Abstract
Children born very preterm (VPT) at less than 32 weeks’ gestational age (GA) are prone to disrupted white matter maturation and impaired cognitive development. The aims of the present study were to identify differences in white matter microstructure and connectivity of children born VPT compared to term-born children, as well as relations between white matter measures with cognitive outcomes and early brain injury. Diffusion images and T1-weighted anatomical MR images were acquired along with developmental assessments in 31 VPT children (mean GA: 28.76 weeks) and 28 term-born children at 4 years of age. FSL’s tract-based spatial statistics was used to create a cohort-specific template and mean fractional anisotropy (FA) skeleton that was applied to each child’s DTI data. Whole brain deterministic tractography was performed and graph theoretical measures of connectivity were calculated based on the number of streamlines between cortical and subcortical nodes derived from the Desikan-Killiany atlas. Between-group analyses included FSL Randomise for voxel-wise statistics and permutation testing for connectivity analyses. Within-group analyses between FA values and graph measures with IQ, language and visual-motor scores as well as history of white matter injury (WMI) and germinal matrix/intraventricular haemorrhage (GMH/IVH) were performed. In the children born VPT, FA values within major white matter tracts were reduced compared to term-born children. Reduced measures of local strength, clustering coefficient, local and global efficiency were present in the children born VPT within nodes in the lateral frontal, middle and superior temporal, cingulate, precuneus and lateral occipital regions. Within-group analyses revealed associations in term-born children between FA, Verbal IQ, Performance IQ and Full scale IQ within regions of the superior longitudinal fasciculus, inferior fronto-occipital fasciculus, forceps minor and forceps major. No associations with outcome were found in the VPT group. Global efficiency was reduced in the children born VPT with a history of WMI and GMH/IVH. These findings are evidence for under-developed and less connected white matter in children born VPT, contributing to our understanding of white matter development within this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Premature birth affects cortical and cognitive development by perturbing the critical foundations of early neurodevelopment. Children born very preterm [VPT, < 32 weeks gestational age (GA)] are at risk for developing difficulties in aspects of cognitive function including intelligence, visual and perceptual abilities, language, attention, learning and memory, processing speed and executive function (Anderson 2014). By 4 years of age, almost half of those with one developmental impairment also have difficulties in multiple developmental domains (Woodward et al. 2009). Lower cognitive scores in children born preterm have been found to be generally stable beginning from 4 years of age through early adolescence, yet with greater intra- and inter-individual variability (Mangin et al. 2017). Additional behavioural problems such as hyperactivity/inattention, emotional symptoms and peer problems are also associated with cognitive impairments, which become apparent by 5 years of age (Delobel-Ayoub et al. 2009). As cognitive difficulties children born VPT experience are widespread, it is likely that there is a global pattern of cortical dysmaturation involving multiple brain regions.
Disturbances of grey matter development have been associated with the preterm brain. Beginning from infancy, those born VPT have reduced subcortical and cortical grey matter volumes (Ball et al. 2012; Boardman et al. 2006) as well as altered diffusion properties in grey matter compared to term-born infants (Ball et al. 2013). Explanations for these findings in grey matter have been attributed to dysmaturation of white matter. Animal models of the preterm brain have revealed delayed dendritic arborisation and synaptic formations of projection neurons, which undergo critical development during the preterm period (Dean et al. 2013; Mcclendon et al. 2014). In particular, the thalamocortical projections of sensory systems have an intricate relationship with preterm birth (Krsnik et al. 2017). Ingrowth of axons from the thalamus to the somatosensory, frontal and occipital regions are especially active during the time of VPT birth (23–34 post conception weeks) and are vulnerable to early brain injury such as hypoxia–ischaemia induced white matter injury (Krsnik et al. 2017).
Diffusion imaging facilitates the study of white matter microstructure on a voxel-wise level by modelling a diffusion tensor to measure water diffusion within the brain (Jones et al. 2013). Fractional anisotropy (FA) quantifies the degree of restricted water diffusion based on the relation between axial diffusivity (AD) and radial diffusivity (RD), which represent the extent of water mobility along the principle and perpendicular directions of the fibre axis, respectively (Jones et al. 2013). With increasing GA and ongoing development, FA is found to increase while RD and mean diffusivity (MD) decrease, due to contributing factors such as increasing myelination, improved coherence of fibres, increasing axonal density and reduced axonal caliber (Beaulieu 2002; Jones et al. 2013; Pannek et al. 2014).
In children born VPT, differences in diffusion properties compared to full-term children have been identified across infancy, late childhood and adolescence. At term-equivalent age, preterm infants exhibited reduced FA as well as elevated RD within frontal white matter regions (Anjari et al. 2007). Furthermore, voxel-wise whole brain analyses have identified measures of FA and RD in regions of the corpus callosum, internal and external capsule to be correlated with global developmental measures of cognitive and motor outcomes at 18 months and 2 years of age (Counsell et al. 2008; Duerden et al. 2015; van Kooij et al. 2012). In late childhood, reduced FA has also been reported in regions including the external capsule, superior longitudinal fasciculus, uncinate fasciculus and inferior fronto-occipital fasciculus (Duerden et al. 2013). Altered FA within these regions in addition to the corpus callosum and internal capsule persists into adolescence (Constable et al. 2008; Loe et al. 2013; Mullen et al. 2012; Travis et al. 2015; Vangberg et al. 2006).
Tractography methods and graph theoretical analyses using diffusion imaging have contributed to our understanding of topological characteristics of structural brain connectivity across development. The organization of grey matter regions (nodes) and their connections (edges) in white matter can be described through graph measures such as strength, how connected nodes are to adjacent nodes and global efficiency, how integrated and efficient networks are within the whole brain (Bullmore and Sporns 2012; Rubinov and Sporns 2010). Local efficiency and clustering coefficient describe segregation, based upon the path length and clustered connectivity between a node’s adjacent neighbours, respectively (Achard and Bullmore 2007). Increased integration and decreased segregation as well as changes towards a more organized network construction are hallmark features of typical brain development (Cao et al. 2016, 2017a, b). Accordingly, changes in network properties due to early brain maturation occur in conjunction to changes in whole brain FA (Huang et al. 2015; Yap et al. 2011).
Premature birth impacts the organization of structural connections beginning from birth. Infants imaged within the preterm period demonstrated associations between local connectivity and GA involving the thalamus, cerebellum, superior frontal and cingulate gyrus (Batalle et al. 2017). At preterm birth and childhood, greater GA was related to higher connection strength, clustering coefficient and global efficiency as well as lower local efficiency (Brown et al. 2014; Pandit et al. 2014; Thompson et al. 2016). With respect to cognitive outcomes, children born VPT at 7 years of age were found to have associations between impaired performance on intelligence testing with lower connection strength in nodes primarily within the right hemisphere (Thompson et al. 2016).
At present, there is a distinct gap in the literature of children born VPT during early childhood and before school-age. Delayed white matter maturation and impaired outcomes at 4 years of age provide important indications of children who may experience persistent cognitive difficulties as they enter school-age as well as those who begin learning at a disadvantage. The primary aim of the present study was to characterise altered maturation of white matter microstructure and connectivity in children born VPT compared to full-term children at 4 years of age. We employed a whole brain approach, utilizing tract-based spatial statistics (TBSS) to analyse DTI metrics and deterministic tractography to analyse graph theoretical measures of connectivity such as strength, clustering coefficient, local and global efficiency between groups. Furthermore, our secondary aim was to examine relations between whole brain white matter measures with developmental outcomes within each group separately. The impact of early brain injury on white matter maturation, such as those with a history of white matter injury (WMI) and germinal matrix/intraventricular haemorrhage (GMH/IVH) detected at birth, was tested in the children born VPT.
Methods
Participants
At 4 years of age, 53 participants born at < 32 weeks GA were recruited as a part of a larger longitudinal study, conducted between 2009 and 2014 at the Hospital for Sick Children in Toronto, Canada. Exclusion criteria included those with any known chromosomal or major congenital abnormalities. Twenty-eight full-term children born > 37 weeks GA were also recruited at 4 years of age. Exclusion criteria for full-term children included prematurity, learning, language, neurological or developmental disabilities as well as MRI incompatibility based on a screening interview. An informed consent to the study was signed by parents and informed assent was provided by the children. The research ethics board at the Hospital for Sick Children approved the study protocol.
Perinatal clinical and radiological measures
The children born VPT have clinical information from their stay in the neonatal intensive care unit. Characteristics such as GA, birth weight, head circumference, sex and delivery type (caesarean-section or vaginal) are described. Significant events during pregnancy were also recorded such as premature rupture of membranes (PROM) and intrauterine growth restriction (IUGR). Measures of illness included Apgar scores at 1 and 5 min, the Clinical Risk Index for Babies II, patent ductus arteriosis, necrotizing enterocolitis and sepsis (defined as a positive blood or cerebrospinal fluid culture and antibiotic therapy for more than 5 days). Medical treatments including the number of days on positive pressure ventilation, endotracheal tube, continuous positive airway pressure, supplemental oxygen and total parenteral nutrition were also collected. These data are summarised in Table 1.
Paediatric neuroradiologists and neurologists assessed each of the structural T1- and T2-weighted images for the children born VPT shortly following birth. White matter injury was graded on a scale of 1–3 for no injury, mild to moderate (T1 signal abnormalities in three or fewer areas) and severe (T1 signal abnormalities in > 5% of hemisphere) levels of injury (Miller et al. 2003). Presence and grades of germinal matrix/intraventricular haemorrhage (GMH/IVH) on a scale from 0 (no haemorrhage) to 4 (periventricular venous haemorrhagic infarction associated with IVH) corresponding to levels of bleeding within the germinal matrix of the brain were also determined. This was based on the Papile scale (GMH 1–4) for computed tomography findings (Papile et al. 1978) and Volpe scale for cranial ultrasonography findings adapted to MRI findings (IVH 1–3, PVHI) (Volpe 2008). More specifically, GMH1/IVH1 is haemorrhage limited to the germinal matrix, GMH2/IVH2 includes an intraventricular haemorrhagic component, GMH3/IVH3 additionally includes ventriculomegaly and GMH4/PVHI is characterized by periventricular venous haemorrhagic infarction associated with IVH (Raybaud et al. 2013). At 4 years of age, paediatric neuroradiologists also assessed each of the children’s structural T1-, T2-weighted and FLAIR images when collected. Any incidental findings were noted.
Maternal education
Levels of maternal education were obtained for all the children. Two categories of education levels were distinguished: the first as those with high school and non-university post-secondary school education and the second as those with university and post-graduate school education.
Developmental assessments
Developmental assessments were performed at 4 years of age for the children born VPT who returned for follow-up as well as the recruited controls. For each assessment, raw scores were converted into standardized scores with a population mean of 100 (50th percentile of typical development) and a standard deviation of 15. Intelligence quotients (IQ) were determined by the Wechsler Preschool and Primary Scales of Intelligence-Third Edition (WPPSI-III; Wechsler 2002) using Canadian norms. Four measures of cognitive abilities were obtained: verbal IQ (VIQ), performance IQ (PIQ), processing speed (PSQ) and full-scale IQ (FSIQ). The Clinical Evaluation of Language Fundamentals-Preschool, Second Edition (CELF-Pre-2; Semel et al. 2004) evaluated receptive and expressive language, yielding a core language (CL) summary score. Visual-motor integration (VMI) ability and supplemental tests of visual perception (VP) and motor coordination (MC) were assessed by the Beery-Buktenica Test of Visual Motor Integration (VMI; Beery et al. 2010).
MRI data acquisition
MRI scans were acquired with a 3.0T Siemens Trio scanner and 12 channel head coil. Sixty-direction diffusion (SE-EPI, b value = 1000) and T1-weighted (MPRAGE) anatomical images (repetition/echo time: 8.8/0.087 and 2.3/0.00296 s; field of view: 244 × 244 × 140 and 192 × 240 × 256 mm; resolution: 2 mm isotropic and 1 mm isotropic; scan time: 20 and 5 min, respectively) were obtained when the children were awake while watching a movie or naturally asleep in the evening. All images were inspected for gross motion artefacts and anatomical abnormalities. We obtained T1-weighted images in 43 children born VPT and diffusion images in 34 children. One child was identified with ventriculomegally and excluded from analyses. Due to the susceptibility of the diffusion sequence to motion, two children had diffusion data with too much motion. Within the group of children born VPT, 31 of the diffusion scans were determined to be useable. All 28 full-term children obtained usable diffusion scans.
Diffusion processing
The diffusion data were pre-processed, which included the alignment of all volumes to a reference volume using FSL tools (Jenkinson et al. 2012) as well as b-vector realignment using custom scripts. Following visual inspection, volumes corrupted with motion were excluded from an individual’s dataset. We allowed up to 30 volumes of the 60-direction sequence to be excluded. Two children were excluded whose data exceeded this threshold. Across groups, the median number of excluded volumes was 3 with a range of 0–24 volumes. The RESTORE algorithm (Chang et al. 2005) was applied to exclude outliers on a voxel-wise basis.
Tract-based spatial statistics (TBSS)
DTI metrics (FA, MD, AD, RD) were calculated and extracted from the output of the RESTORE algorithm (Chang et al. 2005). Analyses of the DTI metrics were conducted using TBSS (Smith et al. 2006). A cohort-specific template of the diffusion data was created following co-registration of all the children’s diffusion images to each other. Subsequently, a mean fractional anisotropy (FA) skeleton was generated and thresholded at 0.25 to represent the centre of major white matter tracts within the whole brain. The skeleton was applied to each child’s FA, MD, AD and RD data.
Connectivity measures
Structural T1-weighted and diffusion images were co-registered using FSL tools (Jenkinson et al. 2012). The structural images were parcellated to obtain 34 cortical and 7 subcortical regions in MNI space using the Desikan–Killiany atlas (Desikan et al. 2006; Fischl et al. 2002). Whole-brain deterministic tractography was performed on the pre-processed diffusion images between each region pair using the fibre assignment by continuous tracking (FACT) algorithm (Mori et al. 1999) with a 45° angular threshold using Diffusion Toolkit and TrackVis (http://www.trackvis.org; Wang et al. 2007). The number of streamlines (edges) between parcellated regions (nodes) was extracted using the UCLA Multimodal Connectivity Package (UCMP) (Brown et al. 2012) and normalized by the average volume of region pairs for estimates of fibre density. A weighted connectivity matrix was generated containing fibre densities between all pairs of regions. All nodes were organized by their respective lobes. Figure 1 provides a visual representation of methods used to obtain connectivity matrices for each child.
Local and global graph measures of connectivity were calculated using the Brain Connectivity Toolbox in MATLAB (The MathWorks, Inc., Natick, Massachusetts, USA). Local strength, the weighted variant of degree, was calculated by summing all of the edge weights of each node together to quantify how nodes are connected to adjacent nodes (Rubinov and Sporns 2010). Weighted local and global efficiency was also calculated (Rubinov and Sporns 2010). Local efficiency is the average of the inverse minimum path length of each node and global efficiency is the average minimum path length (Achard and Bullmore 2007). Higher global efficiency indicates more complex networks with short mean path length (Bullmore and Sporns 2012). Weighted clustering coefficient, the proportion of the node’s neighbours that are also neighbours to each other, was also calculated (Rubinov and Sporns 2010).
Statistical analysis
Participant characteristics
Differences in the sex ratio and age at scan between the children born VPT and full-term children were tested using a Chi-square and t test. Additional t tests were used to assess differences in maternal education levels with developmental outcomes in the two groups separately as well as differences between groups for each of the developmental outcomes. Significance values were held at p < 0.05.
TBSS analyses
Between and within-group voxel-wise analyses were performed with FSL Randomise (Anderson and Robinson 2001) to determine significance, with 5000 permutations and threshold-free cluster enhancement (Smith and Nichols 2009). Significance was defined to be p < 0.05 following corrections for multiple comparisons. Between-group analyses were conducted using a general linear model with group, age at scan, sex and number of excluded volumes due to motion corruption as explanatory variables. This was performed for all DTI metrics: FA, MD, AD and RD. Within the preterm group, additional voxel-wise regression analyses were performed between FA and GA, presence of white matter injury, GMH/IVH and developmental measures. Within the full-term group, voxel-wise regression analyses were performed between FA and developmental measures.
To visualize significant results based upon the voxel-wise regression analyses, the JHU DTI-based tractography atlas (Mori et al. 2005) was applied to the FA skeleton to plot FA values within defined white matter tracts and developmental measures with significant voxels based on the TBSS group difference analysis.
Connectivity analyses
Local graph measures were calculated for each node and each child, while global graph measures were calculated for each child. Both local and global graph measures were averaged across children by group. To assess group differences in local graph measures, average values from the control group were subtracted from the average values of the preterm group at each node (e.g. group difference = preterm − control). Thus, negative values at any given node represents reduced local graph measures in the children born VPT compared to full-term children. To determine statistical significance for individual metrics, permutation testing was used to shuffle group labels 100,000 times. For each permutation, the difference in group average was recalculated to obtain a null distribution. The null distribution was used in a two-tailed z test to determine significance of the observed group difference. Significance was determined based on a false discovery rate (FDR) of 5% to control for multiple comparisons (Benjamini and Hochberg 1995). To visualize the results, the group difference values of the graph measures for all the nodes were organized by lobes and subcortical structures. BrainNet Viewer (Xia et al. 2013; http://www.nitrc.org/projects/bnv/) was used to further depict the nodes with group differences that passed FDR correction.
Associations between graph measures with GA and outcome measures (VIQ, PIQ, FSIQ and VMI) were tested with Pearson correlations within each group separately. Graph measures and presence of white matter injury and GMH/IVH were further tested with t-tests in the preterm group. Significance was reported at FDR of 5%. Correlations at p < 0.01 uncorrected are reported in Supplemental material. R Studio (2015) and MATLAB software were used for statistical analyses.
Results
Participant characteristics
Of the 53 children born VPT who were tested, 31 children (13 females; mean age: 4.2 ± 0.19 years) completed developmental assessments and had usable diffusion images. Perinatal clinical and radiological characteristics for the children born VPT are provided in Table 1. At 4 years of age, 12 children born VPT had incidental findings involving small, focal white matter hyperintensities. Twenty-eight full-term children (15 females; mean age 4.6 ± 0.32 years) were recruited and completed developmental assessments and neuroimaging. No sex differences were found between groups (p = 0.371) but there was a small difference in age at scan (p < 0.05). Of the mothers of the children born VPT, 22.6% had education levels in the first category and 74.2% in the second category. The mothers of the full-term children had education levels of 14.3% in the first category and 82.2% in the second category.
Performance on developmental assessments indicated average levels of ability on most measures, when considering the mean of each group separately as described in Table 2. The only subtest where the children born VPT were below average as a group compared to the general population was in motor coordination. However, full-term children performed significantly higher than the children born VPT on all the developmental measures (p < 0.05) apart from Verbal IQ (see Table 2). There were no differences between the higher and lower maternal education levels and developmental assessments in either group (all p > 0.05).
TBSS analyses
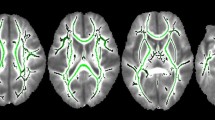
Between-group analyses revealed significant differences between children born VPT and full-term in FA and RD metrics. Full-term children exhibited significantly higher FA than children born VPT within voxels of most tracts, particularly in the left hemisphere (Fig. 2a). These tracts included the bilateral anterior thalamic radiation, corticospinal tract, cingulum, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, uncinate fasciculus as well as the forceps major and minor. Within RD images, children born VPT displayed higher RD than controls within voxels of the corpus callosum, right superior longitudinal fasciculus and inferior fronto-occipital fasciculus (Supplemental Fig. 1). Small clusters within the forceps minor, right cortical spinal tract, anterior thalamic radiation and superior longitudinal fasciculus were found to have differences in FA and RD where more volumes were removed due to motion corruption (Supplemental Fig. 2 and Fig. 3). The number of excluded volumes was not significant between groups (p = 0.298). No significant group differences were found within AD and MD metrics.
a TBSS analyses of group differences revealed widespread areas where full-term children have significantly greater measures of FA compared to children born VPT along the white matter skeleton (p < 0.05). Significant voxels are depicted in red. Colour bars indicate p values. b On the top panel, TBSS analyses identified voxels (in blue) within the full-term group to be significantly associated with VIQ (p < 0.05). On the bottom panel, plots indicate the relation between FA within the forceps minor and right superior longitudinal fasciculus (SLF) and VIQ for both the preterm and full-term (control) groups where significant effects were present in the full-term group. Shaded individuals (in blue) indicate those within the preterm group who experienced any degree of white matter injury at birth
a Connectivity matrix representing group differences in edge weight, the number of streamlines between parcellated regions normalized by the average volume of region pairs. The blue squares represent edge weight values that are smaller in the children born VPT compared to the full-term children. Following permutation testing and FDR correction, results did not survive multiple comparisons. b The group differences by node were averaged across lobes, indicating that children born VPT tend to have reduced edge weights between and within lobes compared to full-term children
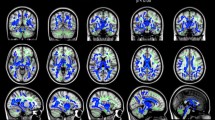
Within the preterm group, no significant associations were found between FA and any of the cognitive measures (FSIQ, VIQ, PIQ, PSQ, CL, VMI, VP and MC). In addition, no significant associations were found between FA and GA, white matter injury or GMH/IVH. Within the control group, significant positive associations between FA and VIQ, PIQ and FSIQ were found. Significant clusters of positive associations between FA and VIQ were present within areas of the forceps major and minor, left anterior thalamic radiation, bilateral inferior fronto-occipital fasciculus and bilateral superior longitudinal fasciculus (Fig. 2b). To visualize this association, mean FA measures extracted within white matter tracts from the JHU-based tractography atlas (Mori et al. 2005) were plotted with VIQ for each group (Fig. 2b). Significant clusters of positive associations between FA, FSIQ and PIQ were present within the left anterior aspects of the inferior fronto-occipital fasciculus, anterior thalamic radiation and forceps minor as well as the right posterior aspect of the forceps major (Supplemental Fig. 4). There were no associations with PSQ, CL, VMI, VP or MC.
Connectivity analyses
The average connectivity matrices for each group indicated that proximal regions were more highly connected to one another than distal regions. Thus, short-range connections were much more prevalent than longer-range connections. The average density of the connectivity matrices was sparse, with 9.49% of region pairs connected (9.42% for preterms, 9.57% for controls). There was no group difference in density (p = 0.183), (Supplemental Fig. 5). Group differences in edge weight were calculated and plotted by node in a connectivity matrix. The average of all the nodal differences by lobe was also plotted in a connectivity matrix as shown in Fig. 3a, b. Although the pattern of edge weights was one of consistently reduced connectivity in the children born VPT, these differences did not survive FDR correction.
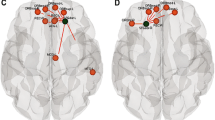
Between-group analyses revealed an overall pattern of reduced strength, local efficiency and clustering coefficient in the children born VPT compared to the term-born children (left panel of Fig. 4a–c). Following FDR correction across all nodes, strength was significantly reduced in the children born VPT in the bilateral middle temporal gyrus (Fig. 4a). Nodes with significantly reduced local efficiency and clustering coefficient included regions across all lobes, but with the majority in the left hemisphere as well as frontal and temporal regions (Fig. 4b, c). Common nodes found between measures of local efficiency and clustering coefficient in the left hemisphere included the lateral orbitofrontal, isthmus of the cingulate, precuneus, lateral occipital, middle temporal, superior temporal and accumbens regions. Common nodes in the right hemisphere included the precentral, isthmus of cingulate, lateral occipital and banks of superior temporal sulcus regions. A summary of all the significant nodes following FDR correction for strength, local efficiency and clustering coefficient are provided in Table 3. Moreover, the children born VPT demonstrated significantly reduced measures of global efficiency (p < 0.0045), (Supplemental Fig. 6).
a Group differences indicate that children born VPT have reduced strength (shown in blue) compared to full-term children. Nodes that were significantly different following permutation testing (p < 0.05) are indicated with black stars. Nodes that survived FDR correction are indicated with pink stars and are plotted on the glass brains. b Group differences of local efficiency by node. c Group differences of clustering coefficient by node. A complete list of the nodes corresponding to the order of regions within the figures from top to bottom is provided in Supplemental Table 3. Each region is ordered bilaterally (right above left)
Within-group analyses for both the children born VPT and full-term revealed relations between local graph measures of strength, local efficiency and clustering coefficient with developmental outcomes at the p < 0.01 uncorrected level (Supplemental Table 1). In the VPT group, correlations between local graph measures with GA, white matter injury and GMH/IVH at the p < 0.01 uncorrected level are reported in Supplemental Table 2. With respect to GMH/IVH, there was a trend for the subcortical structures to have reduced local connectivity. However, these within-group analyses did not survive multiple comparisons.
Following FDR correction, significant correlations between local efficiency and clustering coefficient with GA remained within the right supramarginal gyrus (q = 0.019 and q = 0.0073, respectively). Global efficiency was not found to associate with GA or developmental outcomes. However, significant reductions in global efficiency were found in relation to WMI (p = 0.0022) and GMH/IVH (p = 0.0138) indicating that brain injury detected at birth affected the integration of information.
Discussion
The impact of very preterm birth on the structural organization of white matter during early childhood is widespread and robust. In the present study, we demonstrated that white matter microstructure and connectivity are simultaneously affected in children born VPT compared to children born full-term. Our results of reduced FA and graph measures of strength, clustering coefficient, local and global efficiency support the idea that this atypical profile of white matter in children born VPT reflects one that is less mature and less connected than children born at term. Consequently, our findings suggest that the secondary effects of these structural alterations are expressed as poorer performance in cognitive, language and motor abilities that have a high probability of persisting later in development.
At 4 years of age, white matter maturation is in a period of flux. It is at the beginning of a protracted period of modifications in white matter microstructure and network organization that will persist well into adulthood (Dubois et al. 2014; Hagmann et al. 2010; Paus et al. 2001). The first 2 years of life are known to be a time of dramatic change in brain development that children experience due to increases in myelination, synaptic and axonal pruning that is reflected in rapid increases of FA and decreases in AD, RD and MD (Dubois et al. 2014; Young et al. 2017). While the rate of white matter development is not as steep at 4 years of age as it is from birth to 2 years of age, the microstructure and network connections are far from mature. Parallel processes of myelination will continue to increase as indicated by FA and RD as well as network reorganization towards a more integrated and efficient model of the mature brain (Hagmann et al. 2010; Huang et al. 2015). Children born VPT demonstrate a delay in these maturational processes, which will have downstream implications in their development of more complex and higher-order cognitive abilities as they encounter more demanding environments and responsibilities.
Differences in white matter microstructure
Understanding the role of white matter in preterm brain development is integral in elucidating the basis for their cortical and cognitive differences. Both human and animal studies have supported the hypothesis that overall altered neural connectivity, rather than the number of cortical neurons, is the source of reduced cortical growth and adverse outcomes in preterms (Dean et al. 2014; Kapellou et al. 2006). Our findings of reduced FA across the whole brain in 4-year-old children born VPT compared to those born full-term adds to existing literature that has also found reduced FA within white matter of individuals born preterm at term-equivalent age and older children, adolescents and young adults (Allin et al. 2011; Constable et al. 2008; Duerden et al. 2013). The extensive differences we found may be amplified by our inclusion of lower functioning children, who often could not tolerate the imaging awake and underwent the scans under natural sleep. This occurred for about a third of the total scans and enabled high-quality imaging in children who could otherwise not be scanned.
Our study also found increased RD in the children born VPT within the corpus callosum and aspects of the right superior longitudinal fasciculus and inferior fronto-occipital fasciculus. These tracts are involved in inter-hemispheric communication and intellectual abilities (Tamnes et al. 2010). There was an absence of differences in AD and MD. Thus, as the children born VPT have reduced FA and increased RD, alterations in white matter are likely due to myelin (Anjari et al. 2007; Ball et al. 2013; Song et al. 2005). In addition to reduced myelination, other potential reasons for the widespread reductions in FA could be explained by decreased axonal density and fibre coherence as well as increased axonal size (Jones et al. 2013). Our findings indicate that children born VPT at 4 years of age continue to demonstrate altered white matter microstructure that has been detected with imaging at term-equivalent age (Anjari et al. 2007) and more importantly do not appear to catch up to their term-born peers following the accelerated period of white matter growth between birth and 2 years of age.
The children born VPT included in the present study had non-uniform neonatal clinical courses and incidences of brain injury, representative of the preterm population. Interestingly, we did not find that white matter injury or GMH/IVH directly affected DTI measures following multiple comparisons in our preterm cohort. Furthermore, we did not find a linear relation between DTI metrics and neurodevelopmental outcome measures in the children born VPT at 4 years of age. This finding contrasts others who have found relations in children born VPT between FA at term-equivalent age and early childhood cognitive outcomes (Counsell et al. 2008; Duerden et al. 2015; Keunen et al. 2017; Salvan et al. 2017; Ullman et al. 2015; van Kooij et al. 2012), as well as FA and cognitive measures in late childhood, adolescence and adulthood (Allin et al. 2011; Constable et al. 2008; Feldman et al. 2012, 2013). This may be due to the timing of sampling, as 4 years of age is a dynamic stage of development and more heterogeneous in preterm children than typically developing children. Furthermore, the children born VPT displayed more variability in both their DTI and outcome measures. Associations were found in the term-born group between areas of the forceps major and minor, left anterior thalamic radiation, bilateral inferior fronto-occipital fasciculus and bilateral superior longitudinal fasciculus and VIQ. These tracts facilitate inter-hemispheric communication, connect the thalamus to the frontal lobes and link the frontal and posterior lobes, which have been shown to be related to verbal intelligence abilities in typically developing individuals from late childhood to adulthood (Tamnes et al. 2010).
Network alterations in children born VPT
Connectivity within the infant brain facilitates local information transfer such that structural networks are highly segregated into localized clusters with short path lengths between nodes (Cao et al. 2017). Over time, long-range connections begin to form to allow for greater integration of information between more distal structures while short-range connections are simultaneously fortified (Cao et al. 2017). This common developmental phenomenon has been characterized in normative studies (Hagmann et al. 2010) and should also be present in children born VPT as they follow similar trajectories of white matter microstructural maturation and functional connectivity (Cao et al. 2016; Doria et al. 2010; Young et al. 2017). Our results indicate, however, that those who are born VPT experienced weaker connections relative to term-born children, as reflected in their reduced pattern of connections based on graph measures of strength, clustering coefficient, local efficiency and global efficiency. Hence, children born VPT have network properties that are not only less integrated but also less segregated. It is possible that the VPT children had weaker network segregation than term-born children following birth and are behind in their development of long-range connections. Thus, in our study, children born VPT demonstrated less efficient networks and a potential maturational lag for the integration of networks, even if they eventually mature in a similar pattern to term-born children. Thompson et al. (2016) reported that children born VPT had reduced density, global efficiency, yet higher local efficiency at 7 years of age while we found that our preterm group demonstrated reduced local efficiency. This discrepancy may be due to differences in age or balance between increasing integration and decreasing segregation between the two groups (Hagmann et al. 2010).
The specific nodes that displayed reduced connectivity in the children born VPT were relatively consistent between the graph measures. The left middle temporal region was affected between strength, local efficiency and clustering coefficient. We would expect agreement between local efficiency and clustering coefficient, as they are related measures of local information transfer between neighbouring nodes (Achard and Bullmore 2007). There were also more nodes with group differences in the left hemisphere indicating a weaker connectivity pattern in the left hemisphere, which is typically dominant. Interestingly, the bilateral differences in local efficiency and clustering coefficient were mostly specific to nodes that involved the language network, such as regions within the pars triangularis, middle temporal, superior temporal and banks of the superior temporal sulcus. Moreover, the right supramarginal region was the most affected node with increasing prematurity. In combination, these findings could provide a structural premise for children born VPT to develop compensatory atypical language lateralization involving the right-hemisphere, which has been identified in school-age and adolescent preterms (Gozzo et al. 2009; Schafer et al. 2009).
There are some limitations to consider with the present study. The impact of excluding motion corrupted volumes from the data may have impacted the tractography results. In addition, our sample size may have lacked the power to detect associations between connectivity measures and DTI metrics with cognitive outcomes. We were unable to acquire diffusion images for 41% of the children born VPT who were recruited at 4 years of age. This was due to a combination of reasons such as parental decline to participate in neuroimaging, the diffusion scans being highly sensitive to motion, as well as noisy and long scan durations. Thus, improved scan protocols with shorter scan times would be highly beneficial in this young population.
Conclusions
In conclusion, we have demonstrated that the pattern of white matter in children born VPT, at 4 years of age, is less mature and less connected than term-born children, likely driven by myelination differences. Their white matter development does not appear to catch up following the rapid maturation of white matter in the first years of life. Furthermore, their structural abnormalities reach beyond locally reduced diffusion metrics and encompass network-level architectural differences. Our study provides evidence for both altered brain development and poorer cognitive profiles in children born VPT at this young age, such that they are already disadvantaged and show signs of delay behind their peers. The relation between white matter and cognitive outcomes may involve multiple DTI and connectivity measures, warranting future multivariate analyses with appropriate sample sizes. Importantly, our findings support the need for these children to be followed closely in their first years of life and also provided services such as enrichment programs and early interventions given their poorer cognitive profiles and altered white matter.
References
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):0174–0183
Allin MPG, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RAA et al (2011) White matter and cognition in adults who were born preterm. PLoS One 6(10):1–9
Anderson PJ (2014) Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med 19(2):90–96
Anderson MJ, Robinson J (2001) Permutation tests for linear models. Aust N Zeal J Stat 43(1):75–88
Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ (2007) Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage 35(3):1021–1027
Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N et al (2012) The effect of preterm birth on thalamic and cortical development. Cereb Cortex 22(5):1016–1024
Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV et al (2013) Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci USA 110(23):9541–9546
Batalle D, Hughes EJ, Zhang H, Tournier J-D, Tusor N, Aljabar P et al (2017) Early development of structural networks and the impact of prematurity on brain connectivity. NeuroImage 149(January):379–392
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15(7–8):435–455
Beery K, Buktenica N, Beery N (2010) Beery–Buktenica test of visual motor integration. The Psychological Corporation, San Antonio
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57(1):289–300
Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P et al (2006) Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. NeuroImage 32(1):70–78
Brown JA, Rudie JD, Bandrowski A, Van Horn JD, Bookheimer SY (2012) The UCLA multimodal connectivity database: a web-based platform for brain connectivity matrix sharing and analysis. Front Neuroinform 6(28):1–17
Brown CJ, Miller SP, Booth BG, Andrews S, Chau V, Poskitt KJ, Hamarneh G (2014) NeuroImage Structural network analysis of brain development in young preterm neonates. NeuroImage 101:667–680
Bullmore ET, Sporns O (2012) The economy of brain network organization. Nat Rev Neurosci 13(5):336–349
Cao M, Huang H, Peng Y, Dong Q, He Y (2016). Toward developmental connectomics of the human brain. Front Neuroanat. https://doi.org/10.3389/fnana.2016.00025
Cao M, Huang H, He Y (2017a) Developmental connectomics from infancy through early childhood. Trends Neurosci 40(8):494–506
Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M et al (2017b) Early development of functional network segregation revealed by connectomic analysis of the preterm human brain. Cereb Cortex 27(3):1949–1963
Chang L-C, Jones DK, Pierpaoli C (2005) RESTORE: Robust estimation of tensors by outlier rejection. Magn Reson Med 53(5):1088–1095
Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C et al (2008) Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics 121(2):306–316
Counsell SJ, Edwards AD, Chew ATM, Anjari M, Dyet LE, Srinivasan L et al (2008) Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 131:3201–3208
Dean JM, Mcclendon E, Hansen K, Azimi-zonooz A, Chen K, Riddle A et al (2013) Prenatal cerebral ischemia disrupts MRI-defined cortical micrtostructure through disturbances in neuronal arborization. Sci Transl Med 5(168):1–22
Dean JM, Bennet L, Back S, McClendon E, Riddle A, Gunn AJ (2014) What brakes the preterm brain? An arresting story. Pediatr Res 75(1–2):227–233
Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M et al (2009) Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE study. Pediatrics 123(6):1485–1492
Desikan RS, Se F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980
Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE et al (2010) Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci 107(46):20015–20020
Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L (2014) The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276:48–71
Duerden EG, Card D, Lax ID, Donner EJ, Taylor MJ (2013) Alterations in frontostriatal pathways in children born very preterm. Dev Med Child Neurol 55(10):952–958
Duerden EG, Foong J, Chau V, Branson H, Poskitt KJ, Grunau RE et al (2015) Tract-based spatial statistics in preterm-born neonates predicts cognitive and motor outcomes at 18 months. Am J Neuroradiol 36(8):1565–1571
Feldman HM, Lee ES, Loe IM, Yeom KW, Grill-Spector K, Luna B (2012) White matter microstructure on diffusion tensor imaging is associated with conventional magnetic resonance imaging findings and cognitive function in adolescents born preterm. Dev Med Child Neurol 54(9):809–814
Feldman HM, Lee ES, Yeatman JD, Yeom KW (2013) Language and reading skills in school-aged children adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia 50(14):3348–3362
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002) Whole brain segmentation: Neurotechnique automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355
Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J et al (2009) Alterations in neural connectivity in preterm children at school age. NeuroImage 48(2):458–463
Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ et al (2010) White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA 107(44):19067–19072
Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ et al (2015) Development of human brain structural networks through infancy and childhood. Cereb Cortex 25(5):1389–1404
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012) FSL. NeuroImage 62(2):782–790
Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage 73:239–254
Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J et al (2006) Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med 3(8):1382–1390
Keunen K, Benders MJ, Leemans A, Fieret-Van Stam PC, Scholtens LH, Viergever MA et al (2017). White matter maturation in the neonatal brain is predictive of school age cognitive capacities in children born very preterm. Dev Med Child Neurol. https://doi.org/10.1111/dmcn.13487
Krsnik Ž, Majić V, Vasung L, Huang H, Kostović I (2017) Growth of thalamocortical fibers to the somatosensory cortex in the human fetal brain. Front Neurosci 11(233):1–17
Loe IM, Lee ES, Feldman HM (2013) Attention and internalizing behaviors in relation to white matter in children born preterm. JDBP 34(3):156–164
Mangin KS, Horwood LJ, Woodward LJ (2017) Cognitive development trajectories of very preterm and typically developing children. Child Dev 88(1):282–298
Mcclendon E, Ph D, Chen K, Gong X, Sharifnia E, Hagen M et al (2014) Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann Neurol 75(4):508–524
Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, Vigneron DB, Barkovich AJ (2003) Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. Am J Neuroradiol 24(8):1661–1669
Mori S, Crain BJ, Chacko VP, Van Zijl PCM (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45(2):265–269
Mori S, Wakana S, Van Zijl PCM, Nagai-Poetscher LM (2005) MRI atlas of human white matter. Elsevier, Amsterdam
Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M et al (2012) Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage 54(4):2563–2570
Pandit AS, Robinson E, Aljabar P, Ball G, Gousias IS, Wang Z et al (2014) Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex 29(9):2324–2333
Pannek K, Scheck SM, Colditz PB, Boyd RN, Rose SE (2014) Magnetic resonance diffusion tractography of the preterm infant brain: a systematic review. Dev Med Child Neurol 56(2):113–124
Papile L, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92(4):529–534
Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A (2001) Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull 54(3):255–266
Raybaud C, Ahmad T, Rastegar N, Shroff M, Al Nassar M (2013) The premature brain: developmental and lesional anatomy. Neuroradiology 55(Suppl 2):S23–S40. https://doi.org/10.1007/s00234-013-1231-0
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52(3):1059–1069
Salvan P, Tournier JD, Batalle D, Falconer S, Chew A, Kennea N et al (2017) Language ability in preterm children is associated with arcuate fasciculi microstructure at term. Hum Brain Mapp 38(8):3836–3847
Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC et al (2009) Alterations in functional connectivity for language in prematurely born adolescents. Brain 132:661–670
Semel E, Wiig E, Secord W (2004) Clinical evaluation of language fundamentals—preschool, 2nd edn. The Psychological Corporation, San Antonio
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44(1):83–98
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31:1487–1505
Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26(1):132–140
Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM (2010) Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum Brain Mapp 31(10):1609–1625
Thompson DK, Chen J, Beare R, Adamson CL, Ellis R, Ahmadzai ZM et al (2016) Structural connectivity relates to perinatal factors and functional impairment at 7 years in children born very preterm. NeuroImage 134:328–337
Travis KE, Adams JN, Ben-Shachar M, Feldman HM (2015) Decreased and increased anisotropy along major cerebral white matter tracts in preterm children and adolescents. Plos One 10(11):e0142860. https://doi.org/10.1371/journal.pone.0142860
Ullman H, Spencer-smith M, Thompson DK, Doyle LW, Inder TE, Anderson PJ, Klingberg T (2015) Neonatal MRI is associated with future cognition and academic achievement in preterm children. Brain 138(11):13251–13262
Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O (2006) Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage 32:1538–1548
van Kooij BJM, de Vries LS, Ball G, van Haastert IC, Benders MJNL., Groenendaal F, Counsell SJ (2012) Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR 33(1):188–194
Volpe JJ (2008) Intracranial hemorrhage: germinal matrix-intraventricular hemorrhage of the premature infant. In: Neurology of the newborn, 5th edn. Saunders, Philadelphia, pp 517–588
Wang R, Benner T, Sorensen AG, Wedeen VJ (2007) Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 15:3720
Wechsler D (2002) Wechsler preschool and primary scales of intelligence, 3rd edn. The Psychological Corporation, San Antonio
Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, Austin NC (2009) Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed 94(5):F339–F344
Xia M, Wang J, He Y (2013) BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One 8(7):e68910. https://doi.org/10.1371/journal.pone.0068910
Yap PT, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D (2011). Development trends of white matter connectivity in the first years of life. PLoS One 6(9):e24678
Young JM, Morgan BR, Whyte HEA, Lee W, Smith ML, Raybaud C et al (2017) Longitudinal study of white matter development and outcomes in children born very preterm. Cereb Cortex 27(8):4094–4105. https://doi.org/10.1093/cercor/bhw221
Acknowledgements
We thank all of the families who participated in the study. We also thank Tammy Rayner, Ruth Weiss, Dr. Steven Miller, Dr. Manohar Shroff, Dr. Charles Raybaud, Dr. Aideen Moore, Dr. Hilary Whyte, Dr. Bratislav Misic, Tamara Powell and Wayne Lee for their valuable support and contributions.
Funding
Canadian Institutes of Health Research (MOP-84399 and MOP-137115).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Young, J.M., Vandewouw, M.M., Morgan, B.R. et al. Altered white matter development in children born very preterm. Brain Struct Funct 223, 2129–2141 (2018). https://doi.org/10.1007/s00429-018-1614-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1614-4