Abstract
Previous diffusion tensor imaging (DTI) studies have shown that various factors can affect white matter (WM) tract diffusivity. The aim of the present study was to investigate the effects of childhood adversity (CA), age and gender on WM diffusivity in tracts that are thought to be involved in emotional regulation in individuals with major depressive disorder (MDD) and healthy controls (HC). DTI was obtained from 46 subjects with MDD and 46 HC subjects. Data were pre-processed and deterministic tractography was applied in the cingulum, uncinate fasciculus (UF), fornix, superior longitudinal fasciculus (SLF) and fronto-occipital fasciculus (FOF). In subjects with a history of CA, fractional anisotropy (FA) was greater in the rostral cingulum (RC) and dorsal cingulum, whereas radial diffusivity (RD) was smaller in the RC when compared with subjects with no history of CA. In the UF, FOF and parahippocampal cingulum, FA was greater in the left hemisphere in the subjects with CA when compared with those without CA. Age affected FA, longitudinal diffusivity and RD in the UF, fornix, FOF and SLF, reflecting axonal and myelin degeneration with increasing age. Depression or gender did not have any effects on the diffusivity measures. Due to the cross-sectional nature of the study, a recall bias for CA and possible effects of medical treatment on diffusivity measures could have played a role. CA and age could increase the likelihood to develop WM microstructural anomalies in the brain affective network. Moreover, subjects with CA could be more vulnerable to FA changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is a debilitating mental illness of significant impact on public health. The prevalence of MDD in total national co-morbidity replication samples was recently estimated to be 16.2 % for lifetime and 6.6 % for a 12-month period (Kessler et al. 2003). Other findings indicate that MDD is a leading cause of disease burden, accounting for 4.4 % of total disability and for almost 12 % of all total years lived with disability worldwide (Ustun et al. 2004).
Previous studies suggest that childhood adversity (CA) plays a role in the etiology of adult MDD onset (Sadowski et al. 1999; Aguilera et al. 2009; Klein et al. 2009). In a study that compared individuals with MDD, individuals with anxiety disorder and healthy controls, the interaction between a child’s neurotic traits and CA was found to increase the risk for developing MDD in adulthood (Infrasca 2003). In the same study, the number and frequency of childhood adverse incidents were higher in MDD individuals, when compared to the general population or to individuals with anxiety disorder. Interestingly, exposure to stressful events during childhood was higher in girls, in line with the evidence showing higher prevalence of MDD in women than in men (Sadeghirad et al. 2010; Aaro et al. 2011; Chen et al. 2012). Another study that described the occurrence of CA and stressful life events across the individual’s life span suggests that childhood adversity plays a key role in MDD (Spinhoven et al. 2010). Moreover, stressful life events, such as frequently having witnessed domestic abuse during childhood, are thought to be high risk factors for depression in young adulthood (Russell et al. 2010).
In recent years, much attention has been devoted to studying the relationship between MDD and microstructural abnormalities in the brain. In this respect, diffusion tensor imaging (DTI) has played an important role. DTI is capable of revealing white matter (WM) microstructural anomalies, due to its sensitivity to three-dimensional changes in water diffusion within the fiber tracts (Basser et al. 1994). DTI measures structural brain connectivity in vivo, as opposed to functional connectivity (Tuch et al. 2003; Ramnani et al. 2004; Chanraud et al. 2010; Solano-Castiella et al. 2010) and is sensitive to a wide range of diseases (Lim and Helpern 2002). In particular, fractional anisotropy (FA) is a measure often used in DTI. FA is thought to reflect fiber density, axonal diameter and myelination in the WM and is influenced by the integrity of axons and myelin as well as by axon directional coherence, with reduced FA suggesting either loss of axonal integrity or coherence (Beaulieu 2002). However, recent DTI studies have also explored alternative quantitative methods, in the effort to identify more specific relationships between DTI measurements and WM pathology. In this respect, radial diffusivity (RD) appears to be modulated by myelin integrity in the WM, whereas longitudinal diffusivity (LD) is thought to be affected by axonal degeneration (Song et al. 2002).
Various studies have shown FA reductions in a variety of WM tracts in patients with MDD (Nobuhara et al. 2006; Li et al. 2007; Yang et al. 2007; Cullen et al. 2010; Dalby et al. 2010). Significant FA reductions were found in the WM of the medial orbital prefrontal cortex and external capsule in individuals with refractory MDD (Li et al. 2007). There is also evidence for smaller FA values in the superior longitudinal fasciculus (SLF) (Murphy and Frodl 2011) and prefrontal brain regions (Bae et al. 2006; Murphy et al. 2007; Shimony et al. 2009) of MDD individuals when compared with healthy controls. Additionally, reduced FA in MDD compared to controls has been detected in the cingulate cortex (Kieseppa et al. 2010) and parahippocampal gyrus (Zhu et al. 2011). Tractography studies in patients with MDD have also revealed an increase in FA values in WM tracts involved with mood regulation, i.e., the tracts connecting the right superior frontal gyrus with the right pallidum as well as the tracts between the left superior parietal gyrus and the right superior occipital gyrus (Lu et al. 2010). In MDD individuals, FA anomalies have also been detected in the rostral cingulum (RC) and uncinate fasciculus (UF), two fiber tracts that play key roles in emotion regulation (Laitinen 1979; Hasan et al. 2009). Importantly, the UF connects limbic structures, such as the hippocampus and amygdala, with frontal regions (Taylor et al. 2007) and is thought to be associated with the capacity for autonoetic self-awareness, that is the ability to re-experience previous events as part of one’s past as a continuous entity across time, as well as with the proficiency in auditory-verbal memory and declarative memory (Levine et al. 1998; Mabbott et al. 2009).
Interestingly, the results of DTI studies investigating the effects of childhood abuse—for example, parental verbal abuse—have been found to be associated with FA anomalies in the arcuate fasciculus (a component of the SLF), cingulum and fornix (Choi et al. 2009). Moreover, a 16-week follow-up study on adolescents exposed to childhood maltreatment showed that, at baseline, subjects had significantly lower FA values in the left and right SLF, right cingulum bundle projecting to the hippocampus, left inferior fronto-occipital fasciculus (FOF) and splenium of the corpus callosum, compared with healthy controls. Adolescents who developed MDD at follow-up had significantly lower FA values in the SLF and right cingulum-hippocampal projection compared with their counterparts who did not develop the illness (Huang et al. 2012).
In this context, the aim of the present study was to investigate whether CA can be associated with microstructural anomalies in WM fiber bundles that previous DTI research has shown to be associated with either MDD or CA. In particular, we examined the UF, cingulum, fornix, SLF and fronto-occipital fasciculus (FOF) to examine the main effects of CA, gender and diagnosis. In addition, we explored interactions between gender and CA, gender and diagnosis as well as CA and diagnosis.
Method
Participants
The study included 92 subjects aged between 18 and 65 years, of which 46 had been diagnosed with MDD and were undergoing treatment for first onset or recurrent MDD at the mental health services of the Adelaide and Meath Hospital, incorporating the National Children’s Hospital, Dublin and St. James’s Hospital, Dublin. MDD was clinically diagnosed by consultant psychiatrists based on the Diagnostic and Statistical Manual of Mental Disorders—Revised 4th Edition (DSM-IV TR) (American Psychiatric Association 2000) criteria and was confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al. 2002). Of the 46 MDD patients recruited, only 29 reported CA as assessed by the short form childhood trauma questionnaire (CTQ-SF) (Bernstein et al. 2003). The remaining 46 healthy control (HC) subjects were recruited from the local community via announcements. Of these, 18 reported CA (Table 1). Groups were age and gender-matched. Each subject was carefully screened and examined for medical conditions so that apart from MDD, no subject recruited would have a personal history of neurological or psychiatric disorders (Axis I or Axis II) or a history of severe medical illness, head injury or substance abuse. Demographic variables, inclusion and exclusion criteria were documented using a standardized questionnaire and through a structured interview by a psychiatrist. Differences in demographic variables were tested using the Student t test. A Chi-square test was used for gender distribution analysis and the Mann–Whitney U test to investigate differences in clinical variables.
Rating instruments
Self and observer rated scales were also filled out for all participants included in the study. These included the Hamilton Rating Scale for Depression (HRSD) (Hamilton 1969), Beck’s Depression Inventory (BDI-II) (Beck et al. 1996), SCID-I and CTQ-SF. The 28 item short form of CTQ-SF was used to assess adversity during childhood and teenage years. This questionnaire is a self-report instrument that assesses five types of childhood maltreatment: emotional, physical, and sexual abuse, as well as emotional and physical neglect. The questionnaire also includes a minimization/denial scale for detecting individuals who may be underreporting traumatic events. The cutoff mark applied to determine CA in various domains of CTQ-SF was 12 for emotional abuse, 10 for physical abuse, 8 for sexual abuse, 8 for physical neglect and 15 for emotional neglect. Reliability and validity of the CTQ-SF have been established, including measures of convergent and discriminative validity from structured interviews, stability over time and corroboration (Bernstein et al. 2003).
Written informed consent was obtained from all the participants. The study was designed and performed in accordance with the ethical standards laid out by the Declaration of Helsinki and was approved by the ethics committee of St. James’s and Adelaide and Meath Hospitals, the teaching hospitals affiliated with Trinity College Dublin.
Diffusion tensor imaging (DTI)
Magnetic resonance images were obtained with a Philips Achieva MRI scanner operating at 3 T. High angular resolution diffusion imaging (HARDI) with 61 diffusion directions was obtained [field of view (FOV): 200 × 257 × 126 mm, 60 slices, no gap, spatial resolution: 1.8 × 1.8 × 2.1 mm, TR/TE = 12,561/59 ms, flip angle = 90°, half k-space acquisition was used (half scan factor = 0.68), SENSE parallel imaging factor = 2.5, b values = 0, 1,200 s/mm, with SPIR fat suppression and dynamic stabilization in an image acquisition time of 15 min 42 s].
DTI data pre-processing
Data were pre-processed using Explore DTI (http://www.exploredti.com) with the following steps: (1) data conversion from “par/rec” format to a “nifty” file and a “B-matrix text” file; (2) subsequently, data were converted to an Explore DTI “mat” format and transferred to a voxel size of 2 × 2 × 2 mm. With our acquisition voxel size, there is no significant partial volume effect by doing this. Moreover, diffusion tensor estimation was linear; (3) motion correction was applied to all data to adjust for head movement during scanning using a cubic interpolation and restore function with the lowest speed but highest accuracy; (4) Eddy current correction was used (Leemans and Jones 2009); (5) for data quality check, we first reviewed the DTI data by visually inspecting the slice images displayed in a loop. Explore DTI also allowed us to look at the residuals and outlier profiles, which were in order. Finally, the motion correction parameters were checked. Head movement during scanning was less than 2 mm in x, y, z directions for all subjects.
Tractography
All data were normalized into MNI space. Seed point resolution was 2 × 2 × 2 mm, with a seed FA threshold of 0.2. Deterministic tractography was applied with Explore DTI (Leemans et al. 2009). First, whole-brain tractography was conducted in each subject using a linear interpolation. Then, individual tracts that have been previously shown to be affected by MDD were selected using protocols similar to the knowledge-based multiple region approach described for the association tracts in previous studies (Mori et al. 2002). The Explore DTI software allows the selection of tracts passing through two distinct regions of interest (ROI) using the “AND” operator to include them in the analysis or the “NOT” operator to exclude them.
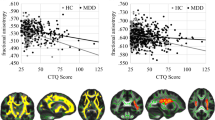
All ROIs were drawn on the FA-weighted colored maps and the investigators were blind to the diagnosis. For all subjects, the same numbers and locations of ROIs were used. The following WM tracts were extracted (Fig. 1):
Three-dimensional view samples of the fiber tracts whose FA was measured and compared across groups (a uncinate fasciculus, b fornix, c rostral cingulum, d dorsal cingulum, e parahippocampal cingulum, f fronto-occipital fasciculus, g superior longitudinal fasciculus). a–e were in sagittal view while f and g were in coronal view. Fibers connecting the right and left hemisphere are shown in red. Fibers passing from the bottom to the top of the brain and vice versa are shown in blue. Finally, fibers running along the fronto-caudal axis are shown in green
-
1.
Rostral cingulum (RC). The first ROI was placed on the most inferior axial slice where the body of the corpus callosum was clearly seen in full profile. The second ROI was located in the axial slice corresponding to the middle of the genu. Finally, the third ROI was placed on the lowest axial slice, where the genu was joined across the midline. NOT regions were placed sagitally to exclude fibers projecting laterally and/or medially.
-
2.
Dorsal cingulum (DC). The tract was identified by placing the first ROI on the most posterior coronal slice, where the genu of the corpus callosum was seen in full profile. The second ROI was located on the most anterior coronal slice, where the splenium of the corpus callosum was seen in full profile. The third ROI was placed on the coronal slice, at the midpoint between the first two ROIs. NOT regions were placed axially to exclude fibers branching out to the cortex and/or other limbic areas.
-
3.
Parahippocampal cingulum (PC). This was first identified on the sagittal slices that provided the clearest profile of the hippocampus. The first ROI was then traced around the hippocampus. The second and the third ROI were then traced on the adjacent medial and lateral slices, respectively. NOT regions were placed on the slice below the lower body of the splenium, excluding the retrosplenial portion and the branches projecting to the retrosplenial cortex.
-
4.
Uncinate fasciculus (UF). The ROIs were placed on the most posterior coronal slice in which the temporal lobe is separated from the frontal lobe. The first ROI included the entire temporal lobe. The second ROI was placed at the same coronal level and included the entire projections toward the frontal lobe. Moreover, a “NOT” ROI was used to exclude fibers from the inferior fronto-occipital fasciculus and the cingulum.
-
5.
Crus of the fornix. The first ROI was placed in the coronal section at the level of the middle hippocampal body, whereas the second ROI was placed where both the body and the crus of the fornix were clearly visible.
-
6.
Superior longitudinal fasciculus (SLF). To select the SLF, the first ROI was placed at the middle of the posterior limb of the internal capsule, whereas the second ROI was placed at middle of the splenium. At these levels, the SLF is seen as a green triangular region, lateral to the superior-to-inferior corticospinal (blue) fibers. “NOT” ROIs were used to exclude fibers crossing from the inferior fronto-occipital fasciculus (FOF) and pyramidal tracts (Luck et al. 2011).
-
7.
Inferior fronto-occipital fasciculus (FOF). Two ROIs were used to select the FOF (Kvickstrom et al. 2011). The first ROI was placed along the course of the FOF in the coronal plane of the DTI images at the level of the anterior commissure and the second ROI was placed on the pontine crossing fibers (Catani et al. 2002).
Inter-rater reliability was calculated after two raters independently performed the tractography of 20 participants. Intra-class correlations were between 0.90 and 0.95 for mean FA values in the tracts. After performing the tractography for all tracts for all individuals, FA mean and variability as well as tract volumes were extracted and fed into SPSS (PASW 1.8) for further data analysis. The ROI method was adjusted to recent published research showing its reliability (Malykhin et al. 2008).
Statistics
Demographic and clinical variables were analyzed using ANOVA with groups (MDD subjects with CA, MDD subjects without CA, HC subjects with CA, HC subjects without CA) as fixed factors. After performing tractography, FA values were subjected to an ANCOVA with hemisphere (left, right) as within subject factor and group (MDD, HC), CA (yes, no) and gender (female, male) as fixed factors, using age as covariate. This design allowed testing for interactive effects of group by CA, group by gender and gender by group within one statistical test. Three-way interactions between CA, group and gender were not considered because of the small group size of male patients without CA. In case of significant interactive effects, post hoc tests were performed to demonstrate between which groups the significant difference was found. To assess the association between age and tract diffusion measures, we used Pearson Product Moment correlations. For the correlation between depression severity and tract diffusion measures, we used Spearman’s correlations. The effect of medication status on diffusion measures was tested with a “repeated measures” ANCOVA. Since we analyzed 5 different tracts, namely the uncinate fasciculus, fornix, cingulum, SLF and FOF, we considered a p < 0.01 to survive Bonferroni correction for multiple testing in the ANCOVA and correlation analyses and have indicated in the results where this is the case.
Results
We found significant effects and/or interactions between CA, age, gender and/or hemispheres in various WM fiber tracts as measured by FA, LD and RD. A summary of the significant effects and interactions is shown in Table 2. The detailed breakdown of the results for each fiber bundle is as follows:
Rostral cingulum (RC)
Childhood adversity had a significant effect on FA (F = 11.52, df = 1,83, p = 0.001, survived Bonferroni correction). The subjects with a history of CA showed greater FA when compared to those without CA. There was also a significant interaction between CA and gender (F = 9.72, df = 1,83, p = 0.003, survived Bonferroni correction). Male subjects with CA had greater FA in the RC when compared with those without CA (F = 12.9, df = 1,32, p = 0.001) (Fig. 2). No other main or interactive effects were observed on FA.
In the subjects with CA, RD was smaller when compared with those without CA (F = 7.2, df = 1,83, p = 0.009, survived Bonferroni correction). Also, there was a significant interaction between CA and gender (F = 8.6, df = 1,83, p = 0.004, survived Bonferroni correction). The males with CA had smaller RD when compared with those without CA (F = 10.5, df = 1,32, p = 0.003). On the other hand, in female subjects, CA had no effects on RD (F = 0.28, df = 1,50, p = 0.6).
Dorsal cingulum
Childhood adversity had an effect on FA (F = 4.06, df = 1,83, p = 0.047) (Fig. 2). The subjects with CA had greater FA when compared with those without ELA.
No effects or interactions were found on LD or RD in the DC.
Parahippocampal cingulum
There was an interaction between group and gender (F = 5.57, df = 1,83, p = 0.021). The male MDD subjects had smaller FA when compared to the male HC subjects (F = 4.6, df = 1,32, p = 0.039). There was also an interaction between CA and gender (F = 4.65, df = 1,83, p = 0.034). In particular, the male subjects with CA had significantly greater FA when compared with those without CA (F = 7.5, df = 1,32, p = 0.01). The above interactions were not observed in the female subjects. However, a three-way interaction was found between CA, group and hemisphere (F = 5.79, df = 1,83, p = 0.018). FA was greater in the left hemisphere of the MDD subjects with CA when compared to the MDD subjects without CA (F = 6.5, df = 1,41, p = 0.015).
No effects or interactions were detected on LD and RD.
Uncinate fasciculi
Age had an effect on FA (F = 4.45, df = 1,83, p = 0.038), which was found to be smaller with increasing age. There was also an interaction between CA and hemisphere (F = 4.06, df = 1,83, p = 0.047). Greater FA values were detected in the left UF in the subjects with CA when compared with those without CA (F = 7.7, df = 1,98, p = 0.007).
Age had an effect on both LD and RD (F = 8.84, df = 1,83, p = 0.004, survived Bonferroni correction), respectively. With increasing age, LD decreased while RD increased. There was also an interaction between CA and hemisphere in the LD (F = 4.6, df = 1,83, p = 0.035). Specifically, LD was greater in the left hemisphere of the subjects with CA when compared with those without CA.
Fornix
Age had an effect on FA in the fornix. Post hoc analysis revealed smaller FA in older subjects (F = 31.2, df = 1,81, p < 0.001, survived Bonferroni correction).
Both age (F = 7.40, df = 1,81, p = 0.008, survived Bonferroni correction) and CA (F = 7.55, df = 1,81, p = 0.008, survived Bonferroni correction) had significant effects on LD and RD. While LD decreased as the subjects got older, RD was proportional to age. The subjects with CA had higher LD than the subjects without CA.
Fronto-occipital fasciculus
There was an interaction between CA and hemisphere (F = 4.75, df = 1,83, p = 0.032). The subjects with CA had increased FA in the FOF on the left hemisphere compared to the subjects without CA (F = 8.8, df = 1,46, p = 0.005). Gender interacted with hemispheres (F = 14.79, df = 1,83, p < 0.001, survives Bonferroni correction). In particular, FA was smaller in the left hemisphere (F = 8.0, df = 1,50, p = 0.007) of the female subjects when compared with the male subjects. The male subjects did not show any difference between hemispheres (F = 2.0, df = 1,32, p = 0.17). CA, group (MDD/HC) and hemisphere also interacted (F = 6.8, df = 1,83, p = 0.011). The female MDD patients with CA had smaller FA when compared to their male counterparts.
Age (F = 8.27, df = 1,83, p = 0.005, survived Bonferroni correction) and CA (F = 4.10, df = 1,83, p = 0.046) had effects on LD resulting in LD being smaller in older subjects and greater in the subjects with CA when compared with those without CA. An interaction between group and gender (F = 6.74, df = 1,83, p = 0.011) was also observed. Male MDD patients had greater LD in the FOF compared with male controls (F = 4.9, df = 1,32, p = 0.034). In addition, an interaction between gender, hemisphere and CA (F = 5.0, df = 1,83, p = 0.028) was found. The male subjects with CA showed smaller LD in the left hemisphere (F = 14.1, df = 1,32, p = 0.001) when compared with those without CA. Gender and CA also interacted (F = 8.0, df = 1,83, p = 0.006, survived Bonferroni correction). The male subjects with CA had smaller LD than those without CA.
A significant interaction between hemisphere, group and CA was detected (i = 10.3, df = 1,83, p = 0.002). The MDD subjects with CA had smaller RD in the right FOF when compared with the MDD subjects without CA (F = 6.6, df = 1,41, p = 0.014). There was also an interaction between gender and CA (F = 5.3, df = 1,83, p = 0.024). The male subjects with CA showed smaller RD in the FOF when compared with those without CA (F = 6.3, df = 1,32, p = 0.017).
Superior longitudinal fasciculi
In the SLF, only age had an effect on FA (F = 8.48, df = 1,83, p = 0.005, survived Bonferroni correction). FA was smaller with increasing age.
Age had an effect on LD (F = 4.90, df = 1,83, p = 0.03), which was proportional to age. Significant interactions were found between group and gender (F = 4.64, df = 1,83, p = 0.034). In the MDD subjects, LD was found to be greater in males when compared with females (F = 4.4, df = 1,41, p = 0.042). No difference between the male and female subjects was found for the HC group (F = 0.41, df = 1,41, p = 0.53). There was also an interaction between CA and gender (F = 8.30, df = 1,83, p = 0.005, survived Bonferroni correction). LD was greater in the male subjects without CA when compared with the female subjects without CA (F = 8.7, df = 1,46, p = 0.005). However, no gender differences were found within the group of subjects with CA (F = 1.6, df = 1,36, p = 0.22).
No main or interactive effects were observed on RD in the SLF.
Correlations between FA and age
Correlation with age was found in the left UF (r = −0.26, p = 0.008), left fornix (r = −0.49, p < 0.001), right fornix (r = −0.36, p < 0.001) (Fig. 3), left dorsal cingulum (r = −0.22, p = 0.027), right dorsal cingulum (r = −0.29, p = 0.003), left SLF (r = −0.38, p < 0.001), right SLF (−0.39, p < 0.001), left FOF (r = −0.21, p = 0.031) and right FOF (r = −0.34, p < 0.001). Most of these correlations achieved a significant level that survives Bonferroni correction. No significant differences between patients and controls were found with respect to correlations between FA and age (Fig. 3).
Scatter plot of correlations between age (years) and FA in different tracts: left UF (r = −0.26, p = 0.008), left fornix (r = −0.49, p < 0.001), right fornix (r = −0.36, p < 0.001) (Fig. 3), left dorsal cingulum (r = −0.22, p = 0.027), right dorsal cingulum (r = −0.29, p = 0.003), left SLF (r = −0.38, p < 0.001), right SLF (−0.39, p < 0.001), left FOF (r = −0.21, p = 0.031), right FOF (r = −0.34, p < 0.001)
Correlations between FA and depression severity
No significant correlations were found between FA values and depression severity scores as measured by the HDRS or BDI questionnaires.
Effect of medication on FA
No medication effect was found on the WM tracts studied (UF: F = 1.1, df = 2,43, p = 0.33; fornix: F = 2.5, df = 2,43, p = 0.10; SLF: F = 2.2, df = 2,43, p = 0.12; FOF: F = 1.8, df = 2,43, p = 0.17; cingulum: F = 1.3, df = 2,43, p = 0.29).
Discussion
The present study provides new evidence for a link between CA and FA anomalies in the cingulum (RC, DC, and PC) and UF. The effects were particularly strong in the RC and survived correction for multiple testing. In the RC and DC, FA was greater in the subjects with CA when compared with those without CA. In addition, we observed a correspondingly smaller RD in the RC in the CA group. Specifically, these effects were found in the male subjects. In the UF and PC, greater FA was detected only in the left hemisphere of the subjects with CA. In the PC, greater FA in the left hemisphere was only observed in male subjects with CA.
These results suggest an effect of CA on the WM tracts connecting limbic and prefrontal regions, previously found to play a role in emotion regulation (Roberts and Wallis 2000; Ghashghaei and Barbas 2002; Burgel et al. 2006; Wager et al. 2008; Thiebaut de Schotten et al. 2012). The role of the cingulate cortex in emotion regulation and MDD has also been demonstrated by studies showing a positive effect of deep brain stimulation in the subgenual cingulate region (Broadmann area 25) in treatment-resistant MDD patients (Mayberg et al. 2005). There is evidence that these effects may be mediated via strong connections between the orbitofrontal cortex, anterior mid-cingulate cortex, hypothalamus, nucleus accumbens and amygdala/hippocampus (Johansen-Berg et al. 2008).
The above findings might be in line with the results of our earlier study showing greater FA in healthy relatives of MDD subjects with CA when compared with healthy relatives of MDD subjects without CA (Frodl et al. 2012). Speculatively, this might suggest that increased diffusivity in the cingulum could play a role in adaptive psychological processes triggered by exposure to stressful challenges during childhood, and might also support previous evidence showing that in adults, FA and mean diffusivity can be modified by experience (Scholz et al. 2009; Lovden et al. 2010). Moreover, the differences in FA and RD between the male subjects with CA and the male subjects without CA suggest that both axonal and myelin microstructural anomalies might have been involved (Song et al. 2002, 2003, 2005; Sun et al. 2005). However, as previous research has shown lower FA in subjects with CA (Paul et al. 2008; Daniels et al. 2013), our results surely need replication.
The role of the interplay between CA and genetic factors should also be considered in explaining our findings. Magnetic resonance imaging (MRI) studies have shown that polymorphism of the brain-derived neurotrophic factor (BDNF) or the serotonin 3A (5-HT3A) gene is associated with smaller volumes in the hippocampus (Gatt et al. 2010; Carballedo et al. 2013) and frontal cortex (Gatt et al. 2010) in individuals with CA. Moreover, resilience to adverse social conditions experienced in early years may be influenced by gene–environment interactions (Kaufman et al. 2006; Carli et al. 2011; Das et al. 2011; Grabe et al. 2012). Future DTI research should investigate the effects of these complex interactions on WM tract in subjects with CA, especially with regard to male subjects.
In the FOF, FA was greater in the left hemisphere only in the subjects that reported CA. Interestingly, even though this was an interactive effect, it might be in line, at least in part, with previous electroencephalogram (EEG) findings suggesting that the degree of left-sided bias in prefrontal activity may be a good indicator of an individual’s ability to regulate emotions (Jackson et al. 2003; Kim and Bell 2006), and also with the evidence supporting inter-hemispheric metabolic differences and variability in emotion regulation across individuals (Kim et al. 2012).
Recent data on brain connectivity measured in normal human subjects indicate gender differences in the pattern of neuronal communication, with adult males consistently exhibiting higher overall FA than females in all WM regions, perhaps as a result of differences in myelination (Menzler et al. 2011; Pal et al. 2011). Similarly, in several brain regions, including the cingulum and SLF, FA was greater in males when compared with females (Inano et al. 2011), whereas in the column of the fornix, FA was greater in females. These results are in line with evidence indicating substantial gender difference in brain connectivity (Gong et al. 2011). In our study, we observed differences between male and female subjects (i.e., higher FA in the cingulum of male subjects) only when gender interacted with CA, suggesting a possible mediating role of CA. Several factors beyond the scope of this study could have differentially influenced gender WM differences. For instance, previous studies have demonstrated that testosterone mediated an increase in WM volume in male subjects compared with female subjects and that this effect was in turn moderated by the androgen receptor gene (Perrin et al. 2008; Paus et al. 2010; Herting et al. 2012). Normal inter-individual variation in lipid levels has also been suggested to be associated with compromised regional WM integrity, even in individuals below clinical thresholds for hyperlipidemia (Williams et al. 2013). Moreover, light to moderate alcohol consumption has been reported to affect both the gray and WM volumes in male but not female individuals (Sachdev et al. 2008). It is unclear if any and/or to what extent the above factors or other unknown factors were implicated. However, considering that WM anomalies were only found in males with CA, it is possible that CA, at least partially, played a role.
In addition, there were no significant main effects of diagnosis status (MDD, controls). This is at odds with previous research showing WM anomalies in MDD subjects (Bae et al. 2006; Zou et al. 2008; Murphy and Frodl 2011). However, interactions between diagnosis and gender in the PC were found only in male subjects. The male MDD subjects had smaller FA when compared with the male HC subjects, in line with recent studies that found smaller FA in the cingulate cortex (Kieseppa et al. 2010) and parahippocampal gyrus (Zhu et al. 2011). Moreover, the male MDD subjects had larger LD in the FOF compared to the male HC subjects, supporting previous findings indicating an increase in diffusivity in the tracts that connect the right superior frontal gyrus with the right pallidum and also in the tracts between the left superior parietal gyrus and the right superior occipital gyrus (Lu et al. 2010). There were no interactions within the female group (MDD and HC), suggesting that depressed male individuals might be more vulnerable to WM microstructural insults.
Lastly, another important finding was the effect of age on DTI measures. With increasing age, FA and LD became smaller, while RD was larger in various tracts (Table 2). The associations between age and DTI measures were statistically robust and most of them survived a Bonferroni correction of p < 0.01 for the five different tract areas studied. However, there was no age effect in the RC, in line with previous research reporting age-related FA decline in the prefrontal region, posterior limb of the internal capsule (PLIC) and the genu of the corpus callosum, with concurrent sparing of temporal lobe connections, cingulum, and the parieto-occipital commissure (Salat et al. 2005; Michielse et al. 2010). Regional selective age-related decline in WM FA has also been reported in other studies (Pfefferbaum et al. 2000; Hsu et al. 2008; Davis et al. 2009). Interestingly, LD and RD most consistently show an age-related decrease (Davis et al. 2009; Wu et al. 2011), similarly to what we found in the present study. Abnormalities in LD and RD associated with axonal and myelin degeneration, respectively, have been demonstrated to affect WM integrity in animal models of traumatic brain injury (Song et al. 2002, 2003, 2005; Sun et al. 2005). Thus, our results might strengthen the link between older age and axonal/myelin changes in the brain.
In conclusion, the present study provides new evidence that CA affects WM tract diffusivity as measured by DTI and also that axonal and myelin degeneration increase with age. Our results also suggest that MDD might increase the likelihood to develop WM microstructural anomalies in male individuals and that these effects could be mediated by CA.
Limitations
While some MDD subjects were being treated with antidepressants at the time of their participation, the details of their treatment were not taken into account in this study. Previous research has suggested that antidepressant agents might have protective effects on WM structural integrity (Mostert et al. 2006; Yoo et al. 2007), although opposite effects have also been reported (Steffens et al. 2008). In the present study, however, we did not find any significant medication effect or correlation in the WM tracts that we selected. In addition, while our findings were robust statistically, they must be interpreted with caution. The study was cross-sectional in design and, as such, causal relationship could not be deduced from our findings. Longitudinal DTI studies are surely needed to demonstrate possible causal relationships between CA, age and MDD with WM tract diffusivity abnormalities in the brain. Moreover, the findings in the male subjects with MDD should be interpreted with caution, as gender had no main effect.
Finally, as we used a retrospective method to identify subjects with CA in our study, a recall bias should be considered as a possible limitation. Previous studies have shown that a prospective method is the single most comprehensive approach to evaluate childhood physical abuse, sexual abuse and neglect and that, ideally, a combination of all available identification methods should be used (Shaffer et al. 2008). However, MDD is most often associated with CA (Claes 2004; Dalton et al. 2014), which could explain why in our study MDD subjects reported higher levels of CA than HC subjects.
References
Aaro LE, Herbec A et al (2011) Depressive episodes and depressive tendencies among a sample of adults in Kielce, south-eastern Poland. Ann Agric Environ Med 18(2):273–278
Aguilera M, Arias B et al (2009) Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med 39(9):1425–1432
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn, text rev. Author, Washington, DC
Bae JN, MacFall JR et al (2006) Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry 60(12):1356–1363
Basser PJ, Mattiello J et al (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103(3):247–254
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15(7–8):435–455
Beck AT, Steer RA et al (1996) Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess 67(3):588–597
Bernstein DP, Stein JA et al (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27(2):169–190
Burgel U, Amunts K et al (2006) White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29(4):1092–1105
Carballedo A, Morris D et al (2013) Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Med Genet B Neuropsychiatr Genet 162(2):183–190
Carli V, Mandelli L et al (2011) A protective genetic variant for adverse environments? The role of childhood traumas and serotonin transporter gene on resilience and depressive severity in a high-risk population. Eur Psychiatry 26(8):471–478
Catani M, Howard RJ et al (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17(1):77–94
Chanraud S, Zahr N et al (2010) MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev 20(2):209–225
Chen S, Conwell Y et al (2012) Prevalence and natural course of late-life depression in China primary care: a population based study from an urban community. J Affect Disord 141(1):86–93
Choi J, Jeong B et al (2009) Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65(3):227–234
Claes SJ (2004) CRH, stress, and major depression: a psychobiological interplay. Vitam Horm 69:117–150
Cullen KR, Klimes-Dougan B et al (2010) Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry 49(2):173–183 e171
Dalby RB, Frandsen J et al (2010) Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res 184(1):38–48
Dalton VS, Kolshus E et al (2014) Epigenetics and depression: return of the repressed. J Affect Disord 155:1–12
Daniels JK, Lamke JP et al (2013) White matter integrity and its relationship to ptsd and childhood trauma-a systematic review and meta-analysis. Depress Anxiety 30(3):207–216
Das D, Cherbuin N et al (2011) DRD4-exonIII-VNTR moderates the effect of childhood adversities on emotional resilience in young-adults. PLoS ONE 6(5):e20177
Davis SW, Dennis NA et al (2009) Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46(2):530–541
First MB, Spitzer RL et al (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York
Frodl T, Carballedo A et al (2012) Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J Psychiatry Neurosci 37:37–45
Gatt JM, Williams LM et al (2010) Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depress Anxiety 27(8):752–759
Ghashghaei HT, Barbas H (2002) Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115(4):1261–1279
Gong G, He Y et al (2011) Brain connectivity: gender makes a difference. Neuroscientist 17:575–591
Grabe HJ, Schwahn C et al (2012) Genetic epistasis between the brain-derived neurotrophic factor Val66Met polymorphism and the 5-HTT promoter polymorphism moderates the susceptibility to depressive disorders after childhood abuse. Prog Neuropsychopharmacol Biol Psychiatry 36(2):264–270
Hamilton M (1969) Standardised assessment and recording of depressive symptoms. Psychiatr Neurol Neurochir 72(2):201–205
Hasan KM, Iftikhar A et al (2009) Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res 1276:67–76
Herting MM, Maxwell EC et al (2012) The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex 22(9):1979–1992
Hsu JL, Leemans A et al (2008) Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 39(2):566–577
Huang H, Gundapuneedi T et al (2012) White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 37(12):2693–2701
Inano S, Takao H et al (2011) Effects of age and gender on white matter integrity. AJNR Am J Neuroradiol 32(11):2103–2109
Infrasca R (2003) Childhood adversities and adult depression: an experimental study on childhood depressogenic markers. J Affect Disord 76(1–3):103–111
Jackson DC, Mueller CJ et al (2003) Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci 14(6):612–617
Johansen-Berg H, Gutman DA et al (2008) Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex 18(6):1374–1383
Kaufman J, Yang BZ et al (2006) Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry 59(8):673–680
Kessler RC, Berglund P et al (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289(23):3095–3105
Kieseppa T, Eerola M et al (2010) Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord 120(1–3):240–244
Kim KJ, Bell MA (2006) Frontal EEG asymmetry and regulation during childhood. Ann N Y Acad Sci 1094:308–312
Kim SH, Cornwell B et al (2012) Individual differences in emotion regulation and hemispheric metabolic asymmetry. Biol Psychol 89(2):382–386
Klein DN, Arnow BA et al (2009) Early adversity in chronic depression: clinical correlates and response to pharmacotherapy. Depress Anxiety 26(8):701–710
Kvickstrom P, Eriksson B et al (2011) Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol 11:13
Laitinen LV (1979) Emotional responses to subcortical electrical stimulation in psychiatric patients. Clin Neurol Neurosurg 81(3):148–157
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61(6):1336–1349
Leemans A, Jeurissen B, Sijbers J et al (2009) Explore DTI: a graphical toolbox for processing, analysing, and visualising diffusion MR data. 17th annual meeting of Intl Soc Mag Reson Med, Hawaii
Levine B, Black SE et al (1998) Episodic memory and the self in a case of isolated retrograde amnesia. Brain 121(Pt 10):1951–1973
Li L, Ma N et al (2007) Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res 1168:124–128
Lim KO, Helpern JA (2002) Neuropsychiatric applications of DTI—a review. NMR Biomed 15(7–8):587–593
Lovden M, Bodammer NC et al (2010) Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia 48(13):3878–3883
Lu C, Teng S et al (2010) A neuronal fiber tracking study for major depressive disorder using MR diffusion tensor imaging with fiber tractography. International conference on bioinformatics and biomedical technology (ICBBT). IEEE, Piscataway, pp 106–110
Luck D, Buchy L et al (2011) Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J Psychiatr Res 45(3):369–377
Mabbott DJ, Rovet J et al (2009) The relations between white matter and declarative memory in older children and adolescents. Brain Res 1294:80–90
Malykhin N, Concha L et al (2008) Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Res 164(2):132–142
Mayberg HS, Lozano AM et al (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45(5):651–660
Menzler K, Belke M et al (2011) Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 54(4):2557–2562
Michielse S, Coupland N et al (2010) Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage 52(4):1190–1201
Mori S, Kaufmann WE et al (2002) Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47(2):215–223
Mostert JP, Sijens PE et al (2006) Fluoxetine increases cerebral white matter NAA/Cr ratio in patients with multiple sclerosis. Neurosci Lett 402(1–2):22–24
Murphy ML, Frodl T (2011) Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord 1(1):3
Murphy CF, Gunning-Dixon FM et al (2007) White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry 61(8):1007–1010
Nobuhara K, Okugawa G et al (2006) Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 77(1):120–122
Pal D, Trivedi R et al (2011) Quantification of age- and gender-related changes in diffusion tensor imaging indices in deep grey matter of the normal human brain. J Clin Neurosci 18(2):193–196
Paul R, Henry L et al (2008) The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat 4(1):193–201
Paus T, Nawaz-Khan I et al (2010) Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav 57(1):63–75
Perrin JS, Herve PY et al (2008) Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci 28(38):9519–9524
Pfefferbaum A, Sullivan EV et al (2000) Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 44(2):259–268
Ramnani N, Behrens TE et al (2004) New approaches for exploring anatomical and functional connectivity in the human brain. Biol Psychiatry 56(9):613–619
Roberts AC, Wallis JD (2000) Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex 10(3):252–262
Russell D, Springer KW et al (2010) Witnessing domestic abuse in childhood as an independent risk factor for depressive symptoms in young adulthood. Child Abuse Negl 34(6):448–453
Sachdev PS, Chen X et al (2008) Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: a voxel-based morphometric study. Psychiatry Res 163(1):61–69
Sadeghirad B, Haghdoost AA et al (2010) Epidemiology of major depressive disorder in Iran: a systematic review and meta-analysis. Int J Prev Med 1(2):81–91
Sadowski H, Ugarte B et al (1999) Early life family disadvantages and major depression in adulthood. Br J Psychiatry 174:112–120
Salat DH, Tuch DS et al (2005) Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26(8):1215–1227
Scholz J, Klein MC et al (2009) Training induces changes in white-matter architecture. Nat Neurosci 12(11):1370–1371
Shaffer A, Huston L et al (2008) Identification of child maltreatment using prospective and self-report methodologies: a comparison of maltreatment incidence and relation to later psychopathology. Child Abuse Negl 32(7):682–692
Shimony JS, Sheline YI et al (2009) Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry 66(3):245–252
Solano-Castiella E, Anwander A et al (2010) Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage 49(4):2958–2965
Song SK, Sun SW et al (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17(3):1429–1436
Song SK, Sun SW et al (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20(3):1714–1722
Song SK, Yoshino J et al (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26(1):132–140
Spinhoven P, Elzinga BM et al (2010) The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord 126(1–2):103–112
Steffens DC, Chung H et al (2008) Antidepressant treatment and worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 39(3):857–862
Sun SW, Song SK et al (2005) Detection of age-dependent brain injury in a mouse model of brain amyloidosis associated with Alzheimer’s disease using magnetic resonance diffusion tensor imaging. Exp Neurol 191(1):77–85
Taylor WD, MacFall JR et al (2007) Structural integrity of the uncinate fasciculus in geriatric depression: relationship with age of onset. Neuropsychiatr Dis Treat 3(5):669–674
Thiebaut de Schotten M, Dell’Acqua F et al (2012) Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48(1):82–96
Tuch DS, Reese TG et al (2003) Diffusion MRI of complex neural architecture. Neuron 40(5):885–895
Ustun TB, Ayuso-Mateos JL et al (2004) Global burden of depressive disorders in the year 2000. Br J Psychiatry 184:386–392
Wager TD et al (2008) Neural mechanisms of emotion regulation: evidence for two independent prefrontal-subcortical pathways. Natl Inst Health Public Access 59(6):1037–1050
Williams VJ, Leritz EC et al (2013) Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum Brain Mapp 34(8):1826–1841
Wu YC, Field AS et al (2011) Age- and gender-related changes in the normal human brain using hybrid diffusion imaging (HYDI). Neuroimage 54(3):1840–1853
Yang Q, Huang X et al (2007) White matter microstructural abnormalities in late-life depression. Int Psychogeriatr 19(4):757–766
Yoo SY, Jang JH et al (2007) White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand 116(3):211–219
Zhu X, Wang X et al (2011) Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res 1369:223–229
Zou K, Huang X et al (2008) Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci 33(6):525–530
Acknowledgments
This study was supported by a grant awarded by Science Foundation Ireland (SFI, G20330) to Stoke Professor Thomas Frodl.
Conflict of interest
All authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ugwu, I.D., Amico, F., Carballedo, A. et al. Childhood adversity, depression, age and gender effects on white matter microstructure: a DTI study. Brain Struct Funct 220, 1997–2009 (2015). https://doi.org/10.1007/s00429-014-0769-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0769-x