Abstract
Striatal medium spiny neurons (MSNs) are contacted by glutamatergic axon terminals originating from cortex, thalamus and other regions. The striatum is also innervated by dopaminergic (DAergic) terminals, some of which release glutamate as a co-transmitter. Despite evidence for functional DA release at birth in the striatum, the role of DA in the establishment of striatal circuitry is unclear. In light of recent work suggesting activity-dependent homeostatic regulation of glutamatergic terminals on MSNs expressing the D2 DA receptor (D2-MSNs), we used primary co-cultures to test the hypothesis that stimulation of DA and glutamate receptors regulates the homeostasis of glutamatergic synapses on MSNs. Co-culture of D2-MSNs with mesencephalic DA neurons or with cortical neurons produced an increase in spines and functional glutamate synapses expressing VGLUT2 or VGLUT1, respectively. The density of VGLUT2-positive terminals was reduced by the conditional knockout of this gene from DA neurons. In the presence of both mesencephalic and cortical neurons, the density of synapses reached the same total, compatible with the possibility of a homeostatic mechanism capping excitatory synaptic density. Blockade of D2 receptors increased the density of cortical and mesencephalic glutamatergic terminals, without changing MSN spine density or mEPSC frequency. Combined blockade of AMPA and NMDA glutamate receptors increased the density of cortical terminals and decreased that of mesencephalic VGLUT2-positive terminals, with no net change in total excitatory terminal density or in mEPSC frequency. These results suggest that DA and glutamate signaling regulate excitatory inputs to striatal D2-MSNs at both the pre- and postsynaptic level, under the influence of a homeostatic mechanism controlling functional output of the circuit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The striatum is composed in large part of GABAergic projection neurons known as medium spiny neurons (MSNs). While MSNs in the dorsal aspect of the striatum are a component of the basal ganglia circuits involved in the control of voluntary movements (Mink 1996; Redgrave et al. 1999; Cisek and Kalaska 2010), MSNs in the ventral part of the striatum are a component of the mesolimbic system involved in the regulation of motivated behaviors (Wise and Bozarth 1987; Salamone and Correa 2012). Glutamatergic axon terminals arising mostly from the cortex and thalamus establish synapses on MSN dendrites (Bolam et al. 2000). While the former express the type 1 vesicular glutamate transporter (VGLUT1), the latter express the type 2 isoform VGLUT2. MSNs are also contacted by VGLUT3-positive terminals arising from striatal cholinergic interneurons (El Mestikawy et al. 2011). In addition, MSNs located in the ventral striatum receive VGLUT2-positive glutamatergic terminals arising from mesencephalic neurons including ventral tegmental area (VTA) dopamine (DA) neurons (Dal Bo et al. 2004; Mendez et al. 2008; Stuber et al. 2010; Tecuapetla et al. 2010; Yamaguchi et al. 2011; El Mestikawy et al. 2011). The striatum is also the principal target of VTA and substantia nigra pars compacta DA neurons, projecting respectively to ventral and dorsal aspects of this nucleus. DA, released from a dense plexus of axon terminals in this structure, is a crucial regulator of striatal activity, particularly at the level of the excitatory synapses that form on MSNs (Shen et al. 2008; Gerfen and Surmeier 2011; Surmeier et al. 2011).
Dorsal striatal MSNs send their axons along two efferent pathways: the striatonigral (direct) and striatopallidal (indirect) pathways (Smith et al. 1998; Wu et al. 2000; Bertran-Gonzalez et al. 2010). Striatonigral MSNs express high amounts of D1 DA receptor and very little amounts of D2, while striatopallidal MSNs show the opposite ratio (Gerfen et al. 1990; Lester et al. 1993; Surmeier et al. 1996; Ade et al. 2011). Recent work has shown that this segregation is established early in development (Thibault et al. 2013). Interestingly, depletion of DA induces a dramatic loss of dendritic spines and excitatory synapses on striatopallidal neurons, leaving striatonigral neurons practically intact (Day et al. 2006). This structural and functional plasticity in the striatum seems to be indirectly dependent on DA, since lesions of corticostriatal afferents prior to DA depletion prevents the spine loss in MSNs (Neely et al. 2007; Garcia et al. 2010). Such findings raise the possibility that the density of excitatory glutamate inputs to MSNs is regulated through complex DA-dependent homeostatic mechanisms. In favor of this possibility, chronic depolarization of cortical and striatal neurons in culture induces a decrease in spine density in MSNs that mimics the effect of DA depletion (Tian et al. 2010).
The mechanisms driving synaptogenesis in immature MSNs are still ill-defined. Predictably, co-culture with cortical neurons induces the appearance of dendritic spines and functional glutamatergic synapses in MSNs (Segal et al. 2003; Penrod et al. 2011). To the contrary, inhibition of cortical inputs to MSNs during early brain development induces a decrease in MSN spine density in vivo (Kozorovitskiy et al. 2012). DA afferents innervate the striatum at a very early stage of development (Specht et al. 1981; Voorn et al. 1988; Perrone-Capano and Di Porzio 2000), and there is evidence that the DA release machinery is already functional at birth (Ferrari et al. 2012). Although DA receptor activation has been reported to trigger the formation of filopodia and immature spines on developing MSN dendrites (Fasano et al. 2013), little is currently known about the role of DA in the establishment of excitatory inputs on MSNs.
In the present study, we used primary co-cultures to test the hypothesis that DA and glutamate interact to regulate the homeostasis of developing glutamatergic synapses on MSNs. Our results suggest that DA and glutamate regulate excitatory terminal and spine density, but that alterations in functional excitatory synaptic function is tightly regulated through a homeostatic mechanism.
Materials and methods
Animals
BAC transgenic drd2-GFP hemizygous mice (C57BL/6 background) (Gong et al. 2003) were crossed with wild-type (WT) C57BL/6 (Charles River, Saint-Constant, QC, Canada); 50 % of the offspring were drd2-GFP-positive and 50 % drd2-GFP-negative. For experiments using conditional VGLUT2 knock-out (KO) mice in DA neurons, hemizygous DAT-CRE transgenic mice (129/Sv/J background) (Zhuang et al. 2005) were mated with VGLUT2 flox/flox mice (129, C57Bl/6 background) (Tong et al. 2007) carrying the exon 2 surrounded by loxP sites. A breeding colony was maintained by mating DAT-CRE; VGLUT2 flox/+ mice with VGLUT2 flox/flox mice. As previously described (Bérubé-Carrière et al. 2009; Fortin et al. 2012), 25 % of mice lacked VGLUT2 in DA neurons (i.e., DAT-CRE; VGLUT2 flox/flox mice) and the littermates used as controls (CTRL) were DAT-CRE; VGLUT2 flox/+ mice (25 %). VGLUT3 KO (VGLUT3 −/−) mice, previously described (Gras et al. 2008), were obtained by crossing heterozygous VGLUT3 +/− mice (129/Sv C57/BL6). BAC transgenic drd2-GFP mice were mated with VGLUT3 KO mice resulting in 100 % of drd2-GFP-positive; VGLUT3 +/– mice. A breeding colony was maintained by mating drd2-GFP-positive; VGLUT3 +/– mice with VGLUT3 −/− mice. Twenty-five percent of the offspring from such mating were drd2-GFP-positive; VGLUT3 −/− mice and 25 % of the littermates used as controls (CTRL) were drd2-GFP-positive;VGLUT3 +/– mice.
Cell culture
Cultures were prepared according to a previously described protocol, with minor variations (Fasano et al. 2008). Briefly, dissociated neurons from the dorsal striatum (drd2-GFP-positive mice or drd2-GFP-positive; VGLUT3 −/− mice and their control littermates) and/or ventral mesencephalon (drd2-GFP-negative mice or DAT-CRE; VGLUT2 flox/flox mice and their control littermates) and/or cortex (drd2-GFP-negative) of P0–P2 animals were seeded on a monolayer of cortical astrocytes (C57BL/6) grown on poly-l-lysine-coated glass coverslips. For the four different culture conditions, the total seeded neuron density was always 240,000 cells/mL divided as follows (in cells/mL): 240,000 striatal cells for monocultures (mono); 140,000 striatal cells plus 100,000 mesencephalic cells for co-mesencephalic cultures (CoMs); 140,000 striatal cells plus 100,000 cortical cells for co-cortical cultures (CoCx); 40,000 striatal cells plus 100,000 mesencephalic cells and 100,000 cortical cells for triple cultures (3×). These ratios were selected to maximize the effect of mesencephalic and cortical neurons on MSN spinogenesis, while maintaining the same total neuron density across each culture type. All cultures were incubated at 37 °C in 5 % CO2 and maintained in Neurobasal medium enriched with 1 % penicillin/streptomycin, 1 % Glutamax, 2 % B-27 supplement and 5 % fetal bovine serum (Invitrogen). For chronic drug treatment conditions, the following drugs were added to the culture medium on days 1, 5, 9 and 13 in different combinations of appropriate vehicle: sulpiride (Sulp, D2-like receptor antagonist, 2 μM, Sigma-Aldrich), CNQX (AMPA receptor antagonist, 10 μM, Sigma-Aldrich) and CPP (NMDA receptor antagonist, 20 μM, Ascent Scientific). After 14 days in culture, cells were either used for electrophysiology or fixed in PBS solution with 4 % PFA for 30 min for immunostaining. Four different cultures were analyzed for each of the experiments comparing culture types, effects of DA receptor antagonists and glutamate receptor antagonists, while two cultures were used in each of the VGLUT2- and VGLUT3-KO experiments. For each culture, three to four independent coverslips were evaluated per condition.
Immunostaining and image analysis
Fixed cultures were permeabilized for 20 min in a PBS solution containing 0.1 % Triton X-100 and 0.02 % NaN3, and nonspecific binding sites were blocked in the same solution with an added 5 % of goat serum and 1 % BSA. All antibodies were used in a PBS solution containing 0.1 % Triton X-100 and 0.02 % NaN3, 5 % of goat serum and 0.5 % BSA. Primary antibodies were incubated overnight, while secondary antibodies were incubated for 1 h, all at room temperature. A rabbit anti-GFP primary antibody (1:5000, Abcam), detected with an Alexa 488 goat anti-rabbit secondary antibody (1:500, Molecular Probes), was used to visualize dendrites and spines from D2-MSNs. The type 1 vesicular glutamate transporter (VGLUT1) and VGLUT2 were labeled with guinea pig (1:5000, Millipore) and mouse (1:4000, Millipore) primary antibodies, respectively, coupled with Alexa 647 goat anti-guinea pig (1:500, Molecular Probes) and Alexa 546 goat anti-mouse (1:500, Molecular Probes). TH immunolabelling was performed with a mouse antibody (1:5000, Milipore). 5–10 MSNs per culture coverslip were randomly selected by a blind observer. Confocal z-stack images were acquired on a laser scanning confocal microscope (FV1000 MPE, Olympus) equipped with multi-argon and helium/neon lasers and a 60× oil-immersion objective. Spine density analysis was performed using NeuronStudio software [Mount Sinai School of Medicine, (Rodriguez et al. 2008)]. Dendrites were traced automatically but spines were identified manually: 3–6 dendrites per neuron (secondary dendrites, or segments at least 50 μm from the soma) of at least 30 μm in length were first randomly selected by a blind observer and straightened using ImageJ software (NIH) before analysis with NeuronStudio. As an index of maturity (Yoshihara et al. 2009), spine morphology was attributed manually between three general categories: stubby (mature, large head and no neck), mushroom (mature, large head and short neck) and thin (immature, small head and long neck). VGLUT1 and VGLUT2 excitatory terminal quantification was performed with ImageJ software by first applying a threshold value to obtain binary images, and then counting continuous signal puncta of diameters between 0.5 and 5 μm with the particle analysis function. Average puncta size in this analysis ranged from 0.75 to 1.5 μm across all different conditions (data not shown). For the assessment of VGLUT colocalization with PSD-95, 3× cultures were fixed in 4 % PFA and sucrose and washed 3 times with PBS without potassium. The same PBS was used to wash the cells during immunocytochemistry. Cells were permeabilized and nonspecific binding sites blocked. Cells were then incubated overnight with a primary antibody solution containing 1 % BSA, 0.1 % Triton X-100 in PBS, 5 % goat serum and 0.02 % NaN3. Cells were washed several times in PBS before incubation for 1 h with the appropriate Alexa-labeled secondary antibodies. The primary antibodies used where mouse PSD-95 (6G6 1C9) (1:2000, Pierce antibody) and either guinea-pig VGLUT1 (1:5000, AbCam) and rabbit GFP (1:5000, AbCam) or rabbit VGLUT2 (1:4000, Millipore) and chicken GFP (1:2000, Aves Lab). The secondary antibodies were Alexa 546 goat anti-mouse and either Alexa 647 goat anti-Guinea Pig and Alexa 488 goat anti-rabbit or Alexa 647 goat anti-rabbit and Alexa 488 goat anti-chicken (1:500, Molecular Probe). Confocal images were obtained with sequential scanning of single image planes, selecting randomly 10–15 MSNs per coverslip. We used a homemade macro in image J to perform the colocalisation analyses. After thresholding the GFP, VGLUT and PSD-95 signals, VGLUT terminals on GFP-positive MSNs were isolated using a mask of the GFP signal. Following this, PSD-95 signal present in these VGLUT terminals was isolated using a mask of the remaining VGLUT signal and the proportion of these VGLUT terminals expressing PSD-95 was quantified. Varicosities containing 3–100 pixels were considered as a putative axon terminal. We also excluded terminals with a circularity of 0.1 or less to remove any linear background signal.
Electrophysiology
Neuron culture coverslips were inserted in a recording chamber affixed to the stage of an inverted Nikon Eclipse TE-200 fluorescent microscope and gravitationally perfused with a physiological saline solution composed of (in mM): 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 6 sucrose, and 10 glucose. Whole-cell patch-clamp recordings were performed on GFP expressing cells using borosilicate pipettes (5.5–6.5 MΩ) filled with an intra-pipette solution composed of (in mM): 120 CsMeSO3, 1.1 EGTA, 4 ATP (Mg salt), 0.3 GTP (tris salt), 10 HEPES, 5 NaCl, and 10 TEA-Cl. The signal was amplified and controlled using a PC-505 patch-clamp amplifier (Warner), filtered at 2 kHz, digitized at 10 kHz and analyzed with pClamp10 software (Molecular Devices). Series resistance was compensated to approximately 70 % and membrane potential was always adjusted to account for the calculated liquid junction potential (≈10 mV). Miniature excitatory postsynaptic currents (mEPSCs) were recorded with the membrane voltage-clamped at −60 mV under perfusion of the physiological saline solution containing the sodium channel blocker tetrodotoxin (0.5 μM, Alomone Labs) and the GABAA receptor antagonist SR-95531 (2 μM, Sigma-Aldrich). Analysis of mEPSCs was performed with MiniAnalysis software (Synaptosoft Inc.).
Statistical analysis
Data are always presented as mean ± SEM. All ANOVA analyses were performed with Bonferroni’s multiple comparison test. The level of statistical significance was established at p < 0.05 in ANOVAs and two-tailed t tests performed with the Prism 5 software (GraphPad Software). Statistical outliers were excluded when they differed by more than two standard deviations above or below the mean.
Results
Competitive equilibrium in the establishment of glutamate synapses on cultured striatal neurons
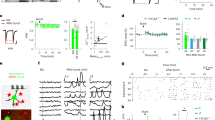
We first characterized a culture system containing striatal, mesencephalic and cortical neurons to test the hypothesis that mesencephalic DA neurons regulate the establishment of spines and excitatory synapses on D2-MSNs. To achieve this goal, we compared striatal monocultures (mono) to co-cultures of striatal neurons together with mesencephalic and/or cortical neurons (CoMs, CoCx and 3×, see “Materials and methods”) at 14 days in vitro. As expected, only CoMs and 3× cultures contained tyrosine hydroxylase (TH)-positive DA neurons (Fig. 1a). In terms of spine density, all co-cultures yielded D2-MSNs with more spines per 10 μm of dendritic length than in monocultures (p < 0.001 in one-way ANOVA; n = 76 neurons for mono, 78 for CoMs, 74 for CoCx and 78 for 3×; Fig. 1b, c). Interestingly, the CoCx condition clearly yielded the greatest spine density of all and was even significantly higher than the 3× condition, even though they both contained the same number of cortical neurons (Fig. 1c). When evaluating the distribution of spine type according to morphology more closely, we noticed that the CoCx condition produced the highest spine density for all spine types (Fig. 1d).
Comparison of four different cultures conditions. a Example of a TH-positive neuron (red) next to a GFP-positive D2-MSN (green) in a CoMs culture condition (scale bar 25 μm). b Example of GFP-labeled D2-MSN dendrites in different culture conditions (scale bar 2 μm). c Spine density analysis of the different culture conditions presented as the average number of spines per 10 μm of dendritic segment. d Average spine density according to morphology. e Assessment of VGLUT1 and VGLUT2 colocalization with PSD-95 (scale bar 2 μm). f Examples of a D2-MSN stained for GFP (green) with labeling for VGLUT1 (blue) and VGLUT2 (red) in the different culture conditions (scale bar 25 μm). g Quantification of the VGLUT1 signal presented as the number of VGLUT1-positive terminals per field in the different culture conditions. h Quantification of the VGLUT2 signal presented as the number of VGLUT2-positive terminals per field in the different culture conditions. i Quantification of the total excitatory terminals presented as the sum of VGLUT1- and VGLUT2-positive terminals per field in the different culture conditions. j Examples of voltage-clamp recordings of D2-MSNs in the different culture conditions. k mEPSC frequency analysis of D2-MSNs recorded in the different culture conditions. l mEPSC amplitude in the different culture conditions (*p < 0.05, **p < 0.01 and ***p < 0.001 in Bonferroni’s multiple comparison test after one- and two-way ANOVAs)
We next evaluated the presynaptic component of these excitatory synapses by quantifying the density of VGLUT1-positive axon terminals, arising from cortical glutamate neurons, and VGLUT2-positive terminals, arising from sub-cortical glutamatergic and DA neurons (El Mestikawy et al. 2011). VGLUT-positive axonal varicosities were likely to reflect the presence of synaptic junctions because more than 80 % of VGLUT1 or VGLUT2-positive terminals were colocalized with the postsynaptic marker PSD-95 in 3× cultures (Fig. 1e). We expected that in CoMs cultures, all excitatory terminals would be VGLUT2-positive, while in CoCx cultures, all excitatory terminals would be VGLUT1-positive. We also hypothesized that in 3× cultures, mesencephalic and cortical neurons should both innervate striatal neurons, leading to a higher total density of excitatory terminals. As expected, only VGLUT1-positive terminals and little if any VGLUT2-positive terminals were detected in CoCx cultures (Fig. 1f, g). Similarly, in CoMs cultures, only VGLUT2-positive terminals and little if any VGLUT1-positive terminals were detected (Fig. 1f, g). Surprisingly, although in 3× cultures both VGLUT1 and VGLUT2-positive terminals were detected, the density of VGLUT1-positive terminals was lower than in the CoCx condition (p < 0.001 in one-way ANOVA; n = 74 fields for mono, 75 for CoMs, 92 for CoCx and 66 for 3×; Fig. 1g). Likewise, in 3× cultures, the density of VGLUT2-positive terminals was lower than in CoMs cultures (p < 0.001 in one-way ANOVA; Fig. 1h). Moreover, the summation of all VGLUT1 and VGLUT2-positive terminals per field revealed that in each co-culture condition, a strikingly identical maximal density of glutamatergic terminals was established (p < 0.001 in one-way ANOVA; Fig. 1i). This result is suggestive of a cap in the maximal density of glutamatergic synapses on D2-MSNs and of a competitive equilibrium between VGLUT1- and VGLUT2-positive terminals on these striatal neurons.
Finally, to provide a functional readout of synapse density and activity, we recorded from D2-MSNs and analyzed the frequency and amplitude of spontaneous glutamate-mediated mEPSCs (Fig. 1j). The frequency of mEPSCs in D2-MSNs was significantly higher in the CoMs, CoCx and 3× conditions compared to the Mono condition, but not different between themselves (p < 0.001 in one-way ANOVA; n = 13 neurons for mono, 12 for CoMs, 13 for CoCx and 10 for 3×; Fig. 1j, k). In terms of mEPSC amplitude, there was no significant difference between the groups (p > 0.05 in one-way ANOVA; n = 5 neurons for mono, 12 for CoMs, 13 for CoCx and 10 for 3×; Fig. 1l). These results are compatible with the possible existence of a homeostatic mechanism maintaining a constant excitatory drive on D2-MSNs, a parameter that under our experimental conditions was more closely related to excitatory terminal density than with spine density.
Contribution of glutamatergic terminals established by DA neurons
Considering, on the one hand, that a subset of mesencephalic DA neurons express VGLUT2 (Dal Bo et al. 2004; Mendez et al. 2008) and contribute to the glutamatergic innervation of MSNs (Bérubé-Carrière et al. 2009; Stuber et al. 2010; Tecuapetla et al. 2010) and on the other hand, that the mesencephalon also contains other non-DAergic VGLUT2-expressing glutamate neurons (Yamaguchi et al. 2007; Mendez et al. 2008; Dobi et al. 2010; Yamaguchi et al. 2011), we sought to determine the importance of glutamate released specifically by DA neurons in D2-MSN synaptogenesis. To test the hypothesis of a contribution of this innervation, we prepared CoMs cultures using cKO mice in which the VGLUT2 gene was selectively deleted in DA neurons. We thus compared CoMs cultures containing striatal neurons from drd2-GFP-positive animals and mesencephalic neurons from either control VGLUT2 f/+;DAT-cre (CTRL) or conditional knock-out VGLUT2 f/f;DAT-cre (cKO) animals (Fig. 2a). A significant reduction of the density of VGLUT2-positive terminals was detected in cKO cultures (p < 0.001 in two-tailed t test; n = 180 fields for CTRL and 189 for cKO; Fig. 2b). This was accompanied by a decrease in the density of spines in D2-MSNs of CoMs cKO cultures in comparison to CTRL (p < 0.05 in two-tailed t test; n = 55 neurons for CTRL and 55 for cKO, Fig. 2c, d), with a similar tendency consistent through every spine category (Fig. 2e). At the functional level, this decrease in terminal and spine density was not accompanied by significant change in mEPSC frequency (p > 0.05 in two-tailed t test, n = 7 neurons for CTRL and 8 for cKO, Fig. 4f, g) or amplitude (p > 0.05 in two-tailed t test, n = 7 neurons for CTRL and 8 for cKO, Fig. 2h). These results show that although glutamate released by mesencephalic DA neurons on D2-MSNs has the potential to contribute to spine formation during the development of striatal synaptic circuits, it does not affect basal mEPSC frequency, arguing in favor of a homeostatic mechanism regulating synaptic excitation of D2-MSNs.
Analysis of CoMs cultures with a conditional KO of VGLUT2 in DA neurons. a Examples of a D2-MSN stained for GFP (green) with labeling for VGLUT2 (red) in the different culture conditions (scale bar 25 μm). b Quantification of the VGLUT2 signal presented as the number of VGLUT2-positive terminals per field in the different culture conditions. c Example of GFP-labeled D2-MSN dendrites in different culture conditions (scale bar 2 μm). d Spine density analysis of the different culture conditions presented as the average number of spines per 10 μm of dendritic segment. e Average spine density according to morphology. f Examples of voltage-clamp recordings of D2-MSNs in the different culture conditions. g mEPSC frequency analysis of D2-MSNs recorded in the different culture conditions. h mEPSC amplitude in the different treatment conditions (*p < 0.05, **p < 0.01 and ***p < 0.001 in two-tailed t tests and two-way ANOVAs)
Contribution of glutamatergic terminals established by striatal cholinergic neurons
Although the frequency of mEPSCs and the density of detected VGLUT1- or VGLUT2-positive terminals were close to zero in striatal monocultures, a small density of spine-like protrusions were detected in these cultures. Considering that striatal cholinergic interneurons have been shown to express VGLUT3 and mediate a form of glutamatergic transmission on MSNs (Gras et al. 2002, 2005; Ding et al. 2010; Higley et al. 2011; El Mestikawy et al. 2011), we evaluated the occurrence of VGLUT3-positive neurons in monocultures and evaluated spine density in mono cultures prepared from VGLUT3 KO mice. Out of 40 microscopic fields examined in CTRL littermates (drd2-GFP-positive, VGLUT3 +/−), only 2 contained VGLUT3-positive terminals. In 40 fields examined in cultures prepared from KO mice (drd2-GFP-positive, VGLUT3 −/−), we could not find any detectable VGLUT3 signal (Fig. 3a). Both culture conditions produced identical D2-MSN spine densities (p > 0.05 in two-tailed t test; n = 46 neurons for CTRL and 40 for KO, Fig. 3b, c), with a similar distribution among the different morphological subtypes (Fig. 3d). Electrophysiological recordings showed no detectable mEPSCs in either culture condition (data not shown), as expected by the very limited amount of VGLUT3 terminals present and the random selection of D2-MSNs for the recordings. Overall, these results suggest that under our experimental conditions, probably related to the low density of cholinergic interneurons, VGLUT3-containing glutamatergic terminals emanating from such neurons play a negligible role in regulating D2-MSN spine density and glutamatergic synaptogenesis.
Analysis of striatal cultures (mono) prepared from VGLUT3 KO mice. a Examples of labeling for VGLUT3 in the different culture conditions (scale bar 25 μm). b Example of GFP-labeled D2-MSN dendrites in different culture conditions (scale bar 2 μm). c Spine density analysis of the different culture conditions presented as the average number of spines per 10 μm of dendritic segment. d Average spine density according to morphology (*p < 0.05, **p < 0.01 and ***p < 0.001 in two-tailed t tests and two-way ANOVAs)
DA receptor activity is not necessary for excitatory synapse formation in D2-MSNs
Based on the previous finding that DA signaling stimulates the formation of immature spines in developing MSNs in monoculture (Fasano et al. 2013), we hypothesized that DA neurons also contribute to functional synaptogenesis in mature mixed cultures through the stimulation of D2-type DA receptors located on D2-MSNs. To test this hypothesis, we exposed 3× cultures chronically to the D2 receptor antagonist sulpiride (Sulp) (2 µM). D2 receptor blockade failed to reduce D2-MSN spine density (p > 0.05 in two-tailed t test; n = 76 neurons for CTRL and 76 for Sulp; Fig. 4a, b). This absence of effect was consistent through every morphological spine category (Fig. 4c).
Analysis of 3× cultures after chronic treatment with a D2 receptor antagonist (sulpiride). a Example of GFP-labeled D2-MSN dendrites in different treatment conditions (scale bar 2 μm). b Spine density analysis of the different treatment conditions presented as the average number of spines per 10 μm of dendritic segment. c Average spine density according to morphology. d Examples of a D2-MSN stained for GFP (green) with labeling for VGLUT1 (blue) and VGLUT2 (red) in the different treatment conditions (scale bar 25 μm). e Quantification of the VGLUT1 signal presented as the number of VGLUT1-positive terminals per field in the different treatment conditions. f Quantification of the VGLUT2 signal presented as the number of VGLUT2-positive terminals per field in the different treatment conditions. g Quantification of the total excitatory terminals presented as the sum of VGLUT1- and VGLUT2-positive terminals per field in the different treatment conditions. h Examples of voltage-clamp recordings of D2-MSNs in the different treatment conditions. i mEPSC frequency analysis of D2-MSNs recorded in the different treatment conditions. j mEPSC amplitude in the different treatment conditions (*p < 0.05, **p < 0.01 and ***p < 0.001 in two-tailed t tests and two-way ANOVAs)
Despite the fact that spine density was unchanged by D2 receptor blockade, Sulp-treated cultures displayed a higher density of VGLUT1-positive terminals (p < 0.001 in two-tailed t test; n = 74 fields for CTRL and 77 for Sulp; Fig. 4d, e). A similar increase in VGLUT2-positive terminals was also induced by chronic D2 receptor blockade (p < 0.001 in two-tailed t test; Fig. 4f). The sum of all VGLUT1 + VGLUT2-positive excitatory terminals was thus increased by Sulp (p < 0.001 in two-tailed t test; Fig. 4g). However, this increase in excitatory presynaptic terminals was not accompanied by a significant change in mEPSC frequency (n = 11 neurons for CTRL and 12 for Sulp; p > 0.05 in one-way ANOVA; Fig. 4h, i) or amplitude (n = 11 for CTRL and 12 for Sulp; p > 0.05 in one-way ANOVA; Fig. 4j). Our results suggest that chronic D2 receptor antagonism, although without effect on spine density, perturbs the regulation of axon terminal density, perhaps by inducing the sprouting of immature, non-functional release sites. Under such conditions, the lack of change in mEPSC frequency further argues in favor of the possibility of a homeostatic mechanism controlling D2-MSN excitation.
Critical role of ionotropic glutamate receptors in striatal synapse formation
We next aimed to determine the role of ionotropic glutamate receptors in regulating spine formation on D2-MSNs. Based on previous reports showing that the establishment of cortical synapses on striatal neurons is activity-dependent (Segal et al. 2003; Penrod et al. 2011), we hypothesized that ionotropic glutamate receptor activation is required for spine and synapse formation. Thus, we performed chronic treatments of the 3× cultures with the AMPA receptor antagonist CNQX (10 µM) and/or the NMDA receptor antagonist CPP (20 µM). Our data show that although CNQX or CPP alone did not alter spine density, combined blockade of AMPA and NMDA receptors significantly reduced spine density in D2-MSNs (p < 0.001 in one-way ANOVA; n = 59 neurons for CTRL, 58 for CNQX, 58 for CPP and 58 for CNQX + CPP, Fig. 5a, b). Once again, the same difference was reflected by a similar pattern across all different spine morphology types (Fig. 5c).
Analysis of 3× cultures after chronic treatments with ionotropic glutamate receptor antagonists. a Example of GFP-labeled D2-MSN dendrites in different treatment conditions (scale bar 2 μm). b Spine density analysis of the different treatment conditions presented as the average number of spines per 10 μm of dendritic segment. c Average spine density according to morphology. d Examples of a D2-MSN stained for GFP (green) with labeling for VGLUT1 (blue) and VGLUT2 (red) in the different treatment conditions (scale bar 25 μm). e Quantification of the VGLUT1 signal presented as the number of VGLUT1-positive terminals per field in the different treatment conditions. f Quantification of the VGLUT2 signal presented as the number of VGLUT2-positive terminals per field in the different treatment conditions. g Quantification of the total excitatory terminals presented as the sum of VGLUT1- and VGLUT2-positive terminals per field in the different treatment conditions. h Examples of voltage-clamp recordings of D2-MSNs in the different treatment conditions. i mEPSC frequency analysis of D2-MSNs recorded in the different treatment conditions. j mEPSC amplitude in the different treatment conditions (*p < 0.05, **p < 0.01 and ***p < 0.001 in Bonferroni’s multiple comparison test after one- and two-way ANOVAs)
Paradoxically, the combined blockade of AMPA and NMDA receptors caused a substantial increase in the density of VGLUT1-positive terminals compared to CTRL (p < 0.01 in one-way ANOVA; n = 88 fields for CTRL, 85 for CNQX, 91 for CPP and 86 for CPP + CNQX; Fig. 5d, e), while all treatment groups showed significantly less VGLUT2-positive terminals compared to the CTRL condition (p < 0.001 in one-way ANOVA; Fig. 5f). Interestingly, the increase in VGLUT1-positive terminals and the parallel decrease in VGLUT2-positive terminals amounted to a lack of significant difference in the total density of VGLUT1- or VGLUT2-positive terminals for all of the drug treatments (p > 0.05 in one-way ANOVA, Fig. 5g). Compatible with the lack of change in total VGLUT1 and VGLUT2 terminals, the treatment protocols produced no significant change in mEPSC frequency (p > 0.05 in one-way ANOVA; n = 11 neurons for CTRL, 10 for CNQX, 11 for CPP and 10 for CNQX + CPP; Fig. 5h, i). However, mEPSC amplitude was significantly higher in the CNQX + CPP group compared to all others (p < 0.01 in one-way ANOVA; n = 11 neurons for CTRL, 10 for CNQX, 11 for CPP and 10 for CNQX + CPP; Fig. 5j). Taken together, our data suggest that ionotropic glutamate receptors play an important role in regulating spinogenesis and axon terminal development in striatal circuitry. However, the lack of effect on mEPSC frequency and the balanced regulation of VGLUT1- and VGLUT2-positive terminals induced by glutamate receptor blockade provide further evidence for global homeostatic regulation of excitatory inputs on D2-MSNs.
Discussion
In the present study, we used primary co-cultures to test the hypothesis that DA and glutamate interact to regulate the homeostasis of developing glutamatergic synapses on MSNs expressing the DA D2 receptor. Our first primary finding is that co-culture of D2-MSNs with mesencephalic neurons increased dendritic spine, VGLUT2-positive axon terminal density and functional synapse formation on D2-MSNs when compared to striatal monocultures. The effect on spine density was due in part to expression of VGLUT2 by double-phenotype DA neurons. Likewise, we also found that co-culture of D2-MSNs with cortical neurons produced a large increase in spines and in the density of VGLUT1-positive glutamatergic axon terminals. Strikingly, the addition of both mesencephalic and cortical neurons produced the same total density of synaptic terminals, compatible with a mechanism capping excitatory synaptic density. Moreover, although there was a significant difference in spine density across culture types, this did not translate to a different functional excitatory drive on D2-MSNs, as estimated by mEPSC frequency. Although other interpretations are possible, one possibility is that a homeostatic mechanism regulates the functional output of glutamatergic synapses on D2-MSNs. Secondly, our data suggest that while glutamate released by VGLUT2-expressing DA neurons has the potential to contribute to D2-MSN spinogenesis, glutamate from VGLUT3-expressing cholinergic interneurons of the striatum does not play a significant role under our culture conditions, perhaps due to the low density of such neurons. Our third major finding is that although blockade of D2 receptors perturbed this homeostatic regulation of axon terminal density and lead to an increase in the density of cortical and mesencephalic glutamatergic terminals, it did not change MSN spine density or mEPSC frequency. These results nonetheless argue for the existence of a negative regulation of glutamate inputs by D2 receptors. Finally, we found that although combined blockade of AMPA and NMDA glutamate receptors reduced the formation of spines, it increased the density of VGLUT1-positive cortical terminals and decreased that of VGLUT2-positive mesencephalic terminals, with no net change in total excitatory terminal density or in mEPSC frequency. Globally, one interpretation of our findings is that a tight homeostatic mechanism controls functional glutamate inputs to D2-MSNs.
Effect of cortical and mesencephalic neurons on spine and synapse formation
We found that adding both mesencephalic and cortical glutamatergic neurons to D2-MSNs did not produce an additive effect on spine density, but rather induced a spine density equivalent to that of cultures including mesencephalic neurons alone. We also found that in such triple cultures, the total density of glutamatergic axon terminals was the same as in co-cultures including mesencephalic or cortical neurons alone. We also observed a mismatch between axon terminal and spine density in these different culture models: spine density was lower in triple cultures and in co-cultures with mesencephalic neurons than in co-cultures with cortical neurons; however, excitatory terminal density was the same across all co-culture conditions. Notably, in this case, mEPSC frequency was better correlated with axon terminal density than with spine density. Considering the previous observation that cortical VGLUT1-positive terminals almost always form synapses on MSN dendritic spines, while thalamic VGLUT2-positive excitatory terminals establish a substantial proportion (20–30 %) of their synapses on dendritic shafts (Moss and Bolam 2008; Smith et al. 2009), it may be hypothesized that mesencephalic neurons establish many of their VGLUT2-positive terminals on dendritic shafts and not on spines. This could explain why synaptic activity in the different culture conditions examined here correlated better with the total excitatory terminal density rather than with spine density. It will be necessary to perform ultrastructural analyses of these VGLUT1 and VGLUT2 synapses to test this hypothesis. A larger number of recordings might also increase the statistical power to detect small differences in mEPSCs frequency between conditions.
As a technical limitation, it should be emphasized that the relative proportion of mesencephalic, striatal and cortical neurons used in the co-cultures of the present study do not reflect the proportions of neurons part of the actual striatal circuitry in vivo. In particular, the proportion of mesencephalic neurons used was likely much higher than that found in vivo. The ratios used were selected, based on preliminary data, to maximize the likelihood of detecting the impact of DA neurons on MSN spine density. To maintain a constant total cell density across all culture conditions, the amount of striatal neurons in the co-cultures used in the present study was adjusted such that the ratio of cortical or mesencephalic neurons to striatal neurons was higher in 3× cultures than in the CoCx or CoMs cultures. In theory, this should have led to an increased potential for excitatory synapse formation per MSN in the 3× cultures. The fact that total excitatory terminal density was the same in 3× cultures compared to CoCx or CoMs suggests that this was not a major problem and actually argues in favor of a cap in the total excitatory synapse load per unit of D2-MSN dendrite.
We found that the combined blockade of AMPA and NMDA glutamate receptors greatly reduced spine density. This observation is compatible with previous work showing that cortical glutamate neurons and their activity play an important role in excitatory synaptogenesis in striatal cultures (Segal et al. 2003; Penrod et al. 2011) and in vivo (Kozorovitskiy et al. 2012). Surprisingly, we found that spine loss was not accompanied by a reduction in the density of excitatory axon terminals: VGLUT1-positive axon terminals increased in density, while VGLUT2-positive axon terminals decreased in density, resulting in a constant total density of excitatory terminals. It thus seems that AMPA and NMDA blockade induces a complex combination of pre- and postsynaptic effects. Presynaptically, there is evidence showing increased axonal sprouting in hippocampal neurons treated with NMDA receptor antagonists (McKinney et al. 1999), which is in line with our present results. Our observation of a decrease in the density of VGLUT2-positive terminals is also compatible with previous work showing that stimulation of both AMPA and NMDA receptors in DAergic neurons promotes axonal growth and branching (Schmitz et al. 2009). Again, ultrastructural analysis would be beneficial to see if glutamatergic receptor antagonists induce a redistribution of synapses from spines to dendritic shafts, which could account for the observed discrepancy between spine and axon terminal density. Further studies would also be needed to explore the mechanism linking glutamate receptor blockade to spine and terminal plasticity. Changes in intracellular calcium could for example be involved. Chronic depolarization with KCl was previously shown to cause MSN spine pruning in a culture system similar to the one used here, through the involvement of L-type calcium channels (Tian et al. 2010). A change in the intrinsic excitability of D2-MSNs could also have resulted from chronic blockade of AMPA and NMDA receptors; such a change in excitability has previously been shown to be associated with structural and functional plasticity in MSNs (Day et al. 2008; Azdad et al. 2009).
Role of dopamine
DA represents a crucial modulator of glutamatergic synapses in the mature striatum (Shen et al. 2008; Gerfen and Surmeier 2011; Surmeier et al. 2011) and DAergic signaling is involved in mature MSN spine maintenance (Ingham et al. 1989, 1993; Stephens et al. 2005; Zaja-Milatovic et al. 2005; Neely et al. 2007; Villalba et al. 2009; Garcia et al. 2010), particularly in D2-MSNs, as demonstrated in a mouse model of Parkinson’s disease (Day et al. 2006). Chronic treatment with haloperidol, a specific D2R antagonist used as an antipsychotic, was shown to reduce striatal dendritic spine density (Kelley et al. 1997). Whether DA receptors localized on D2-MSNs directly or indirectly regulate spines is not completely clear at the present time. Some evidence favors an increase of corticostriatal glutamate transmission as the necessary intermediate in DA depletion-induced spine loss in the striatum (Neely et al. 2007; Garcia et al. 2010). It has also been proposed that changes in spine density represent a homeostatic adaptation resulting from an increase in the intrinsic excitability of D2-MSNs following D2 receptor blockade or loss of DA inputs (Day et al. 2008; Azdad et al. 2009; Tian et al. 2010). Whether DA-dependent regulation of spines on D2-MSNs occurs during development has not received much attention. It is interesting to note that DAergic projections to the striatum are already present and active at birth in rodents (Specht et al. 1981; Voorn et al. 1988; Perrone-Capano and Di Porzio 2000; Ferrari et al. 2012), preceding the postnatal establishment of glutamatergic excitatory connections (Tepper et al. 1998; Sharpe and Tepper 1998). A recent study showed that DA signaling can trigger the formation of filopodia and immature spines on developing MSN dendrites (Fasano et al. 2013). Our present finding of a lack of change in spine density following chronic D2 receptor blockade argues against the hypothesis that DA receptor activation directly promotes spine formation and excitatory synapse formation on D2-MSNs. Rather, D2 receptor blockade lead to an increase in the density of excitatory VGLUT1- and VGLUT2-positive terminals. This observation suggests that D2 receptors perhaps negatively regulate the development of excitatory inputs to the striatum, which is in line with another study showing that chronic activation of the D2 receptor can decrease the formation of DAergic and glutamatergic synapses established by cultured DA neurons (Fasano et al. 2010). Our results are compatible with previous work showing that the selective elimination of DAergic neurons in models of Parkinson’s disease causes an increase in striatal excitatory terminals (Ingham et al. 1993; Raju et al. 2008; Villalba and Smith 2011). It has also previously been shown that pharmacological blockade or genetic deletion of the D2 receptor both increase the density of DAergic terminals in the striatum (Parish et al. 2001, 2002; Tinsley et al. 2009). However, contrary to our expectation, the increase in axon terminal density induced by chronic D2 receptor blockade was not accompanied by any change in spine density or mEPSC frequency. This finding represents an exception to the otherwise relatively strong relationship found in the present work between excitatory terminal density and synaptic activity. Although speculative, this exception could possibly be due to the ability of chronic D2 receptor blockade in our developing culture system to perturb the normal equilibrium between axon terminal and spine development through complex pre- and postsynaptic effects, leading to the accumulation of superfluous terminals that cannot establish functional contacts with spines. The lack of change in mEPSC frequency in this experiment argues in favor of the possibility that it is really the functional excitatory synaptic drive on D2-MSNs that is the subject of homeostatic regulation and not the necessarily the density of terminals or spines. It is possible that DA depletion-dependent striatal spine loss requires a fully mature network to occur. Additional anatomical and physiological data will be required to solve this paradox. Pharmacological treatments in the co-culture system used here have the obvious caveat of being unable to distinguish between presynaptic, postsynaptic or more complex pre- and postsynaptic interactive effects; the selective perturbation of DA receptors on pre- and postsynaptic elements would be essential to define the specific mechanisms involved.
Contribution of mixed phenotype DA neurons and cholinergic neurons
After conditional deletion of the VGLUT2 gene in DA neurons, the density of VGLUT2-positive axon terminals and the density of spines in D2-MSNs were both reduced, demonstrating for the first time that the glutamatergic co-phenotype of DA neurons has the potential to contribute to early excitatory synapse formation on MSNs. This observation is compatible with the recent demonstration conditional KO of VGLUT2 in DA neurons leads to a reduced density of VGLUT2-positive axon terminals in the ventral striatum (Fortin et al. 2012). Interestingly, VGLUT2 expression by DAergic neurons is more abundant at early prenatal stages of development and after 6-hydroxy-DA lesions of DAergic nuclei (Dal Bo et al. 2008; Mendez et al. 2008; Fortin et al. 2012). Furthermore, VGLUT2 conditional KO mice display behavioral changes and altered responses to psychostimulants, which are characteristic of abnormalities in basal ganglia function (Birgner et al. 2010; Hnasko et al. 2010). Although there is evidence for a purely glutamatergic VGLUT2-expressing population in the ventral tegmental area (Dobi et al. 2010; Yamaguchi et al. 2007, 2011), we show that a significant amount of VGLUT2-positive terminals are eliminated in the conditional KO CoMs cultures. However, since this decrease in VGLUT2 terminals did not induce a similar reduction in mEPSC frequency, it would seem that the remaining purely glutamatergic mesencephalic neurons also have an important and plastic contribution to the excitatory inputs to D2-MSNs. Thus, in the model used for the present study, the increase in synaptogenesis attributed to the addition of mesencephalic neurons is quite possibly the result of both DA neurons and purely glutamatergic neurons. In vivo, since DAergic fibers innervate the striatum early in development (Specht et al. 1981; Voorn et al. 1988; Perrone-Capano and Di Porzio 2000; Ferrari et al. 2012), it is conceivable that the glutamate released by mesostriatal afferents produces a priming effect on spine and synapse formation, until cortical and thalamic glutamatergic afferents subsequently generate the bulk of striatal synaptogenesis. It would thus be of interest to examine the origin and anatomical distribution of VGLUT2-positive axon terminals in the striatum of late embryonic and neonatal mice. It would also be important to further examine the impact of the conditional deletion of the VGLUT2 gene in striatonigral DA neurons on striatal excitatory synaptogenesis in vivo.
We found that deletion of the VGLUT3 gene, normally expressed in striatal cholinergic interneurons did not affect D2-MSN synaptogenesis under our experimental conditions. Although we did not quantify the number of cholinergic interneurons present in our cultures, it was apparent that the density of such neurons was very low, perhaps explaining the limited density of VGLUT3-positive glutamatergic terminals detected and the very low basal mEPSC frequency in striatal monocultures. It would nonetheless be of interest to further examine the contribution of glutamatergic terminals established by striatal cholinergic neurons to striatal excitatory synaptogenesis in vivo. Despite the fact that VGLUT3-dependent excitatory transmission has been demonstrated in the striatum (Higley et al. 2011), its functional and developmental roles still remains elusive.
In summary, our data suggest that homeostatic mechanisms tightly regulate the functional output of excitatory connections to D2-MSNs. Furthermore, we find that the early establishment of VGLUT2-positive axon terminals by DA neurons may facilitate early spinogenesis in this MSN population, potentially priming synapse formation for the subsequent establishment of cortical and thalamic inputs. Our results suggest that contrarily to glutamate, DA itself has a dispensable role in excitatory synaptogenesis on D2-MSNs; however, DA negatively regulates the density of both cortical and subcortical glutamate inputs to D2-MSNs by acting through D2 receptors. Further work will be required to validate these conclusions in vivo.
References
Ade KK, Wan Y, Chen M et al (2011) An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci 5:32. doi:10.3389/fnsys.2011.00032
Azdad K, Chàvez M, Don Bischop P et al (2009) Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion. PLoS One 4:e6908. doi:10.1371/journal.pone.0006908
Bertran-Gonzalez J, Hervé D, Girault J-A, Valjent E (2010) What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. doi:10.3389/fnana.2010.00136
Bérubé-Carrière N, Riad M, Dal Bo G et al (2009) The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol 517:873–891. doi:10.1002/cne.22194
Birgner C, Nordenankar K, Lundblad M et al (2010) VGluT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci USA 107:389–394. doi:10.1073/pnas.0910986107
Bolam JP, Hanley JJ, Booth PA, Bevan MD (2000) Synaptic organisation of the basal ganglia. J Anat 196(Pt 4):527–542
Cisek P, Kalaska JF (2010) Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33:269–298. doi:10.1146/annurev.neuro.051508.135409
Dal Bo G, St-Gelais F, Danik M et al (2004) Dopamine neurons in culture express VGluT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem 88:1398–1405
Dal Bo G, Bérubé-Carrière N, Mendez JA et al (2008) Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience 156:59–70. doi:10.1016/j.neuroscience.2008.07.032
Day M, Wang Z, Ding J et al (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9:251–259. doi:10.1038/nn1632
Day M, Wokosin D, Plotkin JL et al (2008) Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci 28:11603–11614. doi:10.1523/JNEUROSCI.1840-08.2008
Ding JB, Guzman JN, Peterson JD et al (2010) Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67:294–307. doi:10.1016/j.neuron.2010.06.017
Dobi A, Margolis EB, Wang H-L et al (2010) Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci 30:218–229. doi:10.1523/JNEUROSCI.3884-09.2010
El Mestikawy S, Wallén-Mackenzie A, Fortin GM et al (2011) From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci 12:204–216. doi:10.1038/nrn2969
Fasano C, Thibault D, Trudeau LE (2008) Culture of postnatal mesencephalic dopamine neurons on an astrocyte monolayer. Curr Protoc Neurosci. doi:10.1002/0471142301.ns0321s44 (Chapter 3:Unit 3.21)
Fasano C, Kortleven C, Trudeau LE (2010) Chronic activation of the D2 autoreceptor inhibits both glutamate and dopamine synapse formation and alters the intrinsic properties of mesencephalic dopamine neurons in vitro. Eur J Neurosci 32:1433–1441. doi:10.1111/j.1460-9568.2010.07397.x
Fasano C, Bourque M-J, Lapointe G et al (2013) Dopamine facilitates dendritic spine formation by cultured striatal medium spiny neurons through both D1 and D2 dopamine receptors. Neuropharmacology 67:432–443. doi:10.1016/j.neuropharm.2012.11.030
Ferrari DC, Mdzomba BJ, Dehorter N et al (2012) Midbrain dopaminergic neurons generate calcium and sodium currents and release dopamine in the striatum of pups. Front Cell Neurosci 6:7. doi:10.3389/fncel.2012.00007
Fortin GM, Bourque M-J, Mendez JA et al (2012) Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci 32:17477–17491. doi:10.1523/JNEUROSCI.1939-12.2012
Garcia BG, Neely MD, Deutch AY (2010) Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb Cortex 20:2423–2432. doi:10.1093/cercor/bhp317
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. doi:10.1146/annurev-neuro-061010-113641
Gerfen CR, Engber TM, Mahan LC et al (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Gong S, Zheng C, Doughty ML et al (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925. doi:10.1038/nature02033
Gras C, Herzog E, Bellenchi GC et al (2002) A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci 22:5442–5451
Gras C, Vinatier J, Amilhon B et al (2005) Developmentally regulated expression of VGluT3 during early post-natal life. Neuropharmacology 49:901–911. doi:10.1016/j.neuropharm.2005.07.023
Gras C, Amilhon B, Lepicard EM et al (2008) The vesicular glutamate transporter VGluT3 synergizes striatal acetylcholine tone. Nat Neurosci 11:292–300. doi:10.1038/nn2052
Higley MJ, Gittis AH, Oldenburg IA et al (2011) Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One 6:e19155. doi:10.1371/journal.pone.0019155
Hnasko TS, Chuhma N, Zhang H et al (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65:643–656. doi:10.1016/j.neuron.2010.02.012
Ingham CA, Hood SH, Arbuthnott GW (1989) Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res 503:334–338
Ingham CA, Hood SH, van Maldegem B et al (1993) Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res 93:17–27
Kelley JJ, Gao XM, Tamminga CA, Roberts RC (1997) The effect of chronic haloperidol treatment on dendritic spines in the rat striatum. Exp Neurol 146:471–478. doi:10.1006/exnr.1997.6552
Kozorovitskiy Y, Saunders A, Johnson CA et al (2012) Recurrent network activity drives striatal synaptogenesis. Nature 485:646–650. doi:10.1038/nature11052
Lester J, Fink S, Aronin N, DiFiglia M (1993) Colocalization of D1 and D2 dopamine receptor mRNAs in striatal neurons. Brain Res 621:106–110. doi:10.1016/0006-8993(93)90303-5
McKinney RA, Lüthi A, Bandtlow CE et al (1999) Selective glutamate receptor antagonists can induce or prevent axonal sprouting in rat hippocampal slice cultures. Proc Natl Acad Sci USA 96:11631–11636
Mendez JA, Bourque M-J, Dal Bo G et al (2008) Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci 28:6309–6318. doi:10.1523/JNEUROSCI.1331-08.2008
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425
Moss J, Bolam JP (2008) A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci 28:11221–11230. doi:10.1523/JNEUROSCI.2780-08.2008
Neely MD, Schmidt DE, Deutch AY (2007) Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience 149:457–464. doi:10.1016/j.neuroscience.2007.06.044
Parish CL, Finkelstein DI, Drago J et al (2001) The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci 21:5147–5157
Parish CL, Stanic D, Drago J et al (2002) Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur J Neurosci 16:787–794
Penrod RD, Kourrich S, Kearney E et al (2011) An embryonic culture system for the investigation of striatal medium spiny neuron dendritic spine development and plasticity. J Neurosci Methods 200:1–13. doi:10.1016/j.jneumeth.2011.05.029
Perrone-Capano C, Di Porzio U (2000) Genetic and epigenetic control of midbrain dopaminergic neuron development. Int J Dev Biol 44:679–687
Raju DV, Ahern TH, Shah DJ et al (2008) Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci 27:1647–1658. doi:10.1111/j.1460-9568.2008.06136.x
Redgrave P, Prescott TJ, Gurney K (1999) The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89:1009–1023
Rodriguez A, Ehlenberger DB, Dickstein DL et al (2008) Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One 3:e1997. doi:10.1371/journal.pone.0001997
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76(3):470–485. doi:10.1016/j.neuron.2012.10.021
Schmitz Y, Luccarelli J, Kim M et al (2009) Glutamate controls growth rate and branching of dopaminergic axons. J Neurosci 29:11973–11981. doi:10.1523/JNEUROSCI.2927-09.2009
Segal M, Greenberger V, Korkotian E (2003) Formation of dendritic spines in cultured striatal neurons depends on excitatory afferent activity. Eur J Neurosci 17:2573–2585
Sharpe NA, Tepper JM (1998) Postnatal development of excitatory synaptic input to the rat neostriatum: an electron microscopic study. Neuroscience 84:1163–1175
Shen W, Flajolet M, Greengard P, Surmeier DJ (2008) Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321:848–851. doi:10.1126/science.1160575
Smith Y, Bevan MD, Shink E, Bolam JP (1998) Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86:353–387
Smith Y, Raju D, Nanda B et al (2009) The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull 78:60–68. doi:10.1016/j.brainresbull.2008.08.015
Specht LA, Pickel VM, Joh TH, Reis DJ (1981) Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. II. Late ontogeny. J Comp Neurol 199:255–276. doi:10.1002/cne.901990208
Stephens B, Mueller AJ, Shering AF et al (2005) Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience 132:741–754. doi:10.1016/j.neuroscience.2005.01.007
Stuber GD, Hnasko TS, Britt JP et al (2010) Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci 30:8229–8233. doi:10.1523/JNEUROSCI.1754-10.2010
Surmeier DJ, Song WJ, Yan Z (1996) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16:6579–6591
Surmeier DJ, Carrillo-Reid L, Bargas J (2011) Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience 198:3–18. doi:10.1016/j.neuroscience.2011.08.051
Tecuapetla F, Patel JC, Xenias H et al (2010) Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci 30:7105–7110. doi:10.1523/JNEUROSCI.0265-10.2010
Tepper JM, Sharpe NA, Koós TZ, Trent F (1998) Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci 20:125–145
Thibault D, Loustalot F, Fortin GM et al (2013) Evaluation of D1 and D2 dopamine receptor segregation in the developing striatum using BAC transgenic mice. PLoS One 8:e67219. doi:10.1371/journal.pone.0067219
Tian X, Kai L, Hockberger PE et al (2010) MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Mol Cell Neurosci 44:94–108. doi:10.1016/j.mcn.2010.01.012
Tinsley RB, Bye CR, Parish CL et al (2009) Dopamine D2 receptor knockout mice develop features of Parkinson disease. Ann Neurol 66:472–484. doi:10.1002/ana.21716
Tong Q, Ye C, McCrimmon RJ et al (2007) Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5:383–393. doi:10.1016/j.cmet.2007.04.001
Villalba RM, Smith Y (2011) Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J Comp Neurol 519:989–1005. doi:10.1002/cne.22563
Villalba RM, Lee H, Smith Y (2009) Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol 215:220–227. doi:10.1016/j.expneurol.2008.09.025
Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ (1988) The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience 25:857–887
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94(4):469–492
Wu Y, Richard S, Parent A (2000) The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci Res 38:49–62
Yamaguchi T, Sheen W, Morales M (2007) Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci 25:106–118. doi:10.1111/j.1460-9568.2006.05263.x
Yamaguchi T, Wang H-L, Li X et al (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31:8476–8490. doi:10.1523/JNEUROSCI.1598-11.2011
Yoshihara Y, De Roo M, Muller D (2009) Dendritic spine formation and stabilization. Curr Opin Neurobiol 19:146–153. doi:10.1016/j.conb.2009.05.013
Zaja-Milatovic S, Milatovic D, Schantz AM et al (2005) Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64:545–547. doi:10.1212/01.WNL.0000150591.33787.A4
Zhuang X, Masson J, Gingrich JA et al (2005) Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods 143:27–32. doi:10.1016/j.jneumeth.2004.09.020
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (MOP-106556) and from the Brain Canada and Krembil Foundations to L.-E. Trudeau, as well as from an infrastructure grant from the Fonds de la recherche en santé du Québec to the Groupe de Recherche sur le Système Nerveux Central (GRSNC). D. Thibault was supported by a Canadian Institutes of Health Research graduate studentship. N. Giguère was supported by a Parkinson Society Canada studentship. We thank Dr. Xiaoxi Zhuang (University of Chicago) for providing the DAT-CRE mice and Dr. Bradford Lowell (Harvard University Medical School) for providing the VGLUT2 flox/flox mice. We thank Dr. James Surmeier (Northwestern University) for helpful comments and suggestions on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thibault, D., Giguère, N., Loustalot, F. et al. Homeostatic regulation of excitatory synapses on striatal medium spiny neurons expressing the D2 dopamine receptor. Brain Struct Funct 221, 2093–2107 (2016). https://doi.org/10.1007/s00429-015-1029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1029-4