Abstract
Structural connectivity among cortical areas provides the substrate for information exchange in the cerebral cortex and is characterized by systematic patterns of presence or absence of connections. What principles govern this cortical wiring diagram? Here, we investigate the relation of physical distance and cytoarchitecture with the connectional architecture of the mouse cortex. Moreover, we examine the relation between patterns of ipsilateral and contralateral connections. Our analysis reveals a mirrored and attenuated organization of contralateral connections when compared with ipsilateral connections. Both physical distance and cytoarchitectonic similarity of cortical areas are related to the presence or absence of connections. Notably, our analysis demonstrates that the combination of these factors relates better to cortico-cortical connectivity than each factor in isolation and that the two factors relate differently to ipsilateral and contralateral connectivity. Physical distance is more tightly related to the presence or absence of ipsilateral connections, but its relevance greatly diminishes for contralateral connections, while the contribution of cytoarchitectonic similarity remains relatively stable. Our results, together with similar findings in the cat and macaque cortex, suggest that a common set of principles underlies the macroscale wiring of the mammalian cerebral cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The connectional architecture of the mammalian cerebral cortex provides the anatomical substrate for the communication of its distinct elements. At the macroscale level, this architecture corresponds to the long-range white matter pathways linking the mosaic of areas of the cortical sheet (Sporns et al. 2005). Extensive invasive studies in animal models, such as the macaque monkey, have uncovered a characteristic pattern of the absence or presence of specific connections among cortical areas (e.g., Pandya and Yeterian 1990; Yeterian et al. 2012). However, few studies have aimed at uncovering the principles underlying the cortico-cortical connectional architecture in a systematic and quantitative way. This endeavor is important for understanding the basic blueprint of the wiring of the cortex and identifying fundamental candidate neurodevelopmental mechanisms of the cortical connectome.
At least two wiring principles appear to underlie the cortico-cortical connectional architecture. The first principle is the physical distance between two cortical areas. That is, areas which are close to each other are likely to be connected, while distant areas are less likely to be connected (e.g., Greilich 1984; Young 1992). This principle reflects a wiring cost reduction design (Ramón y Cajal 1899; Scannell et al. 1995; Kaiser and Hilgetag 2006). The second principle is grounded in the cytoarchitecture of cortical areas, suggesting a “similar prefers similar” wiring rule (Pandya and Yeterian 1990; Barbas 2015). Based on this principle, areas that are more similar in terms of their cytoarchitecture, for instance, two agranular areas (areas lacking layer IV), are more likely to establish a connection, while less similar areas, such as an agranular area and a granular area (an area possessing layer IV), are less likely to be connected. Both principles have been shown quantitatively to relate to the presence or absence of connections between cortical areas of the cat (Beul et al. 2015a) and the macaque monkey (Beul et al. 2015b). Thus, cytoarchitectonic similarity and physical distance (hereafter simply called distance) appear to constitute mammalian-general principles of cortico-cortical wiring. Further examination of the wiring of other mammalian cortices is necessary to substantiate this claim.
A limitation of most studies on cortico-cortical connections is the lack of extensive data on ipsilateral and contralateral connectivity. One exception is a previous study focusing on the macaque prefrontal cortex (Barbas et al. 2005) which revealed a large overlap of the topography and high correlation of the strength of ipsilateral and contralateral connections. Thus, the findings indicated a mirrored organization of ipsilateral and contralateral cortical connectivity, fostering the development of hypotheses on the relation of ipsilateral and contralateral connections in other mammalian species. Moreover, data sets offering information on contralateral connectivity also allow the examination of the relation of cytoarchitectonic similarity and distance to contralateral connections.
Recent efforts have generated valuable and extensive connectivity data of the mouse cortex (Oh et al. 2014; Zingg et al. 2014). These data sets constitute the current best estimate of cortico-cortical connectivity in this species. In this study, we used these wiring diagrams and adopted a quantitative approach to examine the relation of ipsilateral and contralateral connections in the mouse cortex and the relation of connectivity to the distance and cytoarchitectonic similarity of cortical areas.
Materials and methods
Connectivity data
The data set used for our main analysis is the Allen Mouse Connectivity Atlas (http://connectivity.brain-map.org/). The mouse wiring diagram was mapped by employing the recombinant adeno-associated virus expressing enhanced green fluorescent protein as an anterograde tracer. For constructing a large-scale connectivity map, the Allen Reference Atlas (http://mouse.brain-map.org/static/atlas) was used. In total, 295 non-overlapping structures (cortical areas, subcortical nuclei, etc.) were considered. Altogether, 469 injected brains of C57BL/6J male mice were included in the construction of the large-scale connectivity matrix through constrained optimization. The constrained optimization sets about two-thirds of all possible connections to zero (absent). p values were estimated for the remaining non-zero weights by linear regression. See Oh et al. (2014) for details on the estimation of the p values. This procedure resulted in connectivity matrices involving 213 structures. Details on the informatics pipeline, quality controls and estimation of the inter-areal connectivity matrix are provided in Oh et al. (2014). The connectivity matrices from Oh et al. (2014) were obtained from the Open Connectome project (http://www.openconnectomeproject.org/). The .graphml file was converted to .gml with the online Open Connectome project conversion tools (http://openconnecto.me/graph-services/convert/). Last, the data from the .gml file were imported in Matlab and converted into a directed graph with the aid of Matlab scripts (http://www.mathworks.de/matlabcentral/fileexchange/45741-read-gml). In this study, we focused only on cortico-cortical connections involving 38 cortical areas per hemisphere (Fig. 1). The entorhinal cortex was excluded from the analysis, since not all of its sub-compartments were injected. Connections were considered present if they exhibited a p value, obtained from the linear regression, below 0.05 and all remaining connections were considered absent. For the majority of the analyses, the weight of the connections was not considered unless otherwise stated. In cases where connection weights were analyzed, we used the so-called normalized connectivity strength that quantifies the amount of signal detected in a target area after infecting one voxel in the source area (see Oh et al. (2014) for details). Moreover, for assessing if the results are driven primarily by homotopic connections (connections linking the same area in the two hemispheres), we performed the analysis with and without the homotopic connections. The results reported below are derived from the analysis without the homotopic connections [except from the homotopic strength analysis (see “Results”)]. Inclusion of homotopic connections did not change the results.
Schematic depiction of the cortical areas of the mouse cortex based on the Allen Reference Atlas. Note that this drawing is an approximation offering an overview of the cortical areas in the mouse. Colors denote the distinct cortical types on an ordinal scale, with 1 denoting less eulaminated and 4 more eulaminated areas. An intermediate type (2.5) denotes areas that exhibit substantial within-area heterogeneity, that is, a combination of cortical type 2 and 3. The actual Allen Reference Atlas is available online (http://mouse.brain-map.org/static/atlas). For abbreviations of the cortical areas, see Oh et al. (2014). For a discussion on the nomenclature and other parcellation schemes of the mouse cortex, see Van De Werd and Uylings (2014) and Paxinos and Franklin (2013)
Relation of ipsilateral and contralateral connections
Previously, Oh et al. (2014) used simple metrics for comparing the ipsilateral and contralateral connections, that is, the correlation and ratio of ipsilateral and contralateral connection strengths at the whole brain level. Here, we employ such metrics, as well as additional topological metrics, conjointly with statistical inference for examining the similarity of ipsilateral and contralateral cortico-cortical connections. We adopt a similar approach as previously done for the macaque prefrontal cortex (Barbas et al. 2005). Specifically, the following metrics were computed. For assessing the overall topological similarity of the ipsilateral and contralateral matrices, we used the edit distance (e.g., Trusina et al. 2005) that assesses the number of insertion/deletion operations needed to convert one matrix into the other. Smaller edit distance values indicate higher similarity between two matrices (since fewer insertion/deletion operations are needed to convert one matrix into the other). This analysis considers only the topology, that is, the presence or absence of connections. To assess the relation of the strength of ipsilateral and contralateral connections, we computed Spearman’s rank correlation between the two matrices. In addition to this global analysis, we performed an area-wise analysis by computing the following metrics. First, for each area, the ratio of the sum of the strength of ipsilateral versus contralateral connections was computed (Barbas et al. 2005). Second, the number of areas common to the ipsilateral and contralateral projection patterns of an area was assessed with the Jaccard index, which is defined as the intersection versus the union of ipsilaterally and contralaterally connected areas. Third, we estimated the similarity between the ipsilateral and contralateral connectivity patterns by considering the weights of the connections and calculating Spearman’s rank correlation between the contralateral and ipsilateral connectivity profiles. The null values for these metrics were computed from 1000 null models for the ipsilateral and contralateral connectivity matrices, matched in node (number of areas), edge (number of total connections), and degree distribution (number of connections of an area) (Rao and Bandyopadhyay 1996). Last, we assessed if connections between homotopic areas (e.g., the connection between the frontopolar cortex in the left and right hemisphere) were significantly stronger than the remaining contralateral connections.
Relating cytoarchitectonic similarity and distance to cortico-cortical connections
For evaluating the role of distance, we used the Euclidean distance between the center of mass of the cortical areas. This distance is a proxy of the length, and consequently wiring cost, of the potential anatomical connection between two areas. Euclidean distances between the center of masses of the cortical areas are provided in (Oh et al. 2014). In addition, we used an alternative distance measure, namely, an estimate of the axonal path length, by computing the geodesic distances between the areas constrained by a white matter mask based on the Allen Reference Atlas (see Supplementary Material). We expected that areas separated by short distances are more frequently connected, while distant areas are less frequently connected. For examining the relation between the presence or absence of connectivity and cytoarchitectonic similarity, the 38 areas were classified in four cortical types. Specifically, the criterion for assigning an area to category 1 was the absence of layer IV, that is, if an area was agranular. Areas assigned to category 2 did not have a clearly discernible layer IV and, hence, were characterized as dysgranular. Cortical areas assigned to category 3 were characterized by the presence of layer IV, and were thus granular areas. Areas assigned to category 4 were more clearly eulaminated, that is, they exhibited a more distinct differentiation of layers accompanied by a thick and dense layer IV (Fig. 1). For this classification, high-resolution Nissl-stained sections were used from a previous cytoarchitectonic study in the mouse (Van De Werd and Uylings 2014) in conjunction with the Paxinos and Franklin’s Mouse Atlas (Paxinos and Franklin 2013). An intermediate cortical type (2.5) was also used, since certain cortical areas from the Allen Reference Atlas exhibit cytoarchitectonic heterogeneity. For instance, the visual posteromedial area mostly corresponds to cortical type 3, but its more medial part is reminiscent of cortical type 2. Thus, the overall assignment was type 2.5 for this area. The assignment of cortical areas to the cortical types was performed by one of the authors (HBMU), an expert on rodent neuroanatomy. It should be noted that such an ordinal scale of cortical types has been shown to exhibit a high positive correlation with objective quantitative measures of cytoarchitecture such as neuronal density (Dombrowski et al. 2001) which constitutes a characteristic fingerprint of cortical architecture. Moreover, a gradual reassignment of cortical areas to cortical types was carried out as control analysis to ensure robustness of the results to these observer-dependent assignments (see Control Analyses in "Materials and Methods" and "Results"). Since the wiring of the cat and macaque cortex obeys a “similar prefers similar” principle (Beul et al. 2015a, b; Pandya and Yeterian 1990), we expected that mouse cortico-cortical connections are more likely to be present between areas of similar cortical types. To express the cytoarchitectonic difference of two areas, the index |Δ| was used, denoting the absolute difference between the cortical type of a pair of areas (e.g., Barbas et al. 2005). Figure 2 offers a visual summary of the overall approach.
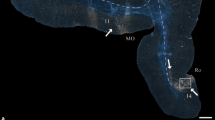
Example of data used and schematic depiction of the factors related to the presence or absence of connectivity. a Ipsilateral and contralateral connectivity pattern of the primary motor area (MOp). b Axial slice depicting the pattern of connectivity of area MOp alongside with a Nissl-stained section and the corresponding parcellation based on the Allen Reference Atlas. c Cytoarchitectonic similarity of cortical areas was estimated by an index |Δ| computed for each pair of areas as the absolute difference of their cortical type. Note that this index is computed for a pair of areas irrespective of the presence or absence of connection(s) (depicted with dashed lines) in between them. d The wiring cost of a potential connection between two areas was approximated by the physical distance between the two areas, i.e., the Euclidean distance between their centers of mass
For illustrating the relation of distance and cytoarchitectonic similarity to the presence or absence of connections in an intuitive manner, we binned the Euclidean distances and the |Δ| values. For each bin, the proportion of present connections was expressed as the number of present connections divided by the number of possible connections for the current bin.
We used nominal logistic regression for examining the relation between the presence or absence of connections (dependent variable) and distance and cytoarchitectonic similarity (predictors). The two predictors range in different units and, thus, were normalized to the [0–1] interval. An initial analysis examined all connections (ipsilateral and contralateral) simultaneously and used all predictors. For this analysis, an additional categorical predictor coding for the contralateral (=1) and ipsilateral (=0) connections was used. The analysis also examined the interactions of this categorical predictor with the predictors of distance and cytoarchitectonic similarity. A subsequent analysis was run separately on the ipsilateral and contralateral connections. In this step, we examined the contribution of distance and cytoarchitectonic similarity separately and then conjointly for assessing the unique contribution of each predictor. The quality of the logistic regression models was assessed with McFadden’s pseudo-R2, which quantifies the improvement of the likelihood of the model when compared with a null model (a model containing only the intercept term). The statistical difference of the likelihood of the bivariate and univariate models was assessed with the likelihood ratio test (e.g., Vidakovic 2011). The likelihood ratio test explicitly addresses if the addition of a predictor significantly improves a model, thus assessing if a more “complex” model (with more predictors) should be favored.
To assess the generalizability of models built with each predictor in isolation or conjointly, a prediction analysis was conducted. To this end, the coefficients from the nominal logistic regression were used to predict the presence or absence of connections with a model built with either distance or cytoarchitectonic similarity, or using both variables as predictors. The quality of such predictions was assessed by computing receiver operating characteristic (ROC) curves and the corresponding areas under curve (AUC) for the original and null (shuffled labels) predictions. It should be noted that in a prediction analysis the addition of a predictor does not necessarily lead to higher AUC values. Thus, a model with statistically significantly higher AUC values should be favored. The predictions were computed 100 times each time using 80 % of the available data to build the model (drawing with replacement) and the rest of the data serving as a test set. The percentage of data used (varying from 80 to 90 %) did not change the results. In addition to ROC curves, precision–recall curves were also computed from the prediction procedure described above, as well as the F1 score, which is the harmonic mean of precision and recall. See Saito and Rehmsmeier (2015) for a discussion on ROC and precision–recall curves. The prediction analysis was carried out separately for the ipsilateral and contralateral connections.
Control analyses
A series of control analyses were performed to ensure robustness of the results and their independence from parameter variations.
First, an additional independent data set on male C57BL/6J mouse cortico-cortical connectivity was examined (Zingg et al. 2014) (see Supplementary Material). This extra data set is derived from different types of anterograde tracers (phaseolus vulgaris leucoagglutinin and dextran tetramethylrhodamine) as well as retrograde tracers (cholera toxin subunit b and Fluorogold). See Zingg et al. (2014) for details.
Second, for the connectivity matrices of the main analysis obtained from the Allen Mouse Connectivity Atlas, a more conservative p value threshold was used, that is, connections with a p value smaller than 0.01 were considered present and all other connections were considered absent.
Third, the Allen Mouse Connectivity Atlas data set was subject to the following control analyses. (1) Connections were randomly inserted, and, in a separate analysis, deleted, in a gradual fashion, as percentages of the already existing connections and all analyses were redone for these “randomly enriched” (or “randomly reduced”) matrices. Thus, this simple scenario simulates a situation where connections are wrongly assumed to be absent or present due to potential biases in the processing pipeline resulting in the connectivity matrices. (2) Cortical areas were randomly assigned to a different (adjacent higher or lower) cortical type in a graded fashion. For instance, at the 10 % level, 4 cortical areas (i.e., 10 % of 38 areas) were randomly reassigned to a different cortical type. This control analysis aimed to capture the effect of a potential different assignment of areas (e.g., as done by a different observer) given the observer-dependent character of the approach. For instance, area MOs might potentially be assigned to cortical type 2 to reflect evidence for a less eulaminated medial part of MOs (Van De Werd et al. 2010).
A last control analysis aimed at taking into account the functional modality similarity of cortical areas. Previous findings at the microscopic connectivity level, that is, pyramidal cell-to-pyramidal cell connectivity in the mouse primary visual cortex, suggest that cells are more frequently connected if they have similar functional profiles (similar orientation preference of visual stimuli) (Ko et al. 2011). At the macroscale level, cortico-cortical connections in the cat cortex have been suggested to obey a functional similarity rule (Lee and Winer 2008a). Therefore, we tested if functional modality similarity of the mouse cortical areas explains the relation between the presence or absence of connections and cytoarchitectonic similarity. To this end, we grouped the cortical areas by functional modalities and used an additional predictor in the nominal logistic regression coding for the functional modality similarity of two cortical areas (see Supplementary Material). All analyses were conducted in Matlab (8.1.0.604 R2013a) (Mathworks).
Results
The results are structured by, first, presenting the ipsilateral and contralateral topological analysis results and, second, the relation of the presence or absence of connections with distance and cytoarchitectonic similarity.
Relation of ipsilateral and contralateral connectivity patterns
Examination of the binary ipsilateral and contralateral connectivity matrices, that is, connectivity matrices denoting only the presence or absence of connections and not the weight of connections, revealed that they are more similar than expected by chance, as quantified by the edit distance measuring the amount of insertions/deletions needed to convert one matrix into the other (Edit DistanceOriginal 0.32 Edit Distancenull mean = 0.71 std = 0.02, p < 0.001). Furthermore, a conserved connectivity strength pattern, that is, correlation of strength of ipsilateral and contralateral connections, was observed (rho = 0.63, p < 0.001). The area-wise analysis revealed that the ipsi/contra ratio of the strength of the areas was much higher than 1, on average 6.14, indicating much stronger ipsilateral connections (Fig. 3a). A striking “outlier” was the ~35 times more prominent strength for ipsilateral versus contralateral connections for two areas (Gu and VISal). Since the strength of the connections in the Oh et al. (2014) data set is obtained through regression analysis that entails certain modeling assumptions (see “Limitations and future directions”), we refrain from assigning a neurobiological interpretation to this “outlier” behavior of areas Gu and VISal. Despite the large differences in strength, the overall pattern of ipsilateral and contralateral connections was moderately to highly correlated (range of positive rho values: 0.32–0.98), indicating a relatively high similarity of the ipsilateral and contralateral connectivity patterns for the majority of cortical areas (Fig. 3b). Moreover, there was a moderate-to-high proportion of common areas in the ipsilateral and contralateral connectivity profile of each area (Fig. 3c). The rendering of the ipsi/contra metrics across the cortical sheet (Fig. 3) indicates no systematic prominence of these metrics for specific sets of cortical areas or lobes, as suggested for the human brain (Wang et al. 2014). Hence, it appears that there is no clear segregation of cortical areas based on the ipsi/contra metrics. Altogether, the above results indicate that as a whole, the topology and strength of ipsilateral and contralateral connection patterns are more similar than expected by chance, despite the fact that contralateral connections are weaker than ipsilateral ones. Hence, the contralateral connectivity pattern appears to be an attenuated mirrored version of the ipsilateral pattern. Last, the homotopic connections were significantly stronger than other contralateral connections (by a factor of × 2.6 compared to contralateral non-homotopic connections, p < 0.001, permutation test). This difference was also significant if distance was taken into account in an analysis of covariance. Importantly, the above analysis involving the strength of connections led to the same qualitative results, if the strength of connections was converted to “connectivity strength”, “connectivity density” or “normalized connectivity density” (see Oh et al. (2014) for details).
Ipsilateral and contralateral connectivity patterns. a Ratio of ipsilateral over contralateral connection strength for each area. b Correlation of the ipsilateral and contralateral connectivity profile for each area. c Proportion of shared areas, i.e., Jaccard index of areas that are part of the ipsilateral and areas that are part of the contralateral connectivity profile. Based on the current topological metrics, no clear segregation of areas suggesting dichotomies, such as primary versus non-primary areas, is evident. The asterisks denote the significance of each area-wise metric in panels b and c. Significance was established via comparisons with metrics derived from null models (see “Materials and methods”). Note that the areas marked with ‘+’ do not have contralateral connections considered as “present” in the current p value threshold (p < 0.05)
Relation of physical distance and cytoarchitecture with the presence or absence of connections
Increasing distance of areas and increasing cytoarchitectonic difference of cortical areas was accompanied by a smaller number of connections between them. This relation was observed both for ipsilateral and contralateral connections (Fig. 4).
Frequency of present connections for different ranges of physical distance and cortical type differences. The connection frequency was estimated by dividing the number of existing connections by the number of possible connections for each interval of cytoarchitectonic similarity and distance. Note that for both ipsilateral and contralateral cases, increasing physical distance of areas and increasing cytoarchitectonic differences of areas were accompanied by a less frequent number of connections
The nominal logistic regression model that simultaneously considered all connections (ipsilateral and contralateral) revealed a significant contribution of distance (beta = −5.33, p < 0.001) and cytoarchitectonic similarity (beta = −1.30, p < 0.001). The negative sign of the regression coefficients indicates that an increase in distance or cytoarchitectonic difference is accompanied by a decrease in the probability of a connection being present, compared with the probability of a connection being absent. In addition, the categorical predictor coding for the ipsilateral and contralateral connections was significant (beta = −1.61, p < 0.001), indicating that fewer contralateral than ipsilateral connections exist. Moreover, the interaction between cytoarchitectonic similarity and the ipsilateral/contralateral categorical predictor was not significant (beta = 0.10, p > 0.1). This result indicates that the influence of cytoarchitectonic similarity on the presence or absence of connections does not depend on whether a connection belongs to the ipsilateral or contralateral category. On the other hand, the interaction between distance and the ipsilateral/contralateral categorical predictor was significant (beta = 4.57, p < 0.001). This result indicates that the influence of distance on the presence or absence of connections depends on whether a connection belongs to the ipsilateral or contralateral category. The same qualitative results were obtained when performing the above analysis while using geodesic distance as a proxy for wiring distance. Specifically, distance (beta = −3.43, p < 0.001) and cytoarchitectonic similarity (beta = −1.46, p < 0.001) were statistically significant, the categorical predictor was statistically significant (beta = −0.65, p < 0.01), the interaction between distance and the ipsilateral/contralateral categorical predictor was statistically significant (beta = 1.74, p < 0.01), while the interaction with cytoarchitectonic similarity was not statistically significant (beta = 0.24, p > 0.1).
Relation of distance and cytoarchitectonic similarity with ipsilateral and contralateral connections
We subsequently examined the contralateral and ipsilateral connections separately. It should be noted that the two predictors were almost orthogonal (rho = 0.11 for ipsilateral connections, rho = 0.01 for contralateral connections). Examination of each predictor in isolation revealed that both distance and cytoarchitectonic similarity were significantly related to the presence or absence of ipsilateral and contralateral connections (univariate model Table 1). The conjoint examination of distance and cytoarchitectonic similarity again highlighted both predictors as significantly related to the presence or absence of connections (bivariate model Table 1). The bivariate model was significantly better than the univariate models for the ipsilateral connections [likelihood ratio test: 184.3, 32.6 (p < 0.001) when comparing the log likelihood of the bivariate model and the model built only on cytoarchitectonic similarity and distance, respectively]. The bivariate model was also significantly better than the univariate models for the contralateral connections [likelihood ratio test: 8.6 (p < 0.05) 29.3 (p < 0.001) when comparing the log likelihood of the bivariate model and the model built only on cytoarchitectonic similarity and distance, respectively]. The same qualitative results were obtained if the approximation of axonal path lengths was used instead of the Euclidean distance as wiring distance (Table S1).
The prediction analysis results were as follows: For the ipsilateral connections, using only distance as predictor led to the highest AUC = 0.76 (p < 0.01, permutation test). Using only cytoarchitectonic similarity as a predictor led to an AUC = 0.64 (p < 0.01, permutation test). The use of both predictors led to slightly better predictions (AUC = 0.78, p < 0.01, permutation test) compared to the ones using each predictor separately (Fig. 5). Comparing the AUC for the ipsilateral predictions revealed that significantly better predictions were obtained with distance when compared to the predictions using cytoarchitectonic similarity. Moreover, the combination of distance and cytoarchitectonic similarity led to significantly better predictions compared to the ones obtained from using each predictor separately (p < 0.001, permutation tests). For the contralateral connections, the highest AUC was observed for cytoarchitectonic similarity (AUC = 0.61, p < 0.01, permutation test). Distance led to an AUC = 0.55 (p < 0.01, permutation test). The use of both predictors led to slightly better predictions (AUC = 0.63, p < 0.01, permutation test) compared to the predictions using each predictor separately (Fig. 5). Comparing the AUC for the contralateral predictions revealed that significantly better predictions were obtained with cytoarchitectonic similarity when compared to the ones using distance. Moreover, the combination of distance and cytoarchitectonic similarity led to significantly better predictions compared to the ones using each predictor separately (p < 0.001, permutation tests). The same picture emerged from ROC curves computed using the approximation of axonal path lengths as wiring distance (Fig. S1) and from the precision–recall curves and the F1 score curves (Fig. S2).
Connectivity prediction analysis based on cytoarchitectonic similarity and physical distance. For each analysis, 100 predictions and corresponding ROC curves were constructed by drawing with replacement. ROC curves are constructed for the original predictions with the true labels (present versus absent connection) as well as for null predictions obtained with shuffled labels. The quality of the prediction was quantified with the AUC. The depicted AUC values are the mean AUC values for the original and null predictions across 100 iterations. All AUC values were significantly higher from the AUC values obtained from the null predictions (p < 0.01). See “Materials and methods” and “Results” for details
Control analyses
The results for the control data set (Zingg et al. 2014) were in line with the above results on ipsilateral connectivity (Table S2). The bivariate model was significantly better than the univariate models [likelihood ratio test: 50.7, 11.2 (p < 0.001) when comparing the log likelihood of the bivariate model and the model built only on cytoarchitectonic similarity and distance, respectively]. The prediction analysis for the control data set demonstrated that significant predictions of connectivity are achieved when using only distance (AUC = 0.66), only cytoarchitectonic similarity (AUC = 0.59) or both (AUC = 0.68) (all AUCs p < 0.01, permutation tests). Predictions based on distance were significantly better than predictions based on cytoarchitectonic similarity, and predictions based on both distance and cytoarchitectonic similarity were moderately but significantly better than predictions obtained with the use of each predictor separately (p < 0.001, permutation tests) (Fig. S3).
The above results were also significant in all other control analyses. First, the use of the axonal path length approximation as a proxy for wiring cost led to qualitatively the same results (Table S1). Second, the main connectivity data set (Allen Mouse Connectivity Atlas) was subjected to a series of gradual perturbations demonstrating the robustness of the findings (Fig. S4). Third, a different p value for determining the presence or absence of connections in the main connectivity dataset (Allen Mouse Connectivity Atlas) was used, that is, p < 0.01, qualitatively giving rise to the same results (Table S3). Last, the addition of a predictor coding for the functional modality similarity of cortical areas did not abolish the effect of cytoarchitectonic similarity and distance (Table S4). Thus, cytoarchitectonic similarity and distance are fundamental wiring principles, the effect of which is not explained by the functional similarity of cortical areas. Moreover, the control analyses highlight the robustness of our findings.
Discussion
The current results provide novel insights into the cortico-cortical connectional architecture of the mouse. We have demonstrated that contralateral connection patterns are significantly similar to the ipsilateral connection patterns and constitute a mirrored but attenuated pattern. Cytoarchitectonic similarity and distance relate to the presence or absence of connections, albeit with a different degree of contribution to ipsilateral and contralateral connections. In addition, the combined use of these factors led to better connectivity prediction models when compared with models built with each factor separately. Thus, our results illustrate that the cortico-cortical connectional architecture (Fig. 6) is related to the cytoarchitecture of cortical areas, in addition to the wiring cost principle (e.g., Rubinov et al. 2015).
Summary of the mouse cortico-cortical connectional architecture based on cytoarchitectonic similarity. Cortical areas are organized in homocentric circles. The innermost circle contains the less eulaminated areas and successively growing circles correspond to increasingly eulaminated areas. Connections between areas are color coded based on the absolute difference of their cortical types. Light shades denote similar cortical types and progressively darker shades denote progressively dissimilar cortical types of the interconnected areas. Note that the wiring diagrams are dominated by lighter shades offering a visual summary of the predominant connectivity between areas of similar cortical type. This holds true for both ipsilateral and contralateral connectivity
Organization of ipsilateral and contralateral connectivity
The topology and strength of ipsilateral compared to contralateral connectivity in the mammalian brain is largely unexamined, especially at the whole brain level. In the macaque prefrontal cortex, it was shown that the overall strength of connections is much higher for ipsilateral than the contralateral connections (Barbas et al. 2005). Our results suggest that this is also true for the cortico-cortical connections of the mouse at the whole cortex level. Despite an overall higher strength of ipsilateral connections, the patterns for ipsilateral and contralateral connections were moderately to strongly correlated. These findings indicate a mirrored but attenuated connectivity pattern in the mouse, in line with previous observations in the macaque prefrontal cortex (Barbas et al. 2005), thus suggesting a similar basic topological organization of ipsilateral and contralateral connections in these two species.
Last, our results further corroborate that homotopic connections are stronger than the average of non-homotopic contralateral connections, in line with findings in the cat (Lee and Winer 2008b) and dog (Rajkowska and Kosmal 1989) cortex. Such findings, obtained from invasive tract-tracing techniques, suggest that the strong homotopic connections observed in the human brain as estimated by functional MRI measurements (e.g., Stark et al. 2008) might also stem from strong homotopic anatomical connections.
Principles of cortico-cortical connectivity
The wiring cost principle, which was already put forward by Ramón y Cajal (1899), has guided much of recent neuroscientific research focusing on the macroscale connectional architecture of the mammalian cortex (Scannell et al. 1995; Chen et al. 2013; Ercsey-Ravasz et al. 2013; Beul et al. 2015a, b). Qualitative observations in the macaque cortex suggest that the cytoarchitecture of cortical areas is linked to the patterns of cortico-cortical connections (Pandya and Yeterian 1990). The current results further corroborate and extend such findings. First, we demonstrate that distance and cytoarchitectonic similarity are wiring principles also characterizing the mouse cortex. This was demonstrated quantitatively by employing two independent data sets that constitute the current best estimate of the macroscale cortico-cortical connectivity of the mouse. Such results resonate well with previous finding in the cat and macaque cortex (Beul et al. 2015a, b), highlighting distance and cytoarchitectonic similarity as potential mammalian-general wiring principles. Second, there is a different degree of association of these principles with the presence or absence of ipsilateral and contralateral connections. The contribution of distance is greatly reduced for the contralateral connections, while the contribution of cytoarchitectonic similarity remains largely unaffected. Moreover, distance, when compared to cytoarchitectonic similarity, appears more tightly linked to the presence or absence of ipsilateral connections, while the opposite holds for the contralateral connections. This demonstrates that contralateral connections are poorly explained by the wiring cost principle. Hence, these results enrich our understanding of the factors related to the macroscale connectional architecture and extend the set of wiring principles of the mammalian cortex beyond the well-documented principle of wiring cost.
Putative neurobiological mechanisms underlying the observed wiring principles
Our findings demonstrate wiring principles of the mouse cortex. However, these principles do not by themselves constitute mechanistic neurobiological explanations. They offer a quantitative “anchoring point” for further investigating how the observed relations between the presence or absence of connections, distance, and cytoarchitectonic similarity emerge. With respect to the length of connections, such wiring economy might arise due to a random axonal growth process previously demonstrated in computational modeling giving rise to realistic distributions of physical length of connections (Kaiser et al. 2009). Hence, connections are more likely to be established between areas that are spatially close, compared to remote areas. With respect to the role of cytoarchitecture, we should note that the correlational nature of the current study cannot determine the causal link between the connectional and cytoarchitectonic architecture of the cortex. However, a plausible scenario is that cytoarchitectonic similarity of areas of the adult cortex might partially reflect similarities in the time window of development during neurogenesis (e.g., Rakic 2002; Charvet et al. 2015). Gradients of neurogenesis that suggest such distinct time windows of neurons populating the distinct cortical areas are documented in the mouse cortex (Smart 1984). Thus, similar time windows in the ontogeny of areas might bias the connections under development to “prefer” areas that exhibit similar cytoarchitecture in the adult cortex, since such areas host neurons that constitute the available origin and more probable targets for establishment of connections (for a schematic depiction of this scenario, see Fig. 7). Such preferential connectivity is observed in C. elegans (Varier and Kaiser 2011), and to a certain extend in the Drosophila connectome (Chiang et al. 2011). While the sketched neurobiological mechanisms are possible scenarios, the current results, in conjunction with similar findings in the cat and macaque cortex, point to a consistent relation between the physical, cytoarchitectonic, and connectional cortical architecture. Thus, a potentially evolutionary conserved neurobiological mechanism must lie at the heart of such prominent and systematic relations.
Diagrams illustrating the scenario that the cytoarchitecture of cortical areas in the adult cerebral cortex reflects distinct time windows of neurogenesis during development resulting in the observed “similar prefers similar” cytoarchitectonic wiring principle. a Gradients of neurogenesis in the mouse cortex. The root of the gradient of neurogenesis in the lateral surface is located near the insula. Gradients in the medial surface have two roots. One near the hippocampus and one in the rostral part of the medial surface. Arrows show the spatial patterns of neurogenesis. These gradients of neurogenesis are based on data presented in Smart (1984). Note that these gradients of neurogenesis can also be accompanied by abrupt differences in proliferation rates and formation of laminar compartments (Polleux et al. 1997). b Assumed overlapping time windows of neurogenesis for three cortical areas with different cortical types. The less eulaminated area has the earliest onset of neurogenesis, while the more eulaminated area has the latest onset of neurogenesis. These differences might arise due to the different position of cortical areas in the gradients of neurogenesis depicted in panel A. The curves schematically show the onset, pick and decline of neurogenesis in the cortical areas. c Establishment of connections at timepoint T1 indicated in panel b might be more plausible between areas that contain neurons generated in similar time windows, since they offer more potential “connection partners”
Functional considerations
The pattern of the presence and absence of connections between cortical areas results in topological network configurations that appear crucial for diverse aspects of brain dynamics (e.g., Sporns et al. 2000; Müller-Linow et al. 2008; Moretti and Muñoz 2013). We have illustrated principles that govern such wiring and also putative neurobiological mechanisms. Hence, miswiring leading to pathological cortical dynamics and function may be conceived of as a failure in sculpting the cortico-cortical landscape based on such principles. Notably, distance and cytoarchitectonic similarity are also tightly related to connectivity features, such as strength (Hilgetag and Grant 2010) and laminar projection patterns (Barbas 1986; Hilgetag and Grant 2010; Beul et al. 2015a). Strength of connections might lead to a differential functional impact (Vanduffel et al. 1997), and laminar patterns appear important for shaping the spectral channels used for exerting influence between cortical areas (Bastos et al. 2015). Hence, physical distance and cytoarchitectonic similarity relate to many features of cortical connections that in turn are closely linked to distinct functional features. Consequently, the currently identified wiring principles constitute an important guiding thread for uncovering and understanding relations between distinct aspects of structural and functional cortical organization.
Limitations and future directions
Certain limitations of our study should be noted. First, the connectivity matrices of Oh et al. (2014) were derived from a constrained optimization approach with assumptions such as homogeneity of areas. An assumption of strict area homogeneity might not be neuroanatomically realistic, since multiple injections in different parts of what is considered as one cortical area might exhibit different connectivity patterns (e.g., Luppino et al. 2003). Moreover, the automated pipelines used in Oh et al. (2014) can lead to spurious connectivity estimates due to misregistration and the inability to clearly distinguish fibers of passage from axon terminals. Importantly, our main results were reproduced in an independent data set (Zingg et al. 2014) that was derived from manual expert annotation and the application of both anterograde and retrograde tracers and thus is not affected by the above methodological limitations. In addition, the results were unaffected by a set of control analyses. Hence, our results are likely not undermined by further updates on the status of specific connections of specific areas, such as the presence of contralateral connections for some areas that currently were considered absent.
We have presently examined the relation of distance and cytoarchitectonic similarity based on prior work in the cat (Beul et al. 2015a) and macaque (Beul et al. 2015b) cortex. Despite the significant relation of these factors to the presence or absence of connections, they do not fully explain the pattern of cortico-cortical connections. Previous studies demonstrate a relationship between connectivity and gene expression (French and Pavlidis 2011; Ji et al. 2014) and topological similarity (Costa et al. 2007). Future predictive models could incorporate such factors, leading to more powerful models explaining the cortico-cortical connectional architecture. Moreover, coactivation of areas, for instance, areas that belong to the same sensory modality, might favor the maintenance and strengthening of the connections between them. In this study, we tentatively addressed this issue with a simple control analysis by incorporating the similarity of the modality of the cortical areas as a factor related to the presence or absence of connections. Despite its marginally significant relation to connectivity, functional similarity indeed appears related to the presence or absence of connections (Table S4). Thus, not only the neurobiological mechanisms giving rise to the observed relation between the connectional, cytoarchitectonic, and physical levels of cortical organization remain to be elucidated, but additional factors explaining more accurately the cortico-cortical landscape remain to be incorporated and interpreted at the neurobiological level.
Conclusions
We examined the topological relation of ipsilateral and contralateral cortico-cortical connectivity in the mouse as well as the degree to which distance and cytoarchitectonic similarity relate to the presence or absence of connections. Remarkably, despite striking differences of mouse, cat and macaque cortices across space (cortex size) and time (phylogeny), a common set of principles appear to underlie their macroscale wiring.
References
Barbas H (1986) Pattern in the laminar origin of corticocortical connections. J Comp Neurol 252:415–422
Barbas H (2015) General cortical and special prefrontal connections: principles from structure to function. Ann Rev Neurosci 38:269–289
Barbas H, Hilgetag CC, Saha S, Dermon CR, Suski JL (2005) Parallel organization of contralateral and ipsilateral prefrontal cortical projections in the rhesus monkey. BMC Neurosci 6:32
Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P (2015) Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85:390–401
Beul SF, Grant S, Hilgetag CC (2015a) A predictive model of the cat cortical connectome based on cytoarchitecture and distance. Brain Struct Funct 220:3167–3184
Beul SF, Barbas H, Hilgetag CC (2015b) A predictive structural model of the primate connectome. http://arxiv.org/abs/1511.07222
Charvet CJ, Cahalane DJ, Finlay BL (2015) Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex 25:147–160
Chen Y, Wang S, Hilgetag CC, Zhou C (2013) Trade-off between multiple constraints enables simultaneous formation of modules and hubs in neural systems. PLoS Comput Biol 9:e1002937
Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC et al (2011) Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol 21:1–11
Costa LF, Kaiser M, Hilgetag CC (2007) Predicting the connectivity of primate cortical networks from topological and spatial node properties. BMC Syst Biol 1:16
Dombrowski SM, Hilgetag CC, Barbas H (2001) Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex 11:975–988
Ercsey-Ravasz M, Markov NT, Lamy C, Van Essen DC, Knoblauch K, Toroczkai Z, Kennedy H (2013) A predictive network model of cerebral cortical connectivity based on a distance rule. Neuron 80:184–197
French L, Pavlidis P (2011) Relationships between gene expression and brain wiring in the adult rodent brain. PLoS Comput Biol 7:e1001049
Greilich H (1984) Quantitative Analyse der cortico-corticalen Fernverbindungen bei der Maus. Thesis, University of Tlibingen
Hilgetag CC, Grant S (2010) Cytoarchitectural differences are a key determinant of laminar projection origins in the visual cortex. NeuroImage 51:1006–1017
Ji S, Fakhry A, Deng W (2014) Integrative analysis of the connectivity and gene expression atlases of the mouse brain. NeuroImage 84:245–253
Kaiser M, Hilgetag CC (2006) Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. PLoS Comput Biol 2:e95
Kaiser M, Hilgetag CC, van Ooyen A (2009) A simple rule for axon outgrowth and synaptic competition generates realistic connection lengths and filling fractions. Cereb Cortex 19:3001–3010
Ko H, Hofer SB, Pichler B, Buchanan K, Sjöström PJ, Mrsic-Flogel TD (2011) Functional specificity of local synaptic connections in neocortical networks. Nature 473:87–91
Lee CC, Winer J (2008a) Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol 507:1920–1943
Lee CC, Winer JA (2008b) Connections of cat auditory cortex: II. Commissural system. J Comp Neurol 507:1901–1919
Luppino G, Rozzi S, Calzavara R, Matelli M (2003) Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur J Neurosci 17:559–578
Moretti P, Muñoz MA (2013) Griffiths phases and the stretching of criticality in brain networks. Nat Commun 4:2521
Müller-Linow M, Hilgetag CC, Hütt MT (2008) Organization of excitable dynamics in hierarchical biological networks. PLoS Comput Biol 4:e1000190
Oh SW et al (2014) A mesoscale connectome of the mouse brain. Nature 508:207–214
Pandya DN, Yeterian EH (1990) Prefrontal cortex in relation to other cortical areas in rhesus monkey: architecture and connections. In: Uylings HBM, van Eden CG, de Bruin JPC, Feenstra MGP, Pennartz CMA (eds) The prefrontal cortex: its structure, function and pathology. Progress in brain research, vol 85. Elsevier, Amsterdam, pp 6394
Paxinos G, Franklin K (2013) The mouse brain in stereotaxic coordinates, 4th edn. Academic Press, London
Polleux F, Dehay C, Kennedy H (1997) The timetable of laminar neurogenesis contributes to the specification of corticla areas in mouse isocortex. J Comp Neurol 385:95–116
Rajkowska G, Kosmal A (1989) Contralateral connections of the dog’s frontal association cortex. Acta Neurobiol Exp 49:141–151
Rakic P (2002) Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 3:65–71
Ramón y Cajal S (1899) Histology of the nervous system of man and the vertebrates, vol 1 (trans: N Swanson and LW Swanson), Ch 5. Oxford University Press, New York
Rao AR, Bandyopadhyay S (1996) A Markov chain Monte Carlo method for generating random (0,1)-matrices with given marginals. Sankhya A 58:225–242
Rubinov M, Ypma RJ, Watson C, Bullmore ET (2015) Wiring cost and topological participation of the mouse brain connectome. Proc Natl Acad Sci USA 112:10032–10037
Saito T, Rehmsmeier M (2015) The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One 10:e0118432
Scannell JW, Blakemore C, Young MP (1995) Analysis of connectivity in the cat cerebral cortex. J Neurosci 15:1463–1483
Smart IHM (1984) Histogenesis of the mesocortical area of the mouse telencephalon. J Anat 138:537–552
Sporns O, Tononi G, Edelman GM (2000) Theoretical neuroanatomy: Relating anatomical and functional connectivity in graphs and cortical connection matrices. Cereb Cortex 10:127–141
Sporns O, Tononi G, Kötter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1:e42
Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, Milham MP (2008) Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci 28:13754–13764
Trusina A, Sneppen K, Dodd IB, Shearwin KE, Egan JB (2005) Functional alignment of regulatory networks: a study of temperate phages. PLoS Comput Biol 1:e74
Van De Werd HJJM, Uylings HBM (2014) Comparison of (stereotactic) parcellations in mouse prefrontal cortex. Brain Struct Funct 219:433–459
Van De Werd HJJM, Rajkowska G, Evers P, Uylings HBM (2010) Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct 214:339–353
Vanduffel W, Payne BR, Lomber SG, Orban GA (1997) Functional impact of cerebral connections. Proc Natl Acad Sci U S A 94:7617–7620
Varier S, Kaiser M (2011) Neural development features: spatio-temporal development of the Caenorhabditis elegans neuronal network. PLoS Comput Biol 7:e1001044
Vidakovic B (2011) Statistics for bioengineering sciences, 1st edn. Springer, New York
Wang D, Buckner RL, Liu H (2014) Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J Neurosci 34:12341–12352
Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M (2012) The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 48:58–81
Young MP (1992) Objective analysis of the topological organization of the primate cortical visual system. Nature 358:152–155
Zingg B, Hintiryan H, Gou L, Song MY, Bay M, Bienkowski MS, Foster NN, Yamashita S, Bowman I, Toga AW, Dong HW (2014) Neural networks of the mouse neocortex. Cell 156:1096–1111
Acknowledgments
This work was supported by a Humboldt research fellowship from the Alexander von Humboldt Foundation to AG and funding by the German Research Council DFG to CCH (SFB 936/A1,Z3; TRR169/A2). The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goulas, A., Uylings, H.B.M. & Hilgetag, C.C. Principles of ipsilateral and contralateral cortico-cortical connectivity in the mouse. Brain Struct Funct 222, 1281–1295 (2017). https://doi.org/10.1007/s00429-016-1277-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1277-y