Abstract
It is generally accepted in neuroscience that anatomy and function go hand in hand. Accordingly, a local morphological variability could lead to a corresponding functional variability. In this study, we tested this hypothesis by linking the variability of the cortical folding pattern of 252 right-handed subjects to the localization or the pattern of functional activations induced by hand motion or silent reading. Three regions are selected: the central sulcus, the precentral sulcus and the superior temporal sulcus (STS). “Essential morphological variability traits” are identified using a method building upon multidimensional scaling. The link between variability in anatomy and function is confirmed by the perfect match between the central sulcus morphological “hand knob” and the corresponding motor activation: as the location of the hand knob moves more or less dorsally along the central sulcus, the motor hand activation moves accordingly. Furthermore, the size of the left hand activation in the right hemisphere is correlated with the knob location in the central sulcus. A new link between functional and morphological variability is discovered relative to the location of a premotor activation induced by silent reading. While this reading activation is located next to the wall of the central sulcus when the hand knob has a ventral positioning, it is pushed into a deep gyrus interrupting the precentral sulcus when the knob is more dorsal. Finally, it is shown that the size of the reading activation along the STS is larger when the posterior branches are less developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is assumed that brain anatomy is the substrate for function. In the human cerebral cortex, this structure–function relationship is difficult to study because of the lack of clear architectural clues in standard in vivo structural images. Recent advances relative to the mapping of the human connectome (Van Essen and Ugurbil 2012) and myeloarchitectony (Van Essen and Glasser 2013) provide new opportunities. For instance, the fiber connectivity pattern was found to predict the localization of functional activations in the fusiform gyrus (Saygin et al. 2012). The reliable mapping of most architectural characterizations, however, requires advanced acquisitions and analyses. Therefore, bolstered by a new methodology, we advocate for revisiting the potential of cortical folding pattern for such predictions, because cortical folding can be visualized easily in standard MR images. The folding patterns of the cortical surface were studied by the first neuroscientists (Broca and Pozzi 1888; Dejerine and Dejerine-Klumpke 1901). However, the exact link between the folding pattern and architecture is a complex issue (Welker 1988; Zilles et al. 2013).
Strong correspondences between cortical folding features and functional activations have been found not only in primary areas but also in higher level areas, such as the visual areas (Watson et al. 1993; Dumoulin et al. 2000), the paracentral sulcus (Grosbras et al. 1999), the dorsal premotor region (Amiez et al. 2006) and the fusiform gyrus (Weiner et al. 2014). The main difficulty limiting the amount of such findings is the complexity and the variability of the folding pattern (Ono et al. 1990; Regis et al. 2005; Petrides 2012). Only experts can reliably label sulci in very variable regions of the cortex (Regis et al. 2005; Zlatkina and Petrides 2010; Segal and Petrides 2012; Mangin et al. 2015b). Hence, identifying key features of the folding patterns that can be linked to the localization of activations is challenging. The first step toward this goal is a better understanding of the main characteristics of morphological variability. For this purpose, a reliable and reproducible model is needed.
In this paper, we show how a new strategy based on computational anatomy overcomes the high morphological variability (Mangin et al. 2004, 2015a; Perrot et al. 2011; Sun et al. 2012). The main variability is demonstrated using a modern multidimensional scaling method called Isomap (Tenenbaum et al. 2000). This approach provides a visual description of the principal variability trait in a given population; we call it an “essential variability”. In terms of cortical folding, while characterizing this variability by visual inspection of a set of individuals is very difficult, our method can provide insights on how the sulcal shapes vary. Using this essential morphological variability representation, the functional activations of each individual can be mapped and analyzed, and potential links between anatomy and function can be observed. The discovery of these essential variability traits thus helps to link morphological variability with functional variability, providing a new understanding of structure–function relationship. In this paper, the link between activations induced by hand motion and the shape of the central sulcus is the starting point of such investigations.
The central sulcus, among the first sulci to appear during cortical development, is also one of the most stable sulci in terms of shape. There are mapped functionalities such as the sensory and motor homunculus surrounding this sulcus (Penfield and Boldrey 1937). A precise structure–function landmark related to the central sulcus shape has been discovered: the hand area in the primary motor cortex is mapped onto a dorsal knob-like structure called today the hand knob (Yousry et al. 1997; Sastre-Janer et al. 1998; Boling and Olivier 2004). The “essential variability” of the central sulcus found in a previous study is the “moving hand-knob” (Sun et al. 2012). Along the central sulcus, the hand knob can be located more ventrally or more dorsally. Hence, each subject in the population can be quantified using the localization of the hand knob. We showed that the hand knob localization depends on the hemisphere and also on the subject handedness. In the dominant hemisphere, the central sulcus of the right-handers has a characteristic double-knob shape that of the left-handers a single-knob shape. In the left hemisphere dominant for the right-handers, the left-handers also tend to have a single-knob central sulcus. The hand-converters (forced right-handers) keep this single-knob shape in the left hemisphere, while their central sulcus elongates to reach the asymmetry level of right-handers (Sun et al. 2012). Considering the large variability of the hand knob localization, the current paper explores first to which extent this morphological landmark really matches the primary hand motor area whatever the knob localization. The activations delineating this motor area are obtained in 252 right-handed subjects from a contrast between a right hand click and a left hand click.
In addition to confirming the link between morphology and function by studying relatively simple anatomical regions and functional tasks such as the central sulcus and the hand click, this paper attempts to perform further exploration with a more complex functional contrast related to silent reading. Reading is one of the most fundamental human-specific behaviors. The network of functional areas activated by reading has been extensively studied; anatomical and functional changes induced when acquiring literacy have been observed (Castro-Caldas et al. 1999; Petersson et al. 2007; Carreiras et al. 2009; Dehaene et al. 2010). Reproducible reading activations can be observed along large sulci such as the central sulcus, the superior temporal sulcus (STS) (Boulenger et al. 2009; Price et al. 2011), and in the precentral gyrus. Compared to the central sulcus, the STS shape is much more variable. A consensus for labeling the branches of the STS is challenging even for an experienced anatomist (Ochiai et al. 2004; Segal and Petrides 2012). The precentral gyrus is also involved in language processing, speech perception, and motor circuits of articulation and phonation, including silent articulation of syllables (Haber and Haber 1982; Brown et al. 2009; Pulvermüller and Fadiga 2010). With respect to the central sulcus, ventral to the hand knob are the regions of the face, lips, larynx and tongue. In terms of folding patterns, the precentral sulcus is more variable in shape compared to the central sulcus (Ono et al. 1990). Certain precentral morphological landmarks linked to function do exist. For example, the left inferior frontal junction (IFJ) is linked to cognitive control (Derrfuss et al. 2005). However, no simple structure–function landmark such as the hand knob is known for the precentral sulcus, likely due to the functional and morphological complexity of the region. The contrast providing the activations used in our study is silent reading minus checkerboard visualization, a component of a database of functional localizers (Pinel et al. 2007) which is freely accessible for a subset of subjects (http://brainomics.cea.fr/localizer). Our localizer protocol is a 5-min fMRI sequence that captures the cerebral bases of auditory and visual perception, motor actions, reading, language comprehension and mental calculation at an individual level. Based on the anatomy-function assumption, our hypothesis is that an observable anatomical variability trait regarding the precentral gyrus or the STS should be linked to an observable functional variability trait.
Materials and methods
We used MRI data collected from 252 right-handed French healthy adult volunteers. The experimental fMRI protocol is a 5-min-long functional localizer (Pinel et al. 2007). FMRI images were analyzed with SPM5 (http://www.fil.ucl.ac.uk) according to the SPM general linear model, two functional contrasts have been considered in our analysis: “right hand click minus left hand click” for motor activations, “visual sentence minus checkerboard” for reading activations.
Essential variability traits of Central sulcus and STS were computed using the procedure described in a previous paper (Sun et al. 2012). First, the sulci of each subject were extracted automatically from the T1-weighted MRI data using the “Morphologist 2012” toolbox of BrainVisa (http://brainvisa.info) (Perrot et al. 2011; Fischer et al. 2012) and brought to Talairach space with a nine parameter affine transform. Right sulci were flipped to become comparable with left sulci. Then, the shape similarity between two sulci was coded by the average quadratic distance between their 3D representations after pairwise rigid alignment. Finally, from the resulting similarity matrix, we used Isomap-based manifold learning to capture a one-dimensional approximation of the high dimensional space spanned by each sulcus (Tenenbaum et al. 2000).
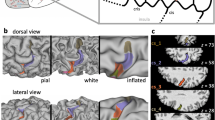
For each Isomap, we then computed a local average sulcus at regularly spaced location along the one-dimensional manifold. During averaging, a weighting scheme is applied: for any specific location, the closer a sulcus lies to this location, the more its shape contributes to the average image. To compute the average shapes, the sulci are aligned with a template sulcus first. The template is the sulcus that minimizes the average distance to the whole set. The 3D representations are then converted to 3D images. These images are summed using the weighting scheme mentioned above in order to obtain a “statistical anatomical probability map”, called further a moving average shape (MAS). Finally, all these MASs are thresholded at the same level to permit 3D rendering. For visualization, each MAS is translated in antero-posterior direction according to its Isomap coordinate, providing a simple description of the shape changes corresponding to the essential variability trait (see Fig. 1). The exact same averaging procedure can be applied to project other information about the same subjects into the Isomap axis, for instance individual functional activations or other sulci. This is our approach for investigating the links between structural and functional variability.
The moving average shape of the central sulcus along its Isomap. From the left to the right of the Isomap axis, observe that the position of the hand knob moves dorsally. As the hand knob (single star) shifts dorsally and flattens, a second distinct lower “knob” (double star) appears and becomes more prominent. An image superimposing the shape at the two extremities of the Isomap is plot for the comparison of the two shapes
To assess the variations of the left and right hand motor areas along the central sulcus manifold, individual contrasts (t-maps) are locally averaged at regularly spaced locations. Three thresholds are applied to represent average activations as a nested set of 3D objects. For the reading contrast, a premotor volume of interest (VOI) and an auditory VOI are defined to extract the individual activations of interest. The premotor VOI (resp. the auditory VOI) is a mask resulting from a dilation of 2 cm of the template central sulcus (resp. the template STS). After masking the reading contrast with the premotor VOI, average premotor activations are computed along the central sulcus manifold. In addition, average precentral sulci are also computed along the central sulcus manifold, to characterize some interactions between both shapes. After masking the reading contrast with the auditory VOI, average auditory activations are computed along the STS manifold.
Finally, to test the structure–function links revealed by the visualization procedure mentioned above, simple tests are performed from the individual activations. The intensity maximum of individual motor activations are projected onto the one-dimensional axis linking the highest and the lowest points of the template central sulcus, resulting in an estimation of activation localization that can be correlated with the localization of the subject in the Isomap axis. The localizations of the intensity maximum of individual reading activations are projected onto the X, Y and Z axis, so that correlation to Isomap values can be studied. The 3D volumes of individual activations are also correlated with the subject coordinates in the central sulcus or in the STS Isomaps.
Results
The variability of the shape of the central sulcus is studied first (Fig. 1). It can be observed that as the “hand knob” representation shifts dorsally along the Isomap axis, a second distinct lower “knob” appears. This trend in shape variation reproduces perfectly the result published with another database (Sun et al. 2012). Hence, the “essential variability trait” of the “moving hand knob” is confirmed in this dataset.
Next, the variability of hand motor activations is analyzed relative to the central sulcus essential variability trait. From Fig. 2, it can be observed that the average right hand motor activation (in red) moves dorsally from the left to the right of the central sulcus manifold in accordance with the position of the left hand knob. This morphological feature is shown to be precisely hosting the primary hand motor area, even for the most extreme morphological configurations. The left hand motor activation is shown in purple in the Isomap representing right hemisphere (Fig. 2). It can be observed that the average activations follow the hand knob as well. Furthermore, when the right knob is located more ventrally, the left hand activation appears to be greater in magnitude. It is found that the location of maximum activation is correlated to Isomap coordinate in both hemispheres (left cor = 0.23; p = 0.0003, right cor = 0.2; p = 0.009). The volume of individual activations defined from a threshold of 4 in the t-map is correlated to Isomap shape for the left hand activation in the right hemisphere (cor = 0.23; p = 0.0002), while no significant correlation is found in the left hemisphere.
The hand motor activation variability along the central sulcus Isomap. Top two rows the manifold of the left central sulcus summarized as in Fig. 1, with two different orientations. The local average t-maps of motor and reading activations are plot onto the sulcus local averages, with three different thresholds (motor: T = 4, 5 and 6; reading: T = 2, 2.5, 3.5) to illustrate the evolution in shape and localization. The right hand motor activation is plot in red and the silent reading activation is plot in blue. From the left to the right of the Isomap axis, observe that as the “hand knob” representation shifts dorsally along the Isomap axis, the average right hand motor activation moves dorsally as well, following the knob. As a second distinct lower “knob” appears toward the right of the axis, the average reading activation moves closer to the precentral sulcus. Bottom two rows the manifold of the right central sulcus, with two different orientations. Left hand motor activation (in purple) is plot onto the sulcus local average. From the left to the right of the Isomap axis, as the “hand knob” representation shifts dorsally along the Isomap axis and flattens, the average left hand motor activation moves dorsally as well, following the knob, and the magnitude of the activation appears to be diminishing simultaneously
With respect to the reading activation (visual sentence minus checkerboard), two regions are studied: the precentral gyrus and the region of the STS. The region of the precentral gyrus is characterized by two sulci: the central sulcus and the precentral sulcus. Regarding the central sulcus, the localization of the reading activation (in blue) is at the level of the ventral or lower knob (Fig. 2). As the lower knob becomes more prominent anatomically, from the left to the right of the manifold, the activation appears to be moving further away or more rostral from the central sulcus, closer to the precentral sulcus. To explore the potential link between the reading activation and the precentral sulcus shape, moving averages of the precentral sulcus are computed along the central sulcus manifold (cf. Fig. 3). The junction of the inferior precentral sulcus and the inferior frontal sulcus, the IFJ, changes in shape along the manifold. From the left to the right of the Isomap axis, a reduction of the depth of the precentral sulcus appears and grows until an interruption forms at the right extremity of the manifold (Fig. 3). This interruption appears in accordance with the central sulcus lower knob. From the left to the right of the Isomap axis, it can be observed that the center of the reading activation moves from the bank of the central sulcus to the interruption of the precentral sulcus (Fig. 3). Regarding the location of the maximum activation, the X (cor = −0.2; p = 0.02) and Z (cor = −0.2; p = 0.003) coordinates are found to be correlated to the Isomap value, not the Y axis. The maximum activation moves dorsally from left to right of the Isomap axis. We did not find any correlation between Isomap and activation volume.
The precentral sulcus variability and the premotor reading activation variability along the central sulcus Isomap. Top row the moving average shape (MAS) of the precentral gyrus along the central sulcus Isomap. The MASs at the left and right extremities of the Isomap are superimposed at the right to facilitate shape comparison. As the hand knob moves dorsally and the second knob appears, the inferior and intermediate precentral sulci become less prominent and an interruption appears splitting them from the superior precentral sulcus. Middle row the forms at the extremities of the Isomap with the functional activation added, threshold T = 2.5. Bottom row the local average t-maps of reading activation (blue) is plot onto the variability trait of the precentral sulcus. The average activation is plot with three different thresholds: T = 2, 2.5 and 3, to illustrate the evolution in the magnitude and location of the activation. It can be observed that the activation is moving from the central sulcus bank at the left of the Isomap to the interruption of the precentral sulcus appearing at the right of the Isomap. The activations at the two extremities shown in the middle row are superimposed to facilitate the comparison of magnitude and location
The second region studied with respect to reading activations is the STS. The essential variability trait that can be observed is related to the length of the main part of the STS, defined from the polar extremity to the fork leading to the two terminal ascending branches reaching the parietal lobe (cf. Fig. 4). From the left to the right of the Isomap axis, the stem of the STS gradually shortens while the terminal branches forming the ‘fork’ get wider apart. The “essential variability trait” can be summarized as the “shortening of the stem and opening of the fork”. The reading activation is found along the main branch (or the stem) of the left STS, not around the terminal ascending branches (Fig. 4). Simple correlation tests are performed from the individual reading activations. There is a correlation between the Z coordinate of the intensity maximum and the Isomap coordinate (cor = 0.2; p = 0.01), no correlation is found along the X and Y coordinates. For a threshold of three in the individual t-map, a correlation of the volume of individual activations and Isomap coordinate is observed (cor = 0.14; p = 0.03). It can be observed that as the stem of the STS shortens and the terminal “fork” opens, the activation size has a tendency to decrease.
The Moving Average shape of the STS and the reading activation variability. Top row from the left to the right of the Isomap axis, the fork leading to the two terminal branches occurs further from the parietal lobe; hence, the main part of the STS gets shorter. In addition, the two terminal branches are more developed toward the right of the axis. Middle row the forms at the extremities of the Isomap with the functional activation added (threshold 2.5). Bottom row the reading activation (blue) is plot onto the variability trait of the left STS. The average t-map of activation is plot with three different thresholds: T = 2, 2.5 and 3.5, to illustrate the evolution in the magnitude and location of the activation. It can be observed that the activation is less extensive toward the right of the axis. The activations at the two extremities shown in the middle row are superimposed to facilitate the comparison
Discussion
This study explores the connections between morphology and function by first characterizing the local essential anatomical variability traits, and then studying the activation patterns within this framework. Building upon the distribution of the subjects along the axis characterizing the anatomical trait, functional maps can be averaged across similar morphologies, leading to morphology-specific activation maps. Hence, observing the evolution of these functional maps along the anatomical trait axis led us to the understanding of several new links between anatomy and function that would have been difficult to reach using conventional methodologies.
The hand knob
The precise link between the hand knob and the hand motor area has been confirmed, despite the huge variability that exists regarding the localization of the knob along the central sulcus. Note that this large variability had never been noticed before the advent of our framework (Sun et al. 2012). It was also shown in this previous study that the left hand knob is located higher (dorsally) in right-handed subjects than in left-handed subjects. Hence, it was of interest to check whether the morphological hand knobs corresponding to the highest (most dorsal) positions were indeed embedding the hand functional motor area. This is what was observed here with a population of right-handed subjects in both hemispheres. The case of a left-handed population should be a future topic of interest.
We have discovered that the volume of the left hand motor activation in the contralateral hemisphere decreases from the one knob configuration at the left of the morphological trait axis to the two knobs configuration at the right. This evolution is consistent with the apparent decrease of the volume of the knob from the left to the right of the Isomap. However, we did not observe such a correlation for the right hand in the left hemisphere. This difference between the dominant and the non dominant hand calls for an evaluation with a population of left-handers (Grabowska et al. 2012).
The precentral reading area
The link between the left central sulcus shape and the localization of the precentral reading area is the most striking discovery reported in this paper. The localization of this functional area can be predicted from the folding pattern of the central sulcus even if it is not always immediately adjacent to the same sulcus. For the subpopulation with a double-knob configuration of the central sulcus, the reading activation maximum is located in a shallowing of the depth of the average precentral sulcus due to the frequent interruption of this sulcus. In return, for the subpopulation with a single-knob configuration, the reading activation maximum is adjacent to the motor bank of the central sulcus, at approximately the height of the lower knob of the double-knob subpopulation. Because of its localization, it is tempting to associate this classical precentral reading activation with the premotor parts of the larynx/phonation area (LPA) and of the lips/articulation area (LAA) (Brown et al. 2008; Pinel et al. 2012).
The interruption of the precentral sulcus by a horizontal gyrus connecting the precentral gyrus to the middle frontal gyrus varies in position and width. In the local averages of the precentral sulcus computed based on the central sulcus Isomap, this interruption appears only in the right part of the manifold (see Fig. 5). Therefore, the double-knob configuration is probably linked to a more stable localization and shape of the interruption in front of the lower knob. In the left hemisphere, dominant for language for most right-handed subjects, the central sulcus shape is shifted toward the double-knob configuration compared to the right hemisphere (Sun et al. 2012). Hence, a hypothesis explaining the colocalization of the central sulcus lower knob and of the precentral sulcus interruption is the differential development of this horizontal gyrus related to language. Indeed this gyrus hosts the premotor and primary motor larynx and lips areas (Brown et al. 2008, 2009) and several studies have shown leftward asymmetries of the motor activations induced by speech (Wildgruber et al. 1996; Funk et al. 2008). With that in mind, the lower knob of the central sulcus could be interpreted as a “lips and larynx knob”, whose presence seems to push the hand knob higher in the central sulcus and the premotor reading activation forward along the horizontal gyrus interrupting the precentral sulcus. This presence of the lower knob would be observed only beyond a given development of the lips and larynx areas more frequent in the left hemisphere because of a larger development of the language network.
Two typical brains at both sides of the central sulcus manifold. Top row the local average t-maps of motor (red) and reading (blue) activations onto the variability trait of the central sulcus. Middle row the local average t-maps of reading activation (blue) and of the precentral sulcus onto the variability trait of the central sulcus. Bottom row two typical subjects at the two extremities of the Isomap axis. For each subject, the left image shows the sulcus recognition in the whole left hemisphere while the right image corresponds to the motor gyrus. The right hand motor activation is in red, the premotor reading activation is in blue, central and precentral sulci in gray. For the subject on the left, the central sulcus has a single-knob configuration and the reading activation is in its motor bank. The lowest part of the precentral sulcus is elongated, which leads to a narrow interruption located not in front but above the reading activation. For the subject on the right, the central sulcus has a double-knob configuration and the reading activation is in the interruption of the precentral sulcus. The lowest part of the precentral sulcus is short, which leads to a wide interruption located directly in front of the lower knob
However, this transversal study with adults does not provide information about the time of apparition of the lowest knob that could either preexist to language or stem from language-induced plasticity. With this regard, longitudinal studies with infants will be required. Furthermore, an interesting future study will aim at clarifying the link between the morphological variability of the precentral area and the variability of the language pathways (Catani et al. 2005; Glasser and Rilling 2008; Aboitiz 2012). The focus will be on the bundles leading to the premotor ventral Brodmann area 6 that probably embeds the premotor reading area.
The auditory reading area
Regarding the superior temporal gyrus on the left hemisphere, the volume of the reading activation is found to be linked to the shape of the STS, particularly the length of the horizontal or main part of the STS where the secondary auditory areas involved in word comprehension (BA 21 in superior temporal gyrus and BA 22 in middle temporal gyrus) are located (Acheson and Hagoort 2013). The morphological variability trait found for the STS is a negative correlation between the length of the horizontal part and the development of the two posterior branches. It is tempting to associate this trait to a proxy for the relative sizes of BA 21–22 areas and BA 39–40 areas (angular gyrus and supramarginal gyrus). Further study providing specific activations within the region BA 39–40 would help to clarify that point.
Folding pattern and functional architecture
Three kinds of links between morphology and function have been considered in this paper:
-
1.
A morphological feature can be a landmark indicating the localization of a specific functional area (the hand knob location and maybe the lips and larynx knob when the lower knob is visible);
-
2.
A morphological variability trait can be correlated with the size of a functional area (central sulcus morphology versus left hand motor area, STS morphology versus auditory reading area);
-
3.
A morphological variability trait can indicate the localization of a functional area that is not directly linked to a specific sulcus (central sulcus morphology versus premotor reading area).
The two first points fit well with the idea that cortical architecture has an impact on the folding pattern, because of interactions between the folding process and the underlying architecture. Several hypotheses have been proposed to support such interactions (Toro 2012; Zilles et al. 2013): the existence of a protomap of the gyri related to the heterogeneous proliferation of radial cells controlled by morphogen-like mechanisms (Lefèvre and Mangin 2010; Borrell and Götz 2014), but also tension-based mechanisms leading the fiber bundles to sculpt the cortical surface (Van Essen 1997). It was also recently shown that cortical folds could simply arise from mechanical instability of a soft tissue that grows non-uniformly (Tallinen et al. 2014). Hence, heterogeneity of the tissue mechanical properties due to architecture could have an influence on the folding pattern.
The last kind of link illustrated by the premotor reading area shows an example of degree of freedom between the functional architecture and the folding pattern. There is a consensus about the link between the bottom of the central sulcus and the transition between primary sensory areas (Brodmann 3) and posterior primary motor area (Brodmann 4p) (Geyer et al. 1996; White et al. 1997) even if area 4p can have extensions into the lowest part of the central sulcus parietal bank (Rademacher et al. 2001). In return, the transition between the anterior primary motor area (Brodmann 4a) and the premotor area (Brodmann 6) does not show clear relationship with the precentral gyrus morphology. Moreover, this transition is not sharp and varies in localization from the top to the bottom of the precentral gyrus (White et al. 1997; Rademacher et al. 2001). It was found that this transition can be located in the posterior bank of the precentral sulcus in the superior third of the precentral gyrus, while it can be located in the anterior bank of the central sulcus elsewhere. Provided that the premotor reading activation has a constant localization inside Brodmann area 6, our finding could mean that the localization of this transition at the level of the lips and larynx areas is largely dependent on the subject but can be predicted from the shape of the central sulcus.
We hope that our framework could provide more of such observations, paving the way to the modeling of the relationship between architecture and folding. The different hypotheses in competition to explain the origin of the folding process could be compared relative to their capacity at predicting the nature of the degrees of freedom between morphological and functional variability. Primary sulci, secondary sulci and their interruptions induced by transverse buried gyri (Regis et al. 2005) are present at term birth. The folding patterns are a priori preserved during postnatal brain development. If the primal sketch of the cortical functional architecture is the driving force behind the folding dynamics, the link between architecture and folding pattern is probably locked during antenatal development. This one-way causality, however, is still an open issue because the deployment of the functional organization of the cortex stems from the complex interaction of genetic and activity-driven but also potentially mechanical processes (Foubet and Toro 2015). The residual stress induced by brain folding could modulate regionally cell proliferation, cell fate and cell shape, influence axonal guidance and even synaptic activity, thus affecting the final functional organization of the neocortex (Franze 2013; Foubet and Toro 2015). Hence, the folding pattern may influence the localization of functional areas with late maturation even after birth.
With regard to the central sulcus pattern, we have observed early sketches of the upper and lower knobs in healthy fetuses and early imaged preterm newborns as early as 32 weeks of gestational age (Dubois et al. 2008; Lefèvre et al. 2015). We plan to perform longitudinal analyses using images of the preterm newborns at term-equivalent age to assess to which extent the pattern can change significantly during the last months of gestation. Observing the presence or absence of some hand knobs sliding up the central sulcus during the growth of the lower knob would have some consequences on the meaning of the early sketch pattern. Future studies shall also focus on the stability of the folding patterns during aging, which has never been explored. Stable patterns could provide some proxy for specific architectural features that could modulate the consequences of aging and pathologies.
The image analysis pipeline called Morphologist (Perrot et al. 2011; Fischer et al. 2012) labels automatically 125 different sulci of the human cerebral cortex. Some of these sulci are too small to have a specific shape, but they can be merged into groups of sulci eligible for the computation of meaningful Isomaps. Hence, our current research program led us to split the cortical folding pattern into 20 such groups for each hemisphere to compute an atlas of essential variability traits inferred from thousands of brains (Mangin et al. 2015a). In addition to the isomap-based representations, this atlas will include a dictionary of patterns (Sun et al. 2007, 2008, 2009; Coulon et al. 2012) in the spirit of Ono’s atlas (Ono et al. 1990) but relying on non-supervised clustering performed with a massive number of brains. We eventually plan to provide information about the architectural value of these patterns using either functional imaging, following the methodology developed in this paper, connectivity (Lefranc et al. 2015), or cytoarchitectony (Weiner et al. 2014; Mangin et al. 2015a).
Our study reiterates the importance of understanding interindividual variability, instead of simply averaging it out. In a field where group analyses of activation maps largely rely on morphology-based spatial normalization, the configuration of the precentral reading activation may lead to loss of sensitivity. Therefore, our observation calls for further development of alternatives aiming at taking into account normalization weaknesses (Coulon et al. 2000; Thirion et al. 2007; Operto et al. 2012). When dealing with large databases, new statistical approaches embedding our manifold-based normalization could be of interest.
Traditionally, the study of anatomy-function connections is done through the discovery of anatomical landmarks or the construction of probability maps in standard spaces. The approach of landmark-finding is relatively difficult. Indeed, discovering an anatomical landmark that can reliably predict the underlining function is a rare accomplishment. The probability map approach is a relatively reliable method of characterization which can be done on any dataset. However, an intuitive and visually observable link between anatomy and function is missing.
In this study, the approach is to build a concrete model of inter-subject variability in anatomy first, and then study the functional variability based on anatomical variability. Instead of finding an anatomy-function landmark such as the hand knob, an “essential variability trait” is found first, such as the “moving hand knob” along the central sulcus, the “opening inferior frontal junction” and the “elongating main branch of STS”. Functional variability is then studied based on the essential anatomical variability trait identified. The utility of such an approach is that for any given region of interest, a framework of variability can be established to characterize the main or essential variability traits. Each individual of the population can be characterized within this framework. This could be of use for researchers and clinicians studying a particular functionality or pathology.
Conclusion and future work
This study provides firm evidence of the link between morphology and function across variability, using a simple and reliable method to characterize population variability.
A subject by subject study focusing on the link between sulcal shape and individual activation patterns would further shed some light on the relationship between morphology and function.
More regions of interest can be explored, regarding different functional activations and sub-populations. Different pathologies, different age groups can be studied. Other interesting descriptors of anatomical structure can be incorporated such as fiber bundles and architectonic information. Furthermore, a better understanding of the relationship between functional organization and morphology would shed some light on the developmental origin of interindividual variability.
References
Aboitiz F (2012) Gestures, vocalizations, and memory in language origins. Front Evol Neurosci 4:2
Acheson DJ, Hagoort P (2013) Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J Cogn Neurosci 25:1664–1677
Amiez C, Kostopoulos P, Champod AS, Petrides M (2006) Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci 26:2724–2731
Boling WW, Olivier A (2004) Localization of hand sensory function to the pli de passage moyen of Broca. J Neurosurg 101:278–283
Borrell V, Götz M (2014) Role of radial glial cells in cerebral cortex folding. Curr Opin Neurobiol 27:39–46
Boulenger V, Hauk O, Pulvermüller F (2009) Grasping ideas with the motor system: semantic somatotopy in idiom comprehension. Cereb Cortex 19:1905–1914
Broca P, Pozzi S (1888) Mémoires sur le cerveau de l’homme et des primates. C. Reinwald, Paris
Brown S, Ngan E, Liotti M (2008) A larynx area in the human motor cortex. Cereb Cortex 18:837–845
Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkeltaub P, Liotti M (2009) The somatotopy of speech: phonation and articulation in the human motor cortex. Brain Cogn 70:31–41
Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ (2009) An anatomical signature for literacy. Nature 461:983–986
Castro-Caldas A, Miranda PC, Carmo I, Reis A, Leote F, Ribeiro C, Ducla-Soares E (1999) Influence of learning to read and write on the morphology of the corpus callosum. Eur J Neurol 6:23–28
Catani M, Jones DK, ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Coulon O, Mangin JF, Poline JB, Zilbovicius M, Roumenov D, Samson Y, Frouin V, Bloch I (2000) Structural group analysis of functional activation maps. NeuroImage 11:767–782
Coulon O, Fonov V, Mangin J-F, Collins DL (2012) Atlas-based clustering of sulcal patterns—application to the left inferior frontal sulcus. In: Proceedings of IEEE ISBI, pp 426–429
Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L (2010) How learning to read changes the cortical networks for vision and language. Science 330:1359–1364
Dejerine J, Dejerine-Klumpke A (1901) Anatomie des centres nerveux. Rueff & Cie, Paris
Derrfuss J, Brass M, Neumann J, von Cramon DY (2005) Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp 25:22–34
Dubois J, Benders M, Cachia A, Lazeyras F, Leuchter RH-V, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Hüppi PS (2008) Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18:1444–1454
Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Le Goualher G, Bruce Pike G, Evans AC (2000) A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex 10:454–463
Fischer C, Operto G, Laguitton S, Perrot M, Denghien I, Rivière D, Mangin J-F (2012) Morphologist 2012: the new morphological pipeline of BrainVISA. In: Proceedings of HBM, Beijing
Foubet O, Toro R (2015) Mechanical morphogenesis and the development of neocortical organisation. bioRxiv. doi:10.1101/021311
Franze K (2013) The mechanical control of nervous system development. Development 140(15):3066–3077
Funk M, Lutz K, Hotz-Boendermaker S, Roos M, Summers P, Brugger P, Hepp-Reymond MC, Kollias SS (2008) Sensorimotor tongue representation in individuals with unilateral upper limb amelia. Neuroimage 43:121–127
Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, Roland PE (1996) Two different areas within the primary motor cortex of man. Nature 382:805–807
Glasser MF, Rilling JK (2008) DTI tractography of the human brain’s language pathways. Cereb Cortex 18:2471–2482
Grabowska A, Gut M, Binder M, Forsberg L, Rymarczyk K, Urbanik A (2012) Switching handedness: fMRI study of hand motor control in right-handers, left-handers and converted left-handers. Acta Neurobiol Exp (Wars) 72:439–451
Grosbras MH, Lobel E, Van de Moortele PF, LeBihan D, Berthoz A (1999) An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb Cortex 9:705–711
Haber LR, Haber RN (1982) Does silent reading involve articulation? Evidence from tongue twisters. Am J Psychol 95:409–419
Lefèvre J, Mangin J-F (2010) A reaction-diffusion model of human brain development. PLoS Comput Biol 6:e1000749
Lefèvre J, Germanaud D, Dubois J, Rousseau F, de Macedo Santos I, Angleys H, Mangin JF, Hüppi PS, Girard N, De Guio F (2015) Are developmental trajectories of cortical folding comparable between cross-sectional datasets of fetuses and preterm newborns? Cereb Cortex
Lefranc S, Sun Z, Roca P, Poupon C, Le Bihan D, Mangin JF, Rivière D (2015) Concomitant variability of the central sulcus morphology and adjacent connectivity patterns. In: Proceedings IEEE ISBI, pp 445–448
Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, Scifo P, Ochiai T, Brunelle F, Regis J (2004) A framework to study the cortical folding patterns. Neuroimage 23:S129–S138
Mangin J-F, Auzias G, Coulon O, Sun ZY, Rivière D, Régis J (2015a) Sulci as landmarks. In: Toga AW (ed) Brain mapping: an encyclopedic reference, vol 2. Academic Press: Elsevier, pp 45–52
Mangin J-F, Perrot M, Operto G, Cachia A, Fischer C, Lefèvre J, Rivière D (2015b) Sulcus identification and labeling. In: Toga AW (ed) Brain mapping: an encyclopedic reference. Academic Press: Elsevier, New York, pp 365–371
Ochiai T, Grimault S, Scavarda D, Roch G, Hori T, Riviere D, Mangin JF, Régis J (2004) Sulcal pattern and morphology of the superior temporal sulcus. Neuroimage 22:706–719
Ono M, Kubik S, Abarnathey CD (1990) Atlas of the cerebral sulci. Georg Thieme, New York
Operto G, Rivière D, Fertil B, Bulot R, Mangin J-F, Coulon O (2012) Structural analysis of fMRI data: a surface-based framework for multi-subject studies. Med Image Anal 16:976–990
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443
Perrot M, Rivière D, Mangin JF (2011) Cortical sulci recognition and spatial normalization. Med Image Anal 15:529–550
Petersson KM, Silva C, Castro-Caldas A, Ingvar M, Reis A (2007) Literacy: a cultural influence on functional left-right differences in the inferior parietal cortex. Eur J Neurosci 26:791–799
Petrides M (2012) The human cerebral cortex: an MRI atlas of the sulci and gyri in MNI stereotaxic space. Academic Press, New York
Pinel P, Thirion B, Meriaux S, Jobert A, Serres J, Le Bihan D, Poline JB, Dehaene S (2007) Fast reproducible identification and large-scale databasing of individual functional cognitive networks. BMC Neurosci 8:91
Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Le Bihan D, Poline JB, Bourgeron T, Dehaene S (2012) Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J Neurosci 32:817–825
Price CJ, Crinion JT, Macsweeney M (2011) A generative model of speech production in Broca’s and Wernicke’s areas. Front Psychol 2:237
Pulvermüller F, Fadiga L (2010) Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci 11:351–360
Rademacher J, Bürgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, Zilles K (2001) Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain 124:2232–2258
Regis J, Mangin J, Ochiai T, Frouin V, Riviere D, Cachia A, Tamura M, Samson Y (2005) “Sulcal root” generic model: a hypothesis to overcome the variability of the human cortex folding patterns. Neurol Med Chir 45:1–17
Sastre-Janer FA, Regis J, Belin P, Mangin J-F, Dormont D, Masure M-C, Remy P, Frouin V, Samson Y (1998) Three-dimensional reconstruction of the human central sulcus reveals a morphological correlate of the hand area. Cereb Cortex 8:641
Saygin ZM, Osher DE, Koldewyn K, Reynolds G, Gabrieli JD, Saxe RR (2012) Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci 15:321–327
Segal E, Petrides M (2012) The morphology and variability of the caudal rami of the superior temporal sulcus. Eur J Neurosci 36:2035–2053
Sun ZY, Rivière D, Poupon F, Régis J, Mangin JF (2007) Automatic inference of sulcus patterns using 3D moment invariants. Med Image Comput Comput Assist Interv MICCAI 10(Pt 1):515–522
Sun ZY, Riviere D, Duchesnay E, Thirion B, Poupon F, Mangin JF (2008) Defining cortical sulcus patterns using partial clustering based on bootstrap and bagging. In: 2008 IEEE international symposium on biomedical imaging. From Nano To Macro, vols 1–4, pp 1629–1632
Sun ZY, Perrot M, Tucholka A, Rivière D, Mangin J-F (2009) Constructing a dictionary of human brain folding patterns. Med Image Comput Comput Assist Interv MICCAI 12(Pt 2):117–124
Sun ZY, Klöppel S, Rivière D, Perrot M, Frackowiak R, Siebner H, Mangin J-F (2012) The effect of handedness on the shape of the central sulcus. Neuroimage 60:332–339
Tallinen T, Chung JY, Biggins JS, Mahadevan L (2014) Gyrification from constrained cortical expansion. Proc Natl Acad Sci USA 111:12667–12672
Tenenbaum J, de Silva V, Langford J (2000) A global geometric framework for nonlinear dimensionality reduction. Science 290:2319–2323
Thirion B, Pinel P, Tucholka A, Roche A, Ciuciu P, Mangin JF, Poline JB (2007) Structural analysis of fMRI data revisited: improving the sensitivity and reliability of fMRI group studies. IEEE Trans Med Imaging 26:1256–1269
Toro R (2012) On the possible shapes of the brain. Evol Biol 39:600–612
Van Essen DC (1997) A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385:313–318
Van Essen DC, Glasser MF (2013) In vivo architectonics: a cortico-centric perspective. Neuroimage 93(Pt 2):157–164
Van Essen DC, Ugurbil K (2012) The future of the human connectome. Neuroimage 62:1299–1310
Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S (1993) Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 3:79–94
Weiner KS, Golarai G, Caspers J, Chuapoco MR, Mohlberg H, Zilles K, Amunts K, Grill-Spector K (2014) The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage 84:453–465
Welker W (1988) Why does cerebral cortex fissure and fold? Cereb Cortex 8B:3–135
White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D (1997) Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cereb Cortex 7:18–30
Wildgruber D, Ackermann H, Klose U, Kardatzki B, Grodd W (1996) Functional lateralization of speech production at primary motor cortex: a fMRI study. Neuroreport 7:2791–2795
Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997) Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120(Pt 1):141–157
Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends Neurosci 36:275–284
Zlatkina V, Petrides M (2010) Morphological patterns of the postcentral sulcus in the human brain. J Comp Neurol 518:3701–3724
Acknowledgments
This work is partially funded by the French National Agency for Research (ANR-09-BLAN-0038-01 ‘BRAINMORPH’, ANR-12-JS03-001-01 ‘MODEGY’, ANR-14-CE30-0014 ‘APEX’) and the European FET Flagship project ‘Human Brain Project’ (SP2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z.Y., Pinel, P., Rivière, D. et al. Linking morphological and functional variability in hand movement and silent reading. Brain Struct Funct 221, 3361–3371 (2016). https://doi.org/10.1007/s00429-015-1106-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1106-8