Abstract
We used magnetic resonance (MR) images obtained in same-sex and opposite-sex dizygotic twins (n = 119, 8 years of age) to study possible effects of prenatal androgens on craniofacial features. Using a principal component analysis of 19 craniofacial landmarks placed on the MR images, we identified a principal component capturing craniofacial features that distinguished females with a presumed differential exposure to prenatal androgens by virtue of having a male (vs. a female) co-twin (Cohen’s d = 0.76). Subsequently, we tested the possibility that this craniofacial “signature” of prenatal exposure to androgens predicts brain size, a known sexually dimorphic trait. In an independent sample of female adolescents (singletons; n = 462), we found that the facial signature predicts up to 8 % of variance in brain size. These findings are consistent with the organizational effects of androgens on brain development and suggest that the facial signature derived in this study could complement other indirect measures of prenatal exposure to androgens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the organizational hypothesis, the prenatal period is a critical window when androgens impact the development of both reproductive and non-reproductive tissues (Phoenix et al. 1959). In human studies, putative effects of prenatal androgens on brain and behavior have been demonstrated using a variety of approaches, including direct (reviewed in van de Beek et al. 2004) and indirect (reviewed in Hönekopp et al. 2007) measures. In previous studies carried out with magnetic resonance imaging (MRI), exposures to prenatal androgens have been associated with inter-individual variations in brain size (Peper et al. 2009), callosal morphology (Chura et al. 2010), and local gray-matter volumes in sexually dimorphic regions (Lombardo et al. 2012).
Given the scarce opportunities for measuring androgen levels directly from amniotic fluid or umbilical-cord blood, one could test for putative effects of prenatal androgens using the discordant-sex twin design (e.g., Peper et al. 2009). This approach is based on the so-called twin-testosterone transfer (TTT) hypothesis: the intrauterine presence of a male (vs. female) co-twin exposes the other twin to higher levels of prenatal androgens (reviewed in Cohen-Bendahan et al. 2005; Miller 1994; Tapp et al. 2011). While female fetuses produce androgens only by fetal adrenal glands (Rainey et al. 2004) and as a by-product of corticosteroid production (Tapp et al. 2011), male fetuses develop testes in the 7–8 week of gestation (Tapp et al. 2011) and start producing increasing levels of testosterone (McIntyre 2006; Sarkar et al. 2007). Androgens produced by the male co-twin can reach the other co-twin by diffusion across amniotic fluid and fetal membranes (Ryan and Vandenberg 2002). Even et al. (1992) showed that injection of silastic capsule containing testosterone into the amniotic sac increased the testosterone levels especially in the adjacent rat fetuses.
Direct evidence for the TTT comes from research on mice and rats; the male sex of (nearby) fetuses is associated with the masculinization of a variety of sexually dimorphic traits in the female offspring (Ryan and Vandenberg 2002; Kinsley et al. 1986; Quadagno et al. 1987; vom Saal and Bronson 1980). Prenatal administration of anti-androgenic flutamide abolished the TTT supporting the fact that masculinization of females by male fetuses is indeed related to exposure to prenatal testosterone and not only to postnatal socialization with male littermates (Clemens et al. 1978). In humans, no direct measurements of prenatal androgens in dizygotic twins have been reported, but discordant twin design studies on digit ratio (Anders and Verhon 2006; Voracek and Dressler 2007) and other sex-specific traits (e.g., Peper et al. 2009; Cohen-Bendahan et al. 2004; Galsworthy et al. 2000) support the existence of intrauterine hormonal transfer in humans. Miller’s (1994) review of the testosterone transfer hypothesis concluded that comparison of female twins with same-sex versus opposite-sex fraternal co-twin provides a reasonable model for studies of prenatal testosterone exposure. Tapp et al. (2011) provided an overview of human studies of phenotypic differences––in a number of domains––between twins with a male or female co-twin and concluded that while the accumulated evidence lacks consistency [e.g., negative findings by Medland et al.’s (2008), Baker et al.’s (2009), Henderson and Berenbaum’s (1997)]––there is sufficient support for the TTT hypothesis to motivate further research.
The current study builds on the interconnected development of the brain and the face; it uses the discordant-sex twin design to identify a “signature” of the prenatal exposure to androgens in the craniofacial morphology. This choice is motivated by the growing availability of magnetic resonance (MR) images in population-based studies of brain development (Paus 2013) and enabled by the current work on MR-based craniofacial morphometry (e.g. Chakravarty et al. 2011; Liu et al. 2012; Marečková et al. 2011, 2013). All skeletal and connective tissues that form the face derive from neural crest cells, which originate along the dorsal margins of the midbrain and rostral hindbrain (Noden 1978; Couly et al. 1993; Kontges and Lumsden 1996). The concurrent phase of the brain and craniofacial development takes place between the 5th and 13th week of gestation (Diewert et al. 1993; Diewert and Lozanoff 1993). Specific genetic factors or exposures (e.g., teratogens) can result in disruptions of molecular signaling between the brain and the face and create malformations in both tissues. In holoprosencephaly, for example, Ssh signaling is reduced, brain can be severely malformed, and facial malformations range from midfacial hypoplasia to complete cyclopia (Muenke and Beachy 2000). Other examples of genetic and environmental perturbations affecting both phenotypes include, respectively, Down’s syndrome (Guihard-Costa et al. 2006) and fetal alcohol syndrome (Larkby and Day 1997).
Sex differences in the craniofacial morphology have been observed as early as in 6-month-old human infants (Bulygina et al. 2006). Studies in adults suggested a relationship between an indirect measure of prenatal androgens, namely, the ratio of the 2nd and 4th finger lengths (2D:4D ratio) and both the face shape (Fink et al. 2005; Meindl et al. 2012) and the perceived masculinity of the face (Neave et al. 2003). Prenatal androgens also appear to influence teeth size: androgenized female monkeys (vs. female controls) had longer and sharper teeth (Zingeser and Phoenix 1978) and human females with a co-twin brother (vs. with a co-twin sister) had larger mesiodistal and, to a lesser extent, buccolingual diameters of the top of the crown (Dempsey et al. 1999).
Here, we used the discordant-sex twin design to identify possible effects of prenatal androgens on craniofacial morphology in prepubertal children (Study 1). This approach allowed us to avoid the possible confounding effect of pubertal androgens. Subsequently, we tested the validity of such a craniofacial “signature” of prenatal androgens by examining its relationship with brain size in adolescent females born as singletons (Study 2). We have restricted the Study 2 to females because our previous work with this sample showed that female (but not male) faces are fully developed at the age of 12 years, with no age-related changes in face morphology occurring during adolescence (Marečková et al. 2011).
Study 1: methods
Participants
A sample of 119 dizygotic 8-year-old twins from the Quebec Newborn Twin Study included 63 females [28 with a twin sister (Same Sex Female; SSF), 35 with a twin brother (Opposite Sex Female; OSF)] and 56 males [20 with a twin brother (Same Sex Male; SSM), 36 with a twin sister (Opposite Sex Male; OSM)]. We were interested in differences between dizygotic twins with same-sex vs. opposite-sex co-twin (SSF vs. OSF, and SSM vs. OSM contrasts). Since individuals within the OSF group (and within the OSM group) were unrelated, our analyses included only one member of each twin pair from the SSF (and SSM) group (chosen at random when both MR images were usable). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed assent and consent forms were obtained from the children and their parents, respectively.
MRI data acquisition
We obtained three-dimensional T1-weighted, sagittal, fast low-angle shot (FLASH) images of the whole head using a 1.5 Tesla system (Magnetom Vision, Siemens Electric, Erlangen, Germany) using TE = 10 ms, TR = 22 ms, flip angle = 30°, 160 contiguous slices, matrix size = 224 × 256, 1 mm × 1 mm × 1 mm voxels.
Landmarks and craniofacial features
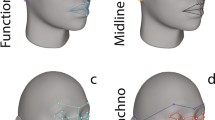
All 119 T1-weighted images were registered, using rigid transformations (3 translations, 3 rotations), to a T1-weighted image of one individual chosen at random (Collins et al. 1994). This ensured similar orientation of all images for the subsequent placement of craniofacial landmarks. Next, we placed 19 skull landmarks (Fig. 1) on each of the 119 images using the register visualization software (http://www.bic.mni.mcgill.ca/ServicesSoftware/HomePage). While it would have been more convenient to place landmarks only on the population-based average and then use the fully automated approaches, as previously developed by our group (Chakravarty et al. 2011) and employed in Marečková et al. (2013), this was not possible in this particular case due to the frequent presence of movement artifacts in T1-weighted images acquired in this sample.
Skull landmarks (19) and their position: 1 inside corner of the left eye socket; 2 outside corner of the left eye socket; 3 inside corner of the right eye socket; 4 outside corner of the right socket; 5 bridge of the nose; 6 middle of the left mandibular sinus; 7 middle of the right mandibular sinus; 8 outside of the right mandibular sinus; 9 outside of the left mandibular sinus; 10 point around 2nd vertebrae where lower teeth most visible; 11 front of the lower teeth; 12 left jaw (unerupted lower 3rd molar); 13 right jaw (unerupted lower 3rd molar); 14 front of the upper teeth; 15 left canine; 16 right canine; 17 left first molar; 18 right first molar; 19 tip of the chin bone
We placed skull landmarks in those non-tissue spaces, represented by a low-intensity signal void that enabled high precision in positioning (e.g., particular teeth, corners of the eye sockets, tip of the chin). Skull rather than facial landmarks were used to capture craniofacial features that are independent of the amount of fat (or muscle) in the face (see Chakravarty et al. 2011; Marečková et al. 2011, 2013 for details).
To remove a possible confounding effect of the overall size of the skull, we used the methods described in Sibson (1978), Chakravarty et al. (2011), and Pujol et al. (2011) to normalize all landmarks with respect to the linear dimensions of the skull (as represented by all of the landmarks of the group). We achieved this by matching the landmarks from each participant to each of the other participants based on the estimation of a 12-parameter affine transformation (3 parameters per translation, rotation, scale and shear). This yields 118 transformations for each participant that are averaged and then applied to the participant’s specific set of landmarks. The result is a distribution of points that define the overall shape variation in the skull across all participants. This is similar to the approach taken in active shape modeling in the computer vision (Cootes and Taylor 1992) and in other craniofacial studies (Liu et al. 2012; Fink et al. 2005). Finally, we extracted X, Y and Z coordinates of the 19 landmarks for each of the 119 individuals.
Variation in craniofacial morphology can be described by a particular constellation of craniofacial landmarks. Principle component analysis (PCA) of the landmark-based data allows one to compute a point-distribution model (Chakravarty et al. 2011), modeling the spatial distribution of the landmarks in all the participants. Principle components describe variability in the landmark positions and define particular craniofacial features. Loadings of the principle components in each individual represent the amount of these particular craniofacial features in one’s craniofacial structure. Here, we normalized the landmark coordinates to a range between 0 and 1, mean centered them, and submitted them to PCA. In addition, 17 craniofacial distances (Table 1) were calculated as Euclidean distances between the normalized mean-centered coordinates. The statistical software package JMP was used to test the effect of twin group on the principal components (PCs) and to determine which of the 17 craniofacial distances correlated with the PC that showed a twin group difference. Bonferroni correction was applied to p values to account for multiple comparisons.
Brain size calculation
Brain volume was estimated with SIENAX (Smith et al. 2001, 2002), part of FSL (Smith et al. 2004). SIENAX starts by extracting brain and skull images from the single whole-head input data (Smith 2002). Next, tissue-type segmentation with partial volume estimation is carried out (Zhang et al. 2001) and total brain volume is calculated.
Study 1: results
Age
All participants were 8 years old (M = 101.1 months, SD = 1.03; range 99.5–106.6 months) and their age did not vary as a function of twin group [F(3,115) = 1.13, p = 0.34].
Craniofacial features
The first 10 PCs from PCA described a total of 78 % of variance in the craniofacial features. As reported in Table 2, only PC2 and PC3 showed sex differences. We followed these two PCs with one-way ANOVA to test for differences in PC loadings across the four groups of twins (SSF, OSF, OSM, SSM). As reported in Table 3, only PC3 skull features showed a main effect of twin group [F(3,115) = 7.3, p = 0.0002 uncorrected; p = 0.002 corrected for 10 comparisons]. Post hoc analyses showed that SSF group had higher loadings of PC3 skull features than any other group [SSF vs. OSF: t(61) = 3.01, p < 0.0001, Cohen’s d = 0.76; SSF vs. OSM: t(62) = 4.35, p < 0.0001, Cohen’s d = 1.08; SSF vs. SSM: t(46) = 2.56, p = 0.01, Cohen’s d = 0.77]. There were no differences in PC3 loadings across the OSF, OSM, and SSM groups; in particular, OSF did not differ from either OSM [t(69) = −1.51, p = 0.14] and SSM [t(53) = −0.003, p = 0.99]. Craniofacial features characteristic for positive range of PC3 values are illustrated in Fig. 2.
Simulation of the third principal component (PC3) craniofacial features. The first column of images represents craniofacial features characteristic for the mean values of PC3, further columns of images represent the positive range of PC3 values (0.5 up to 2 SD from the mean) that reflect craniofacial features characteristic for low exposure to prenatal testosterone. Please notice the increasing width and length of the jaw in the upper and lower row of the images, respectively
Correlations between the 17 craniofacial distances and PC3 features identified the same eight PC3-related distances in both the whole sample of twins (Table 4) and the female twins only. We then evaluated differences between OSF and SSF and found that five of these eight PC3-related features showed an effect of co-twin’s sex (Table 5; we show results for all 17 distances for completeness). Overall, SSF (vs. OSF) had smaller distance between the inside corners of eye sockets, larger distance between left- and right-third molars of the lower jaw, larger “left-third-molar to chin”, “left-third-molar to lower front-teeth”, and “right-third-molar to lower front-teeth” distance. This is consistent with the direction of a simple sex difference: females (vs. males) had also smaller distance between the inside corners of eye sockets [t(116) = 3.37, p = 0.01, Cohen’s d = −0.63], larger distance between left- and right-third molars of the lower jaw [t(116) = −2.00, p = 0.05, Cohen’s d = 0.37], and larger “left-third-molar to chin” [t(116) = −2.75, p = 0.007, Cohen’s d = 0.5], “left-third-molar to lower front-teeth” [t(116) = −4.65, p < 0.0001, Cohen’s d = 0.86], and “right-third-molar to lower front-teeth” [t(116) = −3.88, p = 0.0002, Cohen’s d = 0.72] distance.
Brain size
Brain size was successfully calculated for 106 out of the 119 twins (13 individuals did not pass the quality control). As expected (reviewed in Paus 2010), male brains were larger (by 7.9 %) than female brains [t(104) = 6.42, p < 0.0001]. There was no effect of co-twin’s sex on brain size in either males [t(48) = 1.82, p = 0.07] or females [t(54) = −0.01, p = 0.99]. These results remained the same after correcting brain size for birth weight: Male brains were larger than female brains [t(96) = −6.48, p < 0.0001] and there was no effect of co-twin’s sex on brain size in either males [t(44) = −0.53, p = 0.60] or females [t(51) = −0.06, p = 0.95]. In the full sample (i.e., SSF, OSF, SSM and OSM), we observed a negative relationship between PC3 and brain size [t(105) = −2.31, p = 0.02, r = −0.22]; as predicted, the more “SSF-like” features were associated with lower brain size. When evaluated separately in each sex, the relationship was significant in males [t(50) = −2.96, p = 0.005, r = −0.39] but not in females [t(55) = 1.37, p = 0.18, r = 0.18].
Study 2: methods
In this study, we applied the model of PC3 features in an independent dataset of female adolescents and tested for the presence of a relationship between the PC3-related craniofacial features and brain size. In a previous study, females with a female co-twin had a slightly smaller brain than those with a male co-twin (Peper et al. 2009). We hypothesized that the presence of PC3-related features, an indicator of an absence of prenatal androgens (for PC3: SSF > OSF), would predict smaller brain size. Note that the results of Study 1 vis-à-vis the relationship between PC3 and brain size were inconclusive (present in males but not in females).
Participants
A total of 462 female adolescents born as singletons (age range = 12–18 years; M = 180.02 months, SD = 22.61, see Table S1 for the number of participants per age-bin) were recruited in the context of Saguenay Youth Study (SYS), a community-based sample of typically developing adolescents (Pausova et al. 2007). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed assent and consent forms were obtained from the children and their parents, respectively.
MRI data acquisition
We acquired T1-weighted (T1W) images of the head using a Philips 1T scanner and three-dimensional radio frequency (RF)-spoiled gradient-echo scan (TR = 25 ms, TE = 5 ms, flip angle = 30°, 140–160 slices, resolution 1 × 1 × 1 mm).
Landmarks and craniofacial features
The image-analysis pipeline for deriving craniofacial features uses the methodology described previously (Chakravarty et al. 2011) and employed in our studies of face and skull development during adolescence (Marečková et al. 2011, 2013). In brief, MR images are first corrected for intensity non-uniformity (Sled et al. 1998). Next, we remove the brain using a nonlinear transformation (Avants et al. 2008) that matches each T1-W image to an average model derived from 876 adolescent T1-W images (Grabner et al. 2006). The resulting images are then normalized to an intensity range of 0–10,000.
The goal of the remainder of the pipeline is to build an average model of the craniofacial structure for all of the study participants. Our approach has been inspired by similar pipelines created to estimate average models of brain anatomy (Frey et al. 2011; Borghammer et al. 2010; Grabner et al. 2006). Each image (original T1-W image minus the brain) is matched to a single image (chosen at random from the group) to normalize the spatial position and orientation using a 6-parameter transformation (3 translations and 3 rotations). Next, each image is normalized to the average head size of the entire group through the estimation of a 12-parameter transformation (3 translations, 3 rotations, 3 scales, and 3 shears) (Collins et al. 1994). Note that unlike in Experiment 1, all transformations are estimated using intensity information from the images. An average model is then created that is refined iteratively in subsequent nonlinear registration stages where large differences in craniofacial features are first accounted for followed by the estimation of transformations that progressively capture more subtle differences in craniofacial morphology (<2 mm; Chakravarty et al. 2011). The result is the estimation of a single transformation (a combination of linear and nonlinear transformations) that maps the craniofacial structure of each individual to the average craniofacial representation of the entire group. Using this average image, we then placed 19 landmarks (Fig. 1; the same landmarks as used in Study 1) at anatomically defined locations of the skull. Then, using the inverse nonlinear transformation generated through the hierarchical matching procedure described above, we fit all of the skull landmarks to each participant. This step provided a set of landmarks (and relevant Euclidean distances; Table 1) for each participant’s skull; the 3D position of each landmark is defined by X, Y and Z coordinates, termed below the “original” coordinates.
Projection of the facial signature
We transformed the model of twin PC3 generated in Study 1 to the (SYS) average image as follows. First, we calculated the centroids for each of the 19 landmarks in the previously registered twin dataset and aligned them using affine (12 parameters) registration to the 19 landmarks on the SYS average image. Next, we multiplied these 19 skull coordinates by the PC3 weights and thus created a set of 19 PC3-like coordinates. Finally, for each of the 19 landmarks and each participant, we calculated the difference between the original coordinates and PC3-like coordinates using the root mean square error (RMSE) formula. The RMSE thus determines how similar the craniofacial features of a given person were to those captured by PC3. Individuals from Study 2 whose faces were most similar to the PC3 model had the lowest RMSE values, a possible indicator of low exposure to androgens prenatally. In the manuscript, we refer to these PC3-like features as the facial signature.
Brain size calculation
As in Study 1, brain volume was estimated with SIENAX (Smith et al. 2002), part of FSL (Smith et al. 2004).
Study 2: results
Since we expected to find the relationship between the facial signature of prenatal androgens and brain size only in females, we explored the possible presence of this relationship in a sample of 458 females (4/462 participants were excluded due to failing the image-processing pipeline). As predicted, linear regression showed a positive relationship between RMSE and brain size [t(457) = 2.69, p = 0.007; R 2 = 0.02]; females with faces less similar to the PC3 model (i.e., higher RMSE) had bigger brains.
To identify craniofacial distances with the strongest relationship with brain size, we examined PC3-related distances that showed a difference between the SSF and OSF group (Study 1). Four of the eight PC3-related distances showed significant correlations with brain size: “right-third-molar to chin” (r = −0.29, p = 0.0008), “left-third-molar to chin” (r = −0.24, p = 0.0008), “left-third-molar to lower front-teeth” (r = −0.13, p = 0.03), and “spine to lower front-teeth” (r = −0.19, p = 0.0008).
Finally, we calculated an average of the two symmetrical distances that showed relationship with brain size (“right-third-molar to chin” and “left-third-molar to chin”). Linear regression showed a negative relationship between the average of these distances and brain size [t(457) = −6.34, p < 0.0001]. This fine-tuned facial signature of prenatal androgens explained 8 % variance in brain size.
In a subset of the female participants (n = 192), we were able to investigate also the relationship between digit ratio and brain size. Neither the left nor the right digit ratio showed a relationship with brain size [left 2D4D: t(186) = 1.75, p = 0.08; right 2D4D: t(186) = 1.46, p = 0.15]. There was also no relationship between the facial signature (RMSE indicating the amount of PC3-like features) and digit ratio [left 2D4D: t(191) = 0.82, p = 0.41; right 2D4D: t(191) = −0.36, p = 0.72].
Discussion
We used twin design and head MR images to study the possible effect of prenatal androgens on craniofacial morphology. Females with a female co-twin differed from all the other twin groups that were prenatally exposed to gonadal androgens (OSF, OSM, SSM) and the effect size was large (Cohen’s d ~ 0.8) in all three contrasts (SSF vs. OSF, SSF vs. OSM, SSF vs. SSM). The craniofacial morphology of the females with a female co-twin (vs. all other groups) was characterized by high loadings of PC3 features, mainly the longer and wider jaw that together formed a longer chin (see Fig. 2). We refer to these PC3-like craniofacial features, associated with low exposure to prenatal androgens, as the facial signature. This facial signature is not related to the width of the face, forehead and nose that characterize typically male-like features emerging during male adolescence (see PC1 description in Marečková et al. 2011, Table 1).
To verify the relationship between these craniofacial features and prenatal androgens, we used a large independent dataset of female adolescents (singletons) to explore the relationship between craniofacial features and brain size. Consistently with Peper et al. (2009) who showed that females with a female co-twin had slightly smaller brains than those with a male co-twin, we showed that the facial signature (PC3-like features, associated with low exposure to prenatal androgens) was negatively related to brain size: the set of PC3-related features could explain 2 % variance in brain size, and the length of the jaw could explain 8 % variance in brain size.
Sex differences in skull shape appear already in the first year of life (Bulygina et al. 2006). Male infants had a relatively larger and more globular frontal bone, smaller face, and a more flexed cranial base than female infants (Bulygina et al. 2006). While a comparison of these findings with the results from Study 1 is difficult due to the different sets of skull landmarks, we speculate that the larger frontal bone in male vs. female infants (Bulygina et al. 2006) might be consistent with the greater distance between the inner corners of eye sockets, characteristic for females with a male vs. female co-twin (Study 1). Facial features that showed an influence by prenatal androgens in our study also correspond to the embryonic development. Testes develop at 7–8 weeks of gestation (Tapp et al. 2011), which is a period characterized by the start of mandibular and maxillary ossification, formation of deciduous-tooth buds, and migration of eyes medially (Sperber et al. 2000).
In the twin study (Study 1), we found differences between the same-sex females and each of the other three twin groups, but no difference between the opposite-sex and same-sex males. This is consistent with the effect of co-twin’s sex on teeth size reported by Dempsey et al. (1999). It seems that the effect of prenatal androgens on the skull appears at certain levels of prenatal androgens but does not follow a simple (linear) dose response. Females produce very little endogenous testosterone and therefore gestation with a male co-twin has a relatively greater effect on females compared with males (Tapp et al. 2011).
The large effect of twin group on facial signature (Cohen’s d = 0.76 in SSF vs. OSF, 0.77 in SSF vs. SSM, and 1.08 in SSF vs. OSM) is consistent with Anders and Verhon (2006) study about the effect of co-twin’s sex on digit ratio. The small (2 %) effect of facial signature (RMSE) on brain size in females from Study 2 is consistent with Peper et al. (2009) who reported a small difference in brain size between same- vs. opposite-sex females (Cohen’s d = 0.36, which is R 2 = 0.03). The fine-tuned facial signature (i.e. length of the jaw) explained even more variance in brain size (8 %).
Experimental manipulations in mice showed that digit ratio is related to prenatal exposure to testosterone (Zheng and Cohn 2011). Still, the ability of digit ratio to explain variance in cognition or behavior might be limited. In Study 2, we compared the ability of digit ratio and facial signature to predict brain size and showed that while facial signature predicted small portion of variability in brain size, digit ratio did not predict any. It is possible that facial signature might be a more sensitive indicator of prenatal androgens than 2D:4D ratio. While sex differences in the levels of prenatal testosterone (measured directly) are large (Cohen’s d = 1.9; Knickmeyer et al. 2005; van de Beek et al. 2004), sex differences in 2D:4D ratio are rather small (Cohen’s d = 0.2 in Manning et al. 2007 and 0.3 in Manning et al. 2004). Given the medium effect size of the sex differences in facial signature (Study 1: Cohen’s d = 0.56), the facial signature may provide a useful additional––albeit still indirect––index of prenatal exposure to androgens. In our subset of participants, we compared the digit ratio and facial signature but did not find any relationship. This is consistent with Valla and Halazonetis (2014) who found no relationship between digit ratio and craniofacial shape in prepubertal children.
We are aware of the possible limitation of our study, namely the fact that we were not able to replicate the Peper et al. (2009) findings regarding the effect of co-twin’s sex on brain size. This may be related to the small effect of prenatal androgens on brain size (cca 3 % according to Peper et al. 2009) and possible inter-individual differences in variables known to affect brain size, such as genes (Bartley et al. 1997; Paus et al. 2012), exposures to various toxins during pregnancy, such as cigarette smoke (Anblagan et al. 2013; Paus et al. 2012) or alcohol (Chen et al. 2003) and the duration of breast feeding (Isaacs et al. 2010). The absence of the relationship between PC3 and brain size in Study 1 females is consistent with the lack of the effect of co-twin sex on brain size in this sample. It is possible that the subtle effects of androgens produced by a male co-twin on his female co-twin require relatively large sample size and/or ethnically homogenous populations (the latter important especially for face morphometrics), both of which have been afforded by the SYS sample. It is of interest to note that––in Study 1––PC3 correlated (in the predicted direction) with brain size in male twins (regardless of their co-twin status). It is possible that inter-individual variations in the androgen levels produced by the male fetus influence both PC3 (reducing it) and brain size (increasing it).
Overall, these findings suggest that prenatal androgens did leave their signature in the skull and that this facial signature might be used, in the same way as 2D:4D ratio, as an indirect index of exposure to prenatal androgens. Given the widespread availability of T1-weighted head MRIs, an MR-based facial signature might be easily accessible to many researchers interested in the effects of prenatal androgens. Facial signature could be also estimated based on the PC3-related distances derived from photographs (profile and frontal view) and thus in the absence of MRI data. Study 2 showed that these distances can explain even more variance in brain size (up to 8 %) than the complete set of landmarks characteristic for PC3 (2 %). While the PCA methodology was invaluable for identifying these features, we conclude that the fine-tuned signature is a simpler predictor of prenatal androgens than the PC3-based one and might thus be more applicable for future research.
Conclusion
We used a cohort of 8-year-old dizygotic twins to study the relationship between prenatal androgens and craniofacial shape (Study 1). Head MR images enabled us to describe variability in skull features among same-sex and opposite-sex twins. Females with a female co-twin showed facial features that distinguished them from all other twin groups prenatally exposed to gonadal androgens. To verify the existence of the relationship between prenatal androgens and skull features, we studied relationship of this facial signature with brain size in a large independent sample of female adolescents born as singletons (Study 2). We were able to predict 2 and 8 % variance in brain size by this signature and the jaw length, respectively. We conclude that this signature of prenatal androgens in the skull might be used in future studies as an alternative to digit ratio to investigate further the role of prenatal androgens on brain and disease risk.
References
Anblagan D, Jones NW, Costigan C, Parker AJJ, Allcock K, Aleong R, Coyne LH, Deshpande R, Fenning NR, Bugg G, Roberts N, Pausova Z, Paus T, Gowland PA (2013) Maternal smoking during pregnancy and fetal organ growth: a magnetic resonance imaging study. PLoS One 8(6):e67223. doi:10.1371/journal.pone.0067223
Anders Van, Verhon PA (2006) Finger-length ratios show evidence of prenatal hormone transfer between opposite-sex twins. Horm Behav 49:315–319
Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12(1):26–41
Baker JH, Lichtenstein P, Kendler KS (2009) Intrauterine testosterone exposure and risk for disordered eating. Br J Psychiatry 194:375–376
Bartley AJ, Jones DW, Weinberger DR (1997) Genetic variability of human brain size and cortical gyral patterns. Brain 120(2):257–269
Borghammer P, Ostergaard K, Cumming P, Gjedde A, Rodell A, Hall N, Chakravarty MM (2010) A deformation-based morphometry study of patients with early-stage Parkinson’s disease. Eur J Neurol 17(2):314–320
Bulygina E, Mitteroecker P, Aiello L (2006) Ontogeny of facial dimorphism and patterns of individual development within one human population. Am J Phys Anthropol 131:432–443
Chakravarty M, Aleong R, Leonard G, Perron M, Pike B, Richer L, Veillette S, Pausova Z, Paus T (2011) Automated analysis of craniofacial morphology using magnetic resonance images. PLoS One 6(5):e20241. doi:10.1371/journal.pone.0020241
Chen WJ, Maier SE, Parnell SE, West JR (2003) Alcohol and the developing brain: neuroanatomical studies. Alcohol Res Health 27(2):174–180
Chura LR, Lombardo MV, Ashwin E, Auyenung B, Chakrabarti B, Bullmore ET, Cohen SB (2010) Organizational effects of fetal testosterone on human corpus callosum size and asymmetry. Psychoneuroendocrinology 35(1):122–132
Clemens LG, Gladue BA, Coniglio LP (1978) Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav 10:40–53
Cohen-Bendahan CCC, Buitelaar JK, van Goozen SMH, Cohen-Kettenis PT (2004) Prenatal exposure to testosterone and functional cerebral lateralization: a study in same-sex and opposite-sex twin girls. Psychoneuroendocrinology 29:911–916
Cohen-Bendahan CCC, van de Beek C, Berenbaum SA (2005) Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev 29:353–384
Collins DL, Holmes CJ, Peters TM, Evans AC (1994) Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp 3(3):190–208
Cootes TF, Taylor CJ (1992) Active shape models – ‘smart snakes’. In: Hogg D, Boyle R (eds) BMVC92. Springer-Verlag, London, pp 266–275
Couly GF, Coltey PM, Le Douarin NM (1993) The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117:409–429
Dempsey PJ, Townsend GC, Richards LC (1999) Increased crown size in females with twin brothers: evidence for hormonal diffusion between human twins in utero. Am J Hum Biol 11:577–586
Diewert VM, Lozanoff S (1993) A morphometric analysis of human embryonic craniofacial growth in the median plane during primary palate formation. J Craniofac Genet Dev Biol 13:184–192
Diewert VM, Lozanoff S, Choy V (1993) Computer reconstructions of human embryonic craniofacial morphology showing changes in relations between the face and brain during primary palate formation. J Craniofac Genet Dev Biol 13(3):193–201
Even MD, Dhar MG, vom Saal FS (1992) Transport of steroids between fetuses via amniotic fluid in relation to the intrauterin position phenomenon in rats. J Reprod Fertility 96:709–716
Fink B, Grammer K, Mitteroecker P, Gunz P, Schaefer K, Bookstein FL, Manning IT (2005) Second to fourth digit ratio and face shape. Proc R Soc Lond B Biol Sci 272:1995–2000
Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, Cllins L (2011) An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). Neuroimage 55(4):1435–1442
Galsworthy MJ, Dionne G, Dale PS, Plomin R (2000) Sex differences in early verbal and non-verbal cognitive development. Dev Sci 3:206–215
Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL (2006) Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 9(Pt 2):58–66
Guihard-Costa AM, Khung S, Delbecque K, Menez F, Delezoide AL (2006) Biometry of face and brain in fetuses with trisomy 21. Pediatr Res 59:33–38
Henderson BA, Berenbaum SA (1997) Sex-typed play in opposite-sex twins. Dev Psychobiol 31:115–123
Hönekopp J, Bartholdt L, Beier L, Liebert A (2007) Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: new data and a meta-analytic review. Psychoneuroendocrinology 32(4):313–321
Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A (2010) Impact of breast milk on IQ, brain size and white matter development. Pediatr Res 67(4):357–362
Kinsley C, Miele J, Konen C, Ghiraldi L, Svare B (1986) Intrauterine contiguity influences regulatory activity in adult female and adultmale-mice. Horm Behav 20:7–12
Knickmeyer RC, Wheelwright S, Taylor K, Raggatt P, Hackett G, Baron-Cohen S (2005) Gender-typed play and amniotic testosterone. Dev Psychol 41:517–528
Kontges G, Lumsden A (1996) Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122:3229–3242
Larkby C, Day N (1997) The effects of prenatal alcohol exposure. Alcohol Health Res World 21(3):192–198
Liu F, van der Lijn F, Schurmann C, Zhu G, Chakravarty MM et al (2012) A genome-wide association study identifies five loci influencing facial morphology in europeans. PLoS Genet 8(9):1–13
Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S (2012) Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci 32(2):674–680
Manning JT, Stewart A, Bundred PE, Trivers RL (2004) Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Hum Dev 80(2):161–168
Manning JT, Churchill JGA, Peters M (2007) The effects of sex, ethnicity, and sexual orientation of sef-measured digit ratio (2D:4D). Arch Sex Behav 36(2):223–233
Marečková K, Weinbrand Z, Chakravarty MM, Lawrence C, Aleong R, Leonard G et al (2011) Testosterone-mediated sex differences in the face shape during adolescence: subjective impressions and objective features. Horm Behav 60(5):681–690
Marečková K, Chakravarty MM, Huang M, Lawrence C, Leonard G, Perron M (2013) Does skull shape mediate the relationship between objective features and subjective impressions about the face? Neuroimage 79:234–240
McIntyre MH (2006) The use of digit ratios as markers for perinatal androgen action. Reprod Biol Endocrinol 4(10). doi:10.1186/1477-7827-4-10
Medland SE, Loehlin JC, Willemsen G, Hatemi PK, Keller MC, Boomsma DI, Eaves LJ, Martin NG (2008) Males do not reduce the fitness of their female cotwins in contemporary samples, Twin Res. Hum Genet 11:481–487
Meindl K, Windhager S, Wallener B, Schaefer K (2012) Second-to-fourth digit ratio and facial shape in boys: the lower the digit ratio, the more robust the face. Proc R Soc B 279(1737):2457–2463
Miller EM (1994) Prenatal sex hormone transfer: a reason to study opposite-sex twins. Personal Individ Differ 17:511–529
Muenke M, Beachy PA (2000) Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev 10:262–269
Neave N, Laing S, Fink B, Manning JT (2003) Second to fourth digit ratio, testosterone and perceived male dominance. Proc R Soc Lond B 270:2167–2172
Noden DM (1978) The control of Avian cephalic neural crest cytodifferentiation: skeletal and connective tissues. Dev Bio 96:144–165
Paus T (2010) Sex differences in the human brain: a developmental perspective. Prog Brain Res 186:13–28
Paus T (2013) Population neuroscience. Springer-Verlag, Berlin, Heidelberg, ISBN-10: 3642364497
Paus T, Bernard M, Chakravarty MM, Smith GD, Gillis J, Lourdusamy A, Leonard G, Melka MG, Pavlidis P, Perron M, Pike GB, Richer L, Schumann G, Timpson N, Toro R, Veillette S, Pausova Z (2012) KCTD8 gene and brain growth in adverse intrauterine environment: a genome-wide association study. Cereb Cortex 22:2634–2642
Pausova Z, Paus T, Abrahamowicz M, Almerigi J, Arbour N, Bernard M et al (2007) Genes, maternal smoking, and the offspring brain and body during adolescence: design of the Saguenay Youth Study. Hum Brain Mapp 28:502–518
Peper JS, Brouwer RM, van Baal G, Schnack H, leeuwen M, Boomsma D, Kahn R, Pol H (2009) Does having a twin brother make for a bigger brain? Eur J Endocrinol 160:739–746
Phoenix CH, Goy RW, Gerall AA, Young WC (1959) Organizing action of prenatally administered testosterone propionáte on the tissue mediating mating behavior in the female guinea pig. Endocrinology 65:369–382
Pujol J, Soriano-Mas C, Gispert JD, Bossa M, Reig S, Ortiz H et al (2011) Variation in the shape of the frontobasal brain region in obsessive–compulsive disorder. Hum Brain Mapp 32:1100–1108
Quadagno DM, McQuitty C, McKee J, Koelliker L, Wolfe G, Johnson DC (1987) The effects of intrauterine position on competition and behavior in the mouse. Physiol Behav 41:639–642
Rainey WE, Rehman KS, Carr BR (2004) The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med 22:327–336
Ryan BC, Vandenberg JG (2002) Intrauterine position effects. Neurosci Biobehav Rev 26:665–678
Sarkar P, Bergman K, Fisk NM, O’Connor TG, Glover V (2007) Amniotic fluid testosterone: relationship with cortisol and gestational age. Clin Endocrinol (Oxf) 67:743–747
Sibson R (1978) Studies in the robustness of multidimensional scaling: procrustes statistics. J Roy Stat Soc 40:234–238
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155
Smith SM, De Stefano N, Jenkinson M, Matthews PM (2001) Normalised accurate measurement of longitudinal brain change. J Comput Assist Tomogr 25(3):466–475
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17(1):479–489
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(S1):208–2019
Sperber GH, Sperber SM, Guttmann GD (2000) Craniofacial embryogenetics and development, Shelton, CT: People’s Medical Pub. House, USA
Tapp AL, Maybery MT, Whitehouse AJO (2011) Evaluating the twin testosterone transfer hypothesis: a review of the empirical evidence. Horm Behav 60:713–722
Valla K, Halazonetis DJ (2014) Correlation of 2D:4D digit ratio and craniofacial shape in prepubertal children. Am J Hum Biol 26(3):337–346
Van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM, Buitelaar JK (2004) Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav 46:663–669
vom Saal FS, Bronson FH (1980) Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science 208:597–599
Voracek M, Dressler SG (2007) Digit ratio (2D:4D) in twins: heritability estimates and evidence for a masculinized trait expression in women from opposite-sexpairs. Psychol Rep 100:115–126
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging 20(1):45–57
Zheng Z, Cohn MJ (2011) Developmental basis of sexually dimorphic digit ratios. Proc Nat Acad Sci 108:16289–16294
Zingeser MR, Phoenix CH (1978) Metric characteristics of the canine dental complex in prenatally androgenized female rhesus monkeys (Macaca mulatta). Am J Phys Anthrop 49:187–192
Acknowledgments
The Saguenay Youth Study was funded by the Canadian Institute of Health Research, Heart and Stroke Foundation of Quebec, and the Canadian Foundation of Innovation. The Quebec Newborn Twin Study was funded by Canadian Institute of Health Research and Fonds de la recherche en santé du Quebec. We would like to thank SciNet for access to its supercomputer platform, which is funded by the Canada Foundation for Innovation, NSERC, the Government of Ontario, FedDev Ontario, and the University of Toronto. Finally, we would also like to thank the European Social Fund and the government of Czech Republic who co-financed the project „Employment of Newly Graduated Doctors of Science for Scientific Excellence (grant number CZ.1.07/2.3.00/30.0009).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marečková, K., Chakravarty, M.M., Lawrence, C. et al. Identifying craniofacial features associated with prenatal exposure to androgens and testing their relationship with brain development. Brain Struct Funct 220, 3233–3244 (2015). https://doi.org/10.1007/s00429-014-0852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0852-3