicited moderate titers of JEV-neutralizing antibodies.
Previously Wu et al. [24] expressed JEV envelope domain III fused to thioredoxin protein (Trx-E-DIII) in E. coli. They demonstrated that 100 μg Trx-E-DIII (containing ∼50 μg E-DIII peptide) given with FA generated a PRNT50 of ∼10 after two booster doses. These titers increased to 24 after another booster dose. When 10 μg Trx-E-DIII (containing ∼5 μg E-DIII peptide) was used with FA, PRNT50 was found to be 5. Since FA is not permitted for human use, potential of the liposome formulations was tested for immunization where a PRNT50 of 30 was obtained after three booster doses of 100 μg Trx-E-DIII. Compared to these, in the present study 5.5 μg His-E-DIII (containing ∼5 μg E-DIII peptide) induced a PRNT50 of 54 after two booster doses. Importantly, these titers were obtained using AG as the adjuvant which is permitted for human use.
Virus-neutralizing antibodies are considered important for protection against JEV infection and a neutralization titer of 10 is considered protective in humans [22]. The mean JEV-neutralization titer in His-E-DIII-immunized mice was 54. Although these titers were lower than those induced by the commercial vaccine, the difference was statistically not significant (P = 0.140). Importantly, these antibody titers were sufficient to protect mice against JEV infection indicating the potential of the recombinant His-E-DIII as a vaccine immunogen against JEV.
References
Bharati K, Vrati S (2006) Japanese encephalitis: development of new candidate vaccines. Expert Rev Anti Infect Ther 4:313–324
Cecilia D, Gould EA (1991) Nucleotide changes responsible for loss of neuroinvasiveness in Japanese encephalitis virus neutralization-resistant mutants. Virology 181:70–77
Crill WD, Roehrig JT (2001) Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 75:7769–7773
Halstead SB, Jacobson J (2003) Japanese encephalitis. Adv Virus Res 61:103–138
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8:1668–1674
Kaur R, Sachdeva G, Vrati S (2002) Plasmid DNA immunization against Japanese encephalitis virus: immunogenicity of membrane-anchored and secretory envelope protein. J Infect Dis 185:1–12
Kolaskar AS, Kulkarni-Kale U (1999) Prediction of three-dimensional structure and mapping of conformational epitopes of envelope glycoprotein of Japanese encephalitis virus. Virology 261:31–42
Lin CW, Wu SC (2003) A functional epitope determinant on domain III of the Japanese encephalitis virus envelope protein interacted with neutralizing-antibody combining sites. J Virol 77:2600–2606
Lindenbach BD, Rice CM (2001) Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM (eds) Fields virology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 991–1041
Mason PW, Dalrymple JM, Gentry MK, McCown JM, Hoke CH, Burke DS, Fournier MJ, Mason TL (1989) Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J Gen Virol 70(Pt 8):2037–2049
Plesner AM (2003) Allergic reactions to Japanese encephalitis vaccine. Immunol Allergy Clin North Am 23:665–697
Poland JD, Cropp CB, Craven RB, Monath TP (1990) Evaluation of the potency and safety of inactivated Japanese encephalitis vaccine in US inhabitants. J Infect Dis 161:878–882
Reed LJ, Muench H (1938) A simple method of estimating 50 per cent end-points. Am J Hyg 27:493–497
Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC (1995) The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291–298
Saini M, Vrati S (2003) A Japanese encephalitis virus peptide present on Johnson grass mosaic virus-like particles induces virus-neutralizing antibodies and protects mice against lethal challenge. J Virol 77:3487–3494
Seif SA, Morita K, Igarashi A (1996) A 27 amino acid coding region of JE virus E protein expressed in E. coli as fusion protein with glutathione-S-transferase elicit neutralizing antibody in mice. Virus Res 43:91–96
Seif SA, Morita K, Matsuo S, Hasebe F, Igarashi A (1995) Finer mapping of neutralizing epitope(s) on the C-terminal of Japanese encephalitis virus E-protein expressed in recombinant Escherichia coli system. Vaccine 13:1515–1521
Simmons M, Nelson WM, Wu SJ, Hayes CG (1998) Evaluation of the protective efficacy of a recombinant dengue envelope B domain fusion protein against dengue 2 virus infection in mice. Am J Trop Med Hyg 58:655–662
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99:303–310
Sumiyoshi H, Mori C, Fuke I, Morita K, Kuhara S, Kondou J, Kikuchi Y, Nagamatu H, Igarashi A (1987) Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology 161:497–510
Vrati S, Agarwal V, Malik P, Wani SA, Saini M (1999) Molecular characterization of an Indian isolate of Japanese encephalitis virus that shows an extended lag phase during growth. J Gen Virol 80:1665–1671
World Health Organization (1998) Japanese encephalitis vaccines. Wkly Epidemiol Rec 73:337–344
Wu SC, Lian WC, Hsu LC, Liau MY (1997) Japanese encephalitis virus antigenic variants with characteristic differences in neutralization resistance and mouse virulence. Virus Res 51:173–181
Wu SC, Yu CH, Lin CW, Chu IM (2003) The domain III fragment of Japanese encephalitis virus envelope protein: mouse immunogenicity and liposome adjuvanticity. Vaccine 21:2516–2522
Acknowledgments
This work was supported by the Department of Biotechnology, Government of India grant no. BT/PR2765/Med/14/329/2001 and the core grant of the National Institute of Immunology, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alka and Kaushik Bharati contributed equally to this work.
Rights and permissions
About this article
Cite this article
Alka, Bharati, K., Malik, Y.P.S. et al. Immunogenicity and protective efficacy of the E. coli-expressed domain III of Japanese encephalitis virus envelope protein in mice. Med Microbiol Immunol 196, 227–231 (2007). https://doi.org/10.1007/s00430-007-0043-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-007-0043-4