Abstract
LPA1 receptor is one of the six characterized G protein-coupled receptors (LPA1–6) through which lysophosphatidic acid acts as an intercellular signaling molecule. It has been proposed that this receptor has a role in controlling anxiety-like behaviors and in the detrimental consequences of stress. Here, we sought to establish the involvement of the LPA1 receptor in emotional regulation. To this end, we examined fear extinction in LPA1-null mice, wild-type and LPA1 antagonist-treated animals. In LPA1-null mice we also characterized the morphology and GABAergic properties of the amygdala and the medial prefrontal cortex. Furthermore, the expression of c-Fos protein in the amygdala and the medial prefrontal cortex, and the corticosterone response following acute stress were examined in both genotypes. Our data indicated that the absence of the LPA1 receptor significantly inhibited fear extinction. Treatment of wild-type mice with the LPA1 antagonist Ki16425 mimicked the behavioral phenotype of LPA1-null mice, revealing that the LPA1 receptor was involved in extinction. Immunohistochemistry studies revealed a reduction in the number of neurons, GABA+ cells, calcium-binding proteins and the volume of the amygdala in LPA1-null mice. Following acute stress, LPA1-null mice showed increased corticosterone and c-Fos expression in the amygdala. In conclusion, LPA1 receptor is involved in emotional behaviors and in the anatomical integrity of the corticolimbic circuit, the deregulation of which may be a susceptibility factor for anxiety disorders and a potential therapeutic target for the treatment of these diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emotional regulation is crucial for adapting to environmental changes. The ability to extinguish emotional responses in the face of a no-longer relevant conditioned cue is an essential part of a healthy emotional memory system (Akirav and Maroun 2007). Failure to properly regulate fear responses has been linked with anxiety disorders (Hartley and Phelps 2010) or psychotic disorders (Holt et al. 2009; Konarski et al. 2007). Current efforts to address the emotional deregulation underlying these disorders focus on promoting fear extinction (Hong et al. 2009).
Fear extinction process, as a phenomenon of experience-dependent plasticity, depends on the integrity of the corticolimbic circuit, with a particular emphasis on the amygdala, prefrontal cortex, and hippocampus (Herry et al. 2010; Quirk et al. 2010; Moustafa et al. 2013) and is heavily dependent on GABAergic inhibitory mechanisms, especially in the amygdala (Quirk et al. 2010; Heldt and Mou 2012). In general, the neurobiological mechanism of fear extinction is strikingly similar to that of the adaptative stress response (distress regulation) (Wellman et al. 2007), sharing similar neuroanatomical, neuroendocrine, and neurochemical basis. Inadequate control of the stress response could precipitate or provoke anxiety disorders.
Recently, we described behavioral phenotypes exhibited by maLPA1-null mice (Santin et al. 2009; Castilla-Ortega et al. 2010, 2011, 2012), a stable variant of the original LPA1-null mutant strain (Contos et al. 2000) and developed in our group (Estivill-Torrús et al. 2008). The LPA1 receptor is one of the six G protein-coupled receptors through which lysophosphatidic acid (LPA, 1-acyl-2-sn-glycerol-3-phosphate) acts, with particular relevance to the central nervous system (Choi and Chun 2013). Mice lacking LPA1 receptor exhibit altered adaptative coping of chronic stress (Castilla-Ortega et al. 2011) and increased anxiety-like responses (Santin et al. 2009; Castilla-Ortega et al. 2010). Interestingly, null animals exhibit abnormalities in the structure and plasticity of the hippocampus (Matas-Rico et al. 2008; Castilla-Ortega et al. 2011), disrupted cortical GABAergic neuronal circuits (Cunningham et al. 2006), and synaptic dysfunction in the hippocampus (Musazzi et al. 2011) that may be partially responsible for the cognitive alteration reported in these mice (Santin et al. 2009; Castilla-Ortega et al. 2010, 2011, 2012; rev. in Estivill-Torrús et al. 2013). Despite the emotional changes observed in LPA1-null mice, the integrity of the cortico-amygdala circuit involved in emotional regulation has not yet been examined. Moreover, because the LPA1 receptor plays a role in regulating anxiety-like behaviors (Santin et al. 2009) and in the detrimental consequences of chronic stress (Castilla-Ortega et al. 2011); it may also be involved in fear extinction and acute stress reactivity, two modes of emotional regulation. Furthermore, the stress-hormone systems and amygdala activity are key components of emotional regulation. Taking together, these data make it interesting to examine the immediate responses of these systems following acute stress.
In this context, to elucidate the LPA1 receptor involvement in emotional regulation, we first examined fear extinction in normal wild-type (wt) and maLPA1-null mice (cited as LPA1-null mice, null, in this work) using two different extinction procedures (cued fear extinction and contextual fear extinction). Additionally, to study the role of LPA1 receptor in the absence of developmental abnormalities induced by its permanent loss, the effect of LPA1 antagonist Ki16425 administration was examined in contextual fear extinction on wild-type mice. Next, we studied the consequences of the absence of the LPA1 receptor in two key areas involved in emotional regulation, characterizing the structure and GABAergic composition of the medial prefrontal cortex (mPFC) and the amygdala by immunohistochemical detection of neuron-specific nuclear protein (NeuN), GABA-positive cells and calcium-binding proteins (calretinin (CR), parvalbumin (PV), and calbindin (CB)). Lastly, we examined the corticosterone response and the expression of a marker of neuronal activity, c-Fos protein, in the amygdala and the mPFC after acute stress.
Materials and methods
Animals
The generation and characterization of the Málaga variant of LPA1-null mice, derived from the original colony of Contos et al. (2000), has been described in previous works (Estivill-Torrús et al. 2008; Matas-Rico et al. 2008; see online resource).
For all experimental procedures, 3-month-old male mice were used. Mice were housed in groups of 4 on a 12 h light/dark cycle (lights on at 07:00 hours) with water and food provided ad libitum. Experiments were conducted between 9:00 and 15:00 hours.
Procedures were approved by the Ethics Committee of Malaga University (CEUMA: 2012-0006-A; 2012-0007-A) and performed in compliance with European animal research laws (European Communities Council Directives 2010/63/UE, 90/219/CEE, Regulation (EC) No 1946/2003) and Spanish National and Regional Guidelines for Animal Experimentation and Use of Genetically Modified Organisms (Real Decreto 53/2013, Ley 32/2007, and Ley 9/2003, Real Decreto 178/2004, Decreto 320/2010).
Fear conditioning and extinction in wt and LPA1-null mice
Before experiments, and to ensure that null mice have no different perception and processing of the foot shock or fear response to the tone per se, a pilot study was conducted in both genotypes, recording the stimulus amplitudes required to elicit signs of discomfort, that is, jumping and/or vocalizations. Both genotypes showed the same response at 0.6 mA of shock and freezing was absent when tone was not associated with shock.
For experimental procedure, mice were submitted to three phases of training: fear conditioning (day 1), cued or contextual extinction (day 2), and recall testing (days 3 and 5). The training used was similar for all animals, except for the training on day 2 (extinction), which differed according to the experimental group (Fig. 1a).
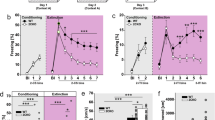
Fear conditioning and extinction training. a Protocol of fear conditioning, cue, and contextual fear extinction. On day 1, fear conditioning was conducted (23 wt and 24 null mice). On day 2, cue extinction (top) (N = 7 wt and 8 LPA1-null mice) or contextual extinction (middle) (N = 8 wt and 8 LPA1-null mice) was carried out. Control animals (N = 8 wt and 8 null mice) remained in their home cage (bottom). On days 3 and 5, all animals were assessed first in ‘context B’ and then ‘in context A’. b Behavior exhibited by animals during fear conditioned procedure. Although the freezing behavior increased in both genotypes across training, it was lower in LPA1-null mice. c Percent of freezing behavior exhibited by animals during cue fear extinction (day 2). The lack of LPA1 receptor impairs cue fear extinction. The initial levels of freezing during the first trial blocks of tone presentation indicate successful fear recall in both genotypes. After repeated tone presentation, only LPA1-wt animals showed reduced percentage of freezing between the first and second blocks of tone presentation, maintaining this reduction in the third trial blocks presentation. d Percent of freezing behavior exhibited by animals during test recall (days 3 and 5). CueEXT- LPA1-null showed no change across the assessment, maintaining high levels of freezing. CueEXT-wt showed significantly less freezing than cueEXT-null, CONT-wt, and CONT-null. e Procedure to examine the specificity of cue fear extinction protocol. Percent of freezing exhibited by animals trained to cue fear extinction and test to context. Only cueEXT-wt showed reduced significantly freezing levels. In contrast, in cueEXT-null, although the levels of freezing were low during the first trial of days 3 and 5, these increased progressively. f On day 2 (context extinction), any genotype reduced the freezing levels during contextual extinction training, probably due to the short time of context exposure. g The exposition to ‘context A’ did not affect cue conditioned fear recall. h Nevertheless, in the first trials of the contextual fear extinction recall (day 3 and 5), contextual extinguished animals showed reduced levels of freezing. However, LPA1-null froze more over time and increased progressively. Post hoc LSD tests: (*p < 0.05); **p < 0.01, significant differences between wt and LPA1-null; (# p < 0.05; ### p < 0.001), significant differences between trials block; (& p < 0.05), significant differences between cueEXT-wt versus its control; (+ p < 0.05), significant differences between CONT-wt and CONT-null. Abbreviations: In b: T trials of 120 s plus 30 s of tone co-terminated with 2 s of shock. In c, T set of 10 blocks of 150 s (30 s of tone plus 120 interval between tones). In d and g, T blocks composed of 30 s of tone plus 5 s between tones. In f and h, T trials of 120 s each
On day 1, fear conditioning was conducted on 23 wt and 24 null mice that received three pairings (120 s of inter-pairing interval) of a 30-s, 80-dB, 3-kHz tone [conditioned stimulus (CS)] and a 2-s, 0.6-mA co-terminating shock [unconditioned stimulus (US)] in a 27 × 27 × 11-cm conditioning chamber (‘context A’); with a floor consisting of 20 steel rods (Fig. 1a).
On day 2, animals were randomly assigned to one of the following conditions: cue extinction (cueEXT), contextual extinction (ctxEXT), or the CONTROL condition (CONT). In total, seven to eight animals per genotype were used for each experimental treatment.
Cue extinction
In animals trained to the tone extinction (hereafter, referred to as cueEXT-wt and cueEXT-null), initial cue recall and subsequent cue extinction were measured in a novel context (black/white-checkered walls and a solid Plexiglas opaque floor) (‘context B’). For tone extinction, after a 120-s acclimation period, mice received 30 tone presentations separated by 120 s (Fig. 1a). There was a 120-s no-stimulus consolidation period after the final tone presentation before mice returned to their home cage.
After both 24 h (day 3) and 72 h (day 5), the cue extinction recall was assessed. It consisted of five tone presentations separated by 5 s in ‘context B’. There was an initial and final 120-s no-stimulus period.
On both days 3 and 5, cue extinction recall was followed by an additional test to asses contextual fear recall and specificity of cue extinction training. It consisted in an exposition of 10 min in the same context of conditioning, ‘context A’ in the absence of tone or shock.
Contextual extinction
Animals trained to the context extinction (hereafter, ctxEXT-wt and ctxEXT-null) were placed in ‘context A’ for 8 min in the absence of any tone or shock (Fig. 1a).
Contextual extinction recall was assessed after both 24 h (day 3) and 72 h (day 5) delays. It consisted of a test of 10 min in ‘context A’. On both days 3 and 5, contextual recall was preceded by an additional test in ‘context B’ to asses cue fear recall and specificity of contextual extinction. The procedure was the same as that used for cue recall in animals extinguished to tone.
Control animals
CONTROL animals were used to examine the effectiveness of the extinction procedure. For this purpose CONTROL animals did not receive extinction training and therefore remained in their home cage on day 2. On days 3 and 5, were assessed first in ‘context B’ and then in ‘context A’ using the same procedure as the experimental groups.
Freezing (an index of conditioned fear) was measured observationally. The behavioral protocol is detailed in online resource.
Pharmacological experiment
To examine the effect of the acute administration of the selective LPA1/3 receptor antagonist Ki16425 (Otha et al. 2003) in extinction of fear conditioning, two doses of Ki16425 (40 and 400 nM, selected on the basis of its ki value of 0.34 μM for the LPA1 receptor, and our previous pilot studies, unpublished) dissolved in vehicle solution (3 % fatty acid free bovine serum albumin/PBS) were i.c.v. injected in wt mice (2 μl/mouse). Injections were done in the right lateral cerebral ventricle, according to Ocaña et al. (1995), as detailed in online resource.
Mice were submitted to three phases of training: contextual fear conditioning (day 1), contextual extinction (day 2), and recall testing (day 3) (Fig. 2a). Wild-type mice were treated with vehicle or Ki16425. No-treated wt and null mice were used as control groups of treatments and administration. Seven to nine animals were used per experimental condition. On day 1, after an initial 120-s acclimation period, animals received a 2-s shock of 0.6 mA followed by a 120-s no-stimulus consolidation period before returning to their home cage. On day 2, initial contextual fear recall and contextual extinction were assessed. For these purposes 10-min context exposures in absence of shock was carried out. Immediately following removal from the context, i.c.v. administration was performed. Control mice were returned to the home cage without any treatment. On day 3, the effect of pharmacological administration on extinction consolidation was examined using 10-min exposures in ‘context A’.
i.c.v. administration of LPA1 receptor antagonist Ki16425 mimicked the behavioral phenotype of LPA1-null mice. a Procedure of contextual fear conditioning (day 1), contextual extinction (day 2), and recall testing (day 3). Behavior exhibited by animals during fear conditioning (day 1) b or during contextual fear extinction (day 2) c. No significant differences were observed among groups. On day 3, the effects of pharmacological administrations on extinction consolidation were examined c. The administration of the antagonist of LPA1 receptor (at dose of 400 nM) impaired extinction recall and induced similar behavioral phenotype of LPA1-null mice. (N = 9 wt; 9 nulls, 8 veh; 9 Ki16425 (400 nM); 6 Ki16425 (40 nM). Post hoc LSD test: (++ p < 0.01), significant differences between initial exploration period and post-conditioning protocol; (*p < 0.05; **p < 0.01), significant differences between wt versus LPA1-null; (# p < 0.05; ## p < 0.01), significant differences between wt mice and animals treated with LPA1 receptor antagonist (Ki16426 at dose of 400 nM); ($ p < 0.01), significant differences between animals treated with vehicle and with Ki16426 at dose of 400 nM; (& p < 0.01), significant differences between animals treated with LPA1 receptor antagonist at dose of 400 versus 40 nM. Abbreviations: c T period of 120 s each
Volume, number of NeuN+ neurons, calcium-binding protein, and GABA+ cells expression in the amygdala and the medial prefrontal cortex of wild-type and LPA1-null mice
To elucidate the neural basis of the fear extinction impairment in null mice we analyzed the populations of mature neurons as well as GABAergic and calcium-binding protein expressing cells in the infralimbic (IL) and prelimbic (PL) prefrontal cortices, the basolateral (BLA) and central (CE) amygdala, and the medial intercalated (ITC) amygdala cells. These brain areas are consistently recruited during extinction or during stress responses in rodents and humans (Quirk et al. 2006; rev. in Hartley and Phelps 2010). LPA1 expression was analyzed in parallel. The hippocampus, a crucial area in contextual processing, was previously examined (Matas-Rico et al. 2008; Castilla-Ortega et al. 2011, 2012).
Six mice from each genotype were transcardially perfused and subsequently processed for immunohistochemistry using the following primary antibodies: mouse monoclonal anti-NeuN antibody (Chemicon, Temecula, CA, USA), and anti-calbindin (Swant, Marly, Switzerland), anti-calretinin (Swant), anti-parvoalbumin (Swant), and anti-GABA (Sigma-Aldrich, St. Louis, MO, USA) rabbit polyclonal antibodies. Detection was carried out using ExtrAvidin®-peroxidase (Sigma-Aldrich; St. Louis, MO, USA) and diamino benzidine. Calcium-binding proteins were chosen because of their common co-localization with GABA, defining a large portion of the GABA population (DeFelipe 1993). These neuronal markers are useful for characterizing morphologically and chemically functional neuronal subpopulations and result essential for amygdala-based emotional facilitation of learning and memory (Spampanato et al. 2011) and cortical processing (Zaitsev et al. 2005). Cell quantifications and volume estimation were performed by stereological procedures using the CAST-Grid software package (Olympus, Glostrup, Denmark) or quantitative planimetry using the ‘Visilog 5’ image analysis system (Noesis, France). Histological, cell counting, and volume estimation procedures are detailed in the online resource.
Response to acute stress
Acute stress protocol
Beginning at 10:00 a.m., mice were subjected to 30 min of restraint in a modified 50-ml clear polystyrene conical centrifuge tube with multiple air holes for ventilation. Control mice remained undisturbed in their home cages.
Corticosterone assay
Corticosterone levels were studied in control and acutely stressed wt and null mice, seven to eight mice per genotype and experimental condition. To obtain plasma samples, after being restrained for 30 min, the mice were rapidly decapitated and trunk blood collected in the presence of EDTA and stored at −80 °C. Control mice were taken directly from their home cage and killed immediately. Plasma corticosterone levels were determined in duplicate, using a commercially available radioimmunoassay kit (intra-assay variability <8 %) following the manufacturer’s instructions (DPC, Los Angeles, CA, USA).
C-Fos immunoreactivity in the amygdala and the medial prefrontal cortex following acute stress
Eight animals per genotype were used for each experimental condition to examine the effect of LPA1 receptor absence on the induction of the neuronal activity marker, c-Fos protein, following acute episodes of stress. Control mice were taken directly from their home cage and transcardially perfused, whereas stressed mice were perfused 90 min after the completion of the stress treatment. Brains were later processed for DAB immunohistochemistry with a rabbit anti-c-Fos primary polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the number of positive cells was determined by stereology. A detailed description of histological and cell counting methodologies is provided in the online resource.
Statistical analysis
Fear conditioning data (day 1) were analyzed by two-way repeated measures ANOVA for the ‘genotype × trials’, in which each trial was 120 s of interval between tones plus 30 s of tone that co-terminated with a shock lasting 2 s. For the cueEXT groups, ANOVA for the ‘genotype × trial block’, in which each ‘trial block’ was a set of 10 blocks of 150 s divided into 30 s of tone and 120 s of interval between tones, was conducted for the session extinction data (day 2). Data of cue extinction recall (days 3 and 5) were analyzed using ANOVA for the ‘training group × trial block’, in which a ‘trial block’ consisted of 30 s of tone and a 5-s interval between tones. For the ctxEXT groups, the same analyses were performed, but the trial blocks were changed to time intervals of 120 s. Data from the initial period of acclimation (on days 1 and 2) were analyzed using Student’s t test. Pharmacological data were analyzed using one-way ANOVA for experimental groups on day 2 and by a two-way ANOVA repeated measures (experimental groups × intervals) on days 1 and 3 (conditioning day and extinction test, respectively). ANOVA was followed by post hoc Fisher’s least significant difference test (LSD).
Data from neuronal quantification were analyzed using Student’s t test. Corticosterone and c-Fos immunoreactivity were analyzed using two-way ANOVA (‘genotype × stress’) followed by the LSD post hoc test.
Only probabilities ≤ 0.05 were considered significant. For the benefit of clarity and brevity, only relevant results of these statistical analyses are reported.
Results
Fear conditioning and extinction
Impaired fear conditioning in LPA1-null mice
Freezing during the conditioning session is shown in Fig. 1b. Both wild-type and null mice showed no freezing during the first 120 s of exploration of the conditioning chamber. All animals showed increased freezing significantly across the three conditioning trials on the conditioning day (‘trials’: F(2,90) = 207.245; p < 0.001). However, we also observed significant differences between genotypes (‘genotype’: F(1,45) = 5.922; p = 0.018; ‘trials × genotype’: F(2,90) = 5.123; p = 0.007). Post hoc analysis revealed that all animals increased their freezing behavior during training. Nevertheless, null animals showed a lower rate of freezing after the second and third trials of conditioning compared with wild-type animals (Fig. 1b).
Absence of the LPA1 receptor affects cued fear extinction
On day 2, during the cued extinction procedure, 24 h after conditioning, cueEXT-animals rarely froze during the initial exploratory 120 s in the new chamber in the absence of tone presentation. During the subsequent block of tone presentation, however, both genotypes showed the same amount of freezing (Fig. 1c), suggesting equal association and consolidation in response to cued fear conditioning. Notwithstanding this, only wild-type animals showed reduced freezing between the first and second block of tone presentation, which was maintained in the third trial block presentation. Two-way ANOVA revealed differences between trial blocks (F(2,26) = 5.034; p = 0.010) and in the interaction ‘genotype × trial blocks’ (F(2,26) = 3.014, p = 0.039) (LSD results, Fig. 1c), indicating that null mice showed problems in cued fear extinction within the session.
Two-way repeated measures ANOVA confirmed the role of the LPA1 receptor during recall to cued fear extinction on days 3 and 5 (Fig. 1d, LSD results). On day 3, there were significant differences in the ‘training group’ (F(3,27) = 3.3, p = 0.030), ‘trial blocks’ (F(4,108) = 3.374, p = 0.012) and ‘trial blocks × training’ (F(12,108) = 3.210, p = 0.000). On day 5, differences were demonstrated between ‘training groups’. (F(3,27) = 6.484, p = 0.001). Using this protocol, cueEXT-wt exhibited low levels of freezing. Although these animals showed decreased freezing in all trial blocks, cueEXT-null mice failed to extinguish the freezing response, thus exhibiting similar freezing levels to the CONTROL groups.
On days 3 and 5, after recall of cued fear extinction, animals were tested in context A to examine the selectivity of the procedure. Two-way ANOVA showed differences among the ‘training groups’ (day 3: F(3,27) = 15.599, p = 0.000; day 5: F(3,27) = 9.685, p = 0.000) and ‘trials’ (day 3: F(4,108) = 14.746, p = 0.000; day 5: F(4,108) = 25.961, p = 0.000). Moreover, our analysis revealed an interaction with ‘training groups × trials’ (day 3: F(12,108) = 4.713, p = 0.000; day 5: F(12,108) = 3.859, p = 0.000). CueEXT-wt showed an extinction response to the context that was previously paired with the US (Context A), but it was not extinguished. However, in cueEXT-null mice, freezing increased progressively over time, showing a similar amount of freezing during the last intervals of the experiment as that of the CONTROL group (Fig. 1e).
Lack of the LPA1 receptor affects contextual fear extinction
On day 2, freezing responses were similar in the ctxEXT-animals from both genotypes. Two-way ANOVA revealed no significant effect associated with genotype based on intervals or interactions. Collectively, these data suggest that the lack of the LPA1 receptor did not affect the consolidation of conditioned contextual fear (Fig. 1f). During repeated context exposure on day 3, however, ctxEXT-wt significantly reduced their freezing percentage across intervals (F(4,120) = 6.592, p = 0.000; LSD p < 0.05 interval 3 versus 4) (Fig. 1h). In contrast, LPA1-null mice maintained their freezing percentage. On day 5, the repetitive exposure to the fear conditioning context, which could be considered a contextual extinction procedure, was effective in ctxEXT-wt mice. In fact, on day 5, significant differences in freezing responses among groups were observed (F(3,28) = 5.479, p = 0.010); among intervals (F(4,112) = 17.370, p = 0.001) and for interactions between training groups and intervals (F(12,112) = 2.500, p = 0.006). The LSD results are shown in Fig. 1h. These data show that ctxEXT-wt mice display reduced freezing responses over the duration of the test. In contrast, although ctxEXT-null mice showed reduced levels of freezing during the first ‘trial block’, they had progressively elevated freezing responses across the test. In fact, as time passed, null animals increased their percentage of freezing whether or not they had received contextual fear extinction training. Taken together, these data suggest that the LPA1 receptor is involved in contextual fear extinction.
In contrast, training to contextual fear extinction did not affect cued fear recall. Levels of freezing responses were similar among groups, persisting throughout the third (F(3,28) = 0.997, p = 0.409) and fifth (F(3,28) = 2.693, p = 0.065) day of the test. Only ctxEXT-wt mice showed a slight, although statistically nonsignificant, decrease of freezing on the last interval of the fifth day (Fig. 1g).
I.c.v. administration of LPA1 receptor antagonist Ki16425 impaired fear extinction and mimicked the behavioral null phenotype
All mice showed no freezing during the first exploratory 120 s before conditioning training. After conditioning, all groups increased significantly their freezing behavior, indicating a successful conditioning process (F(1,37) = 100.854; p = 0.000) and without differences between groups (F(4,37) = 0.293; p = 0.880) (Fig. 2b). Animals that did not satisfy conditioning criteria were ruled out of the analysis. On day 2, data revealed no significant differences among groups in consolidation of contextual conditioned fear (F(4,37) = 2,012; p = 0.105).
Pharmacological treatments (vehicle and LPA1 receptor antagonist Ki16425, 40 and 400 nM) were i.c.v. administrated immediately after finishing extinction training on day 2, and the effects were examined on day 3. Two-way repeated measures ANOVA revealed significant differences among the ‘treatments’ (F(4,37) = 6.612, p = 0.000), ‘intervals’ (F(4,148) = 4.7805, p = 0.001), and interaction ‘treatments × intervals’ (F(16,148) = 2.6666 p = 0.001). Ki16426, at a dose of 400 nM, impaired extinction recall and induced similar behavior to that observed in LPA1-null mice, exhibiting a progressive increment of freezing (Fig. 2c). These data largely confirm the phenotype of LPA1-null mice, indicating a participation of the LPA1 receptor in extinction of aversive memories.
Morphological characterization of the cortico-amygdala circuit in LPA1-null mice
Given our previous works on LPA1-null mice reporting defective hippocampal and cortical neurogenesis (Estivill-Torrús et al. 2008; Matas-Rico et al. 2008; Castilla-Ortega et al. 2011), associated to altered responses to chronic stress (Castilla-Ortega et al. 2011), or changes in anxiety-like behavior (Santin et al. 2009; Castilla-Ortega et al. 2010), we sought to examine the integrity of the cortico-amygdala circuit in these mice. Immunohistochemical analysis of the expression of mature neuronal marker NeuN showed that both genotypes displayed similar mPFC structure. In the IL and PL regions, neuronal density, volume, and total number of neurons were not different between genotypes (Figure S1). Consistent with this finding, morphological differences between genotypes were not observed in CE (Fig. 3b–d). In the BLA, there was no significant effect of genotype on the neuronal density (t = 1.737; p = 0.125) (Fig. 3d). However, LPA1-null mice display a dramatic reduction of volume (t = 3.474, p = 0.010) and number of total neurons (t = 4.235, p = 0.003) in the BLA (Fig. 3b, c respectively). These anomalies could be explained by a defective development from a life-long absence of the LPA1 receptor, considering the specific presence of the LPA1 receptor in fibers and neuronal bodies in this region in adult and younger ages under normal circumstances (Fig. 3a).
The absence of LPA1 receptor induces morphological abnormalities in BLA but not in CE. a Representative picture of BLA and CE image immunostaining by anti-LPA1 in adult and postnatal day 30. In LPA1-null mice, the expression of LPA1 receptor was absent. The images were taken at ×4 (left and right) or ×40 magnification (middle). b LPA1-null mice showed reduced BLA volume and c a smaller number of neurons in this region. d There are no differences in the density of neuron between genotypes in BLA, and the absence of LPA1 receptor did not affect any parameter examined in CE. T Student: (*p < 0.05; ***p < 0.001), significant differences between wt versus LPA1-null. e Representative picture of BLA and CE image immunostaining by NeuN. The image was taken at ×4 magnification and square image (bottom) was taken at ×40 magnification. Bar scale in a 200 μm (middle) and 50 μm (right), respectively; in d 200 μm (top) and 50 μm (bottom), respectively
In the absence of the LPA1 receptor, we observed a reduction in the number of GABA+ cells and calcium-binding proteins in the amygdala (Fig. 4), showing, as compared with wt, a slight, but not significant reduction of GABA+ cells (Fig. 4e–g) and a significant reduction of PV+ (t = 2,890; p = 0.020) and CR+ (t = 4.715; p = 0.000) cells in the BLA (Fig. 4h, i), and of CB + cells in the CE amygdalar medial nucleus (Fig. 4j) (t = −2,536; p = 0.020). The absence of LPA1 receptor did not affect the expression of GABA+ cells in the medial ITC neurons. In PFC, although no differences were observed between genotypes in any of the calcium-binding proteins (Figure S2), quantification of GABA+ cells revealed a significant reduction in LPA1-null mice (t = 2.210; p = 0.043).
Reduced expression of calcium-binding protein in amygdala of LPA1-null mice. a A representative picture of GABA+ cells in BLA, CE, and medial ITC neurons in amygdala. b A representative PV+, c CR+ staining cells in BLA and d CB+ staining cells in CE amygdala in both wt and null animals are shown. The quantification of GABA+ cells in BLA, CE, and medial ITC neurons in amygdala revealed no significant differences between genotypes (e–g, respectively). However calcium-binding proteins quantification revealed reduced expression of PV h and CR in BLA i and CB in CE j in the absence of LPA1 receptor. T Student: (*p < 0.05; ***p < 0.001), significant differences between wt versus LPA1-null mice. All images of the left panel in a, e, g, and i were taken at ×4 magnification. In the right panels the images were taken at ×40 magnifications. Bar scale in i (also valid for a, e, and g): 200 μm (left) and 50 μm (right), respectively
Acute stress response
Acute stress induces an exaggerated corticosterone response in the absence of LPA1 receptor
The analysis of plasma corticosterone levels did not show any significant difference between genotypes in control conditions. After acute stress, corticosterone levels were significantly increased in both wt and, particularly, null mice. In this genotype, an enhanced corticosterone response was observed. ANOVA revealed significant effect of ‘stress’ (F(1,30) = 333.670, p = 0.000), ‘genotype’ (F(1,30) = 20.610, p = 0.000), and ‘genotype × stress’ (F(1,30) = 9.940, p = 0.000) (Fig. 5c).
The absence of LPA1 receptor induces exaggerated corticosterone response and exacerbated c-Fos expression in amygdala after an acute episode of stress. a An illustrative c-Fos immunostaining of the amygdala is shown, indicating the BLA and CE nucleus. The image was taken at ×4 magnification. b Representative examples of c-Fos-stained nucleus of BLA and CE sections from every genotype and treatment are shown. The images were taken at ×40 magnification. c Corticosterone levels in control and after acute stress conditions. Basal serum corticosterone levels were similar between wt and LPA1-null, but stressed null mice showed a marked corticosterone response. d Estimation of the numerical density of c-Fos positive cells (c-Fos/mm3) in BLA. Stress increases c-Fos expression in both genotypes, but the increase was more marked in the case of LPA1-null mice. e Estimation of the numerical density of c-Fos positive cells (c-Fos/mm3) in the CE nucleus. Stress increases c-Fos expression in both genotypes, but in the case of LPA1-null mice the central amygdala showed increased responsivity. Post hoc LSD test: (*p < 0.05; ***p < 0.001), significant difference of the stressed group versus its control; (&& p < 0.005; &&& p < 0.001), significant differences between wt versus LPA1-null; $ p = 0.08, tendency to signification. Bar scale in a: 50 μm; in b: 200 μm
Absence of the LPA1 receptor induces aberrant c-Fos expression in the amygdala after an acute episode of stress
Estimation of the numerical density of c-Fos positive cells (c-Fos/mm3) revealed that stress increased the number of c-Fos+, i.e., activated, cells in the BLA in both genotypes (‘stress’: F(1,32) = 12,314, p = 0.001; Fig. 5d). We observed a trend toward significance using genotype as a variable (F(1,32) = 3.1280, p = 0.086) in which LPA1-null animals had an exaggerated c-Fos response. In the CE, after acute stress, both genotypes showed significant c-Fos expression, but the increase was more marked in null mice. ANOVA revealed significant effect of ‘genotype’ (F(1,32) = 7.761, p = 0.009), ‘treatment’ (F(1,32) = 26.430, p = 0.000), and ‘genotype × treatment’ (F(1,32) = 4.486, p = 0.042). LSD results are shown in Fig. 5e. In the IL and PL cortex, null mice showed increased expression of c-Fos+ cells compared to wt mice under basal conditions; however, only wt mice showed increased expression of this protein after acute stress (Figure S3).
Discussion
Our study clearly indicates the involvement of the LPA1 receptor in the extinction of aversive memories, demonstrating for the first time a crucial role in the extinction of fear conditioning. Thus, the lack of LPA1 receptor or its pharmacological blocking produced significant deficits in this process. In null animals, morphological abnormalities such as reduced volume, number of neurons, and expression of calcium-binding proteins were observed in the amygdala, a key locus of a corticolimbic circuit that mediates the processing of emotional stimuli. Finally, we demonstrated that lack of LPA1 receptor coupled with acute stress induces an altered endocrine response and abnormal patterns of activity in the amygdala supporting increased emotional reactivity.
The expression of emotional responses elicited by context or cued CS was identical in genotypes (first trials during day 2; see T1 in Fig. 1c, f), suggesting equally successful association and consolidation of cued or contextual fear conditioning. The lack of the LPA1 receptor did not affect the expression/consolidation of fear 24 h after fear conditioning, suggesting that the LPA1 receptor was not primary involved in these processes.
In terms of extinction, we first examined the role of the LPA1 receptor in auditory fear extinction. CueEXT-wt mice exhibited a reduction of freezing during extinction or recall test. By contrast, cueEXT-null mice did not show reduced freezing levels and apparent short-term extinction learning over 30 mass extinction sessions and across days. This finding is not an artifact of increased fear to tone per se or a result of increased pain perception. Our pilot studies (unpublished data) ruled out this possibility as no differences were observed between genotypes, indicating that the absence of the LPA1 receptor significantly impairs auditory fear extinction.
Concerning contextual extinction, wt mice, after repeated exposition to context, barely showed freezing behavior. The modest cue extinction observed in cueEXT-maLPA1-wt mice in comparison with the better contextual extinction could be explained, at least in part, by genetic factors, because the background strain for the LPA1 deletion was a hybrid of C57BL/6J and 129X1/SvJ. Consistent with this hypothesis, the rate of extinction observed in our animals was comparable to that observed in other laboratories using a similar 30-s tone mass fear conditioning extinction protocol (Herry et al. 2006; Hefner et al. 2008) in the C57BL/6J strain and was better than that observed in the 129X1/SvJ strain (Hefner et al. 2008). Moreover, a strong persistent cue-related fear memory compared with contextual fear memory has been observed in C57BL/6J mice, which display higher cue than context-related freezing during memory test (Brinks et al. 2009). By contrast, when LPA1-null mice were assessed in the context of conditioning, they showed a progressive increase in fear responses during recall sessions to extinction, independent of the extinction training received (contextual or auditory). Consistent with this, in the pharmacologic study, LPA1-null animals, trained to contextual conditioning and extinction, showed a progressive increment of fear. Pharmacological blocking of LPA1 receptor induced a dramatic impairment of extinction behavior and, similarly to null mice, a progressive increment of freezing. This profile is reminiscent of fear incubation, defined as an increase in the conditioned response over a period of time without further exposure to the aversive stimulus (Eysenck 1968; Camp et al. 2009). Nevertheless, this study was not designed to directly test this hypothesis, so other mechanisms cannot be ruled out.
Taking into account the extinction experiments, our findings strongly suggest that LPA1 receptors are involved in emotional regulation and might be particularly important for the short-term extinction and for the extinction over repeated tone presentation or recurrent exposure to context of conditioning. It should be noted that the resistance to the extinction of conditioned fear responses and the incubation or paradoxical enhancement of fear are crucial in anxiety-related disorders (Sandin and Chorot 1989). The lack of LPA1 receptors engages therefore a variety of neural control mechanisms regulating fear extinction.
The specific nature of the behavioral deficits observed in LPA1-null mice suggests that dysfunction of the BLA, PFC, and hippocampus are involved. Previous works have evidenced that acquisition and short-term extinction are principally amygdala mediated (Maren and Quirk 2004; Pare and Duvarci, 2012) and that consolidation of extinction is PFC dependent (Maren and Quirk 2004; Stafford et al. 2012). The hippocampus, particularly affected in these null mice (Castilla-Ortega et al. 2011; Musazzi et al. 2011), plays a fundamental role in contextual fear extinction by modulating BLA activity (Ji and Maren 2007). Our present data indicate specific changes in PFC and BLA in the absence of LPA1, which might be related to deficits in extinction behavior. Anatomical studies provide support for this hypothesis. Thus, a smaller hippocampal volume may predispose an animal to acquire stronger and/or more persistent conditioned emotional responses (Gilbertson et al. 2002) resulting in contextual fear extinction impairment (Schimanski et al. 2002). Moreover, variation of BLA volume has been associated with differences in specific measures of mouse fear-, anxiety-, and stress-related phenotypes. Increased anxiety-like responses (Santin et al. 2009) and enhanced vulnerability to stress (Castilla-Ortega et al. 2011) reported in LPA1-null mice are common features observed in mouse strains with reduced amygdala volume, which show significantly greater conditioned freezing (Yang et al. 2008). Nevertheless, LPA1-null mice did not exhibit increased fear recall (day 2), likely reflecting a ceiling effect of freezing behavior due to aversiveness of the conditioning protocol, obscuring the effect of reduced amygdala volume. However, this genotype displayed more persistent conditioned emotional responses that it might lead to extinction resistance.
One plausible explanation for the impairment of fear extinction is that a smaller amygdala volume might be coupled with exaggerated amygdala activity during emotional processing (Meyer-Lindenberg et al. 2006). In line with this assumption, and consistent with data from strains with reduced amygdala (Yang et al. 2008), null mice following acute stress increased significantly their corticosterone levels and showed a striking activation of amygdala nuclei (BLA and CE), enhancing thus their emotional reactions. Afferent inputs from the BLA to the CE constitute an important pathway in the induction of different kinds of emotional responses (Everitt et al. 2000; Amano et al. 2010), and it is well known that amygdala stimulation can increase glucocorticoid release (Shepard et al. 2000, 2003). In this way, increased amygdala activity was generally found to cause augmented fear expression (Davis et al. 1994) which is consistent with observations in anxiety disorders showing amygdala hyperresponsiveness (Etkin and Wager 2007). Based on these data, we concluded that null animals have an altered intra-amygdala inhibitory mechanism.
It is widely recognized that an increase in the recruitment of inhibitory circuits in the BLA reduces pyramidal neuron activity (Spampanato et al. 2011) constraining the impact of sensory input. Likewise, GABAergic circuits within the CE may be a point of convergence for central stress promoting and anxiolytic/stress coping systems, and reduced CEm output suppresses fear responses (Ehrlich et al. 2009). Despite the slight reduction of GABA+ cells in the amygdala of LPA1-null mice, a more specific interneuron characterization revealed important changes on GABAergic neurons expressing calcium-binding proteins that control the in- and outflow of information of the amygdala (Davis et al. 1994) which, in turn, influences the generation of emotional responses (Reznikov et al. 2008). Specifically, in the absence of LPA1 receptor, we have observed a significant reduction of PV+ and CR+ cells in BLA and CB+ cells in CE. Considering that a reduction of GABAergic inhibitory control in the amygdala may be a common mechanism to generate a heightened emotional state (Rodríguez-Manzanares et al. 2005), the reduction of calcium-binding proteins may contribute to the abnormal emotional response reported in LPA1-null mice. However, the contribution of the mPFC should also be considered, given that extinction may require strengthening of GABAergic neurotransmission in this region (Akirav et al. 2006), and in null’s mPFC area, we observed a reduced expression of GABA+ cells.
Our findings raise the question: what are the potential mechanisms underlying the neural abnormalities observed in null mice? Our previous works demonstrated the involvement of LPA1 receptor in the development of cortical and hippocampal precursors (Estivill-Torrús et al. 2008; Matas-Rico et al. 2008), and its expression profile after birth is parallel to those observed in other markers associated with interneuron function (Cunningham et al. 2006). Considering its expression in amygdala, and similarly to cortex or hippocampus, its chronic absence would result, as shown, in specific developmental defects, including the reduction of volume and number of neurons, and impaired inhibitory mechanisms which may, in turn, induce hyperresponsiveness of the amygdala to emotional stimuli, leading to the observed phenotype. Dysfunction of this brain area affects the ability to regulate emotion, specifically fear extinction and appropriate response to stress, central features of anxiety disorders (Wellman et al. 2007; Hartley and Phelps 2010).
In summary, our findings suggest for the first time that the LPA1 receptor is required for conditioned fear extinction and that its absence could increase the risk for developing affective disorders, especially posttraumatic stress disorders, by compromising the morphological and functional integrity of the key limbic circuit. Moreover, lack of LPA1 induces exaggerated amygdala reactivity and endocrine responses to emotional stimuli (e.g., an acute episode of stress). Considering that a downregulation of the expression of the Lpar1 gene has been involved in neuropsychiatry diseases, such as schizophrenia (Bowden et al. 2006), these data support a role for LPA signaling via LPA1 receptors as a vulnerability factor for anxiety disorders and a potential therapeutic target for the treatment of these diseases.
References
Akirav I, Maroun M (2007) The role of the medial prefrontal cortex–amygdala circuit in stress effects on the extinction of fear. Neural Plast 2007:1–11
Akirav I, Raizel H, Maroun M (2006) Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci 23:758–764
Amano T, Unal CT, Pare D (2010) Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13:489–494
Bowden NA, Weidenhofer J, Scott RJ, Schall U, Todd J, Michie PT, Tooney PA (2006) Preliminary investigation of gene expression profiles in peripheral blood lymphocytes in schizophrenia. Schizophr Res 82:175–183
Brinks V, de Kloet ER, Oitzl MS (2009) Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp Neurol 216:375–382
Camp M, Norcross M, Whittle N, Feyder M, D’Hanis W, Yilmazer-Hanke D et al (2009) Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strains. Genes Brain Behav 8:744–752
Castilla-Ortega E, Sánchez-Lopez J, Hoyo-Becerra C, Matas-Rico E, Zambrana-Infantes E et al (2010) Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol Learn Mem 94:73–82
Castilla-Ortega E, Hoyo-Becerra C, Pedraza C, Chun J, Rodríguez de Fonseca F, Estivill-Torrús G et al (2011) Aggravation of chronic stress effects on hippocampal neurogenesis and spatial memory in LPA1 receptor knockout mice. PLoS ONE 6(9):e25522. doi:10.1371/journal.pone.0025522
Castilla-Ortega E, Pedraza C, Chun J, Rodríguez de Fonseca F, Estivill-Torrús G, Santín LJ (2012) Hippocampal c-Fos activation in normal and LPA1-null mice after two object recognition tasks with different memory demands. Behav Brain Res 231:400–405
Choi JW, Chun J (2013) Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta 1831:20–32
Contos JJ, Ishii I, Chun J (2000) Lysophosphatidic acid receptors. Mol Pharmacol 58:1188–1196
Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH et al (2006) Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci 26:2767–2776
Davis M, Rainnie D, Cassell M (1994) Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci 17:208–214
DeFelipe J (1993) Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex 3:273–289
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A (2009) Amygdala inhibitory circuits and the control of fear memory. Neuron 62:757–771
Estivill-Torrús G, Llebrez-Zayas P, Matas-Rico E, Santín L, Pedraza C, De Diego I et al (2008) Absence of LPA1 signaling results in defective cortical development. Cereb Cortex 18:938–950
Estivill-Torrús G, Santín LJ, Pedraza C, Castilla-Ortega E, Rodriguez de Fonseca F (2013) Role of lysophosphatidic acid (LPA) in behavioral processes: implications for psychiatric disorders. in: Chun J (ed) lysophospholipid receptors: signaling and biochemistry. Wiley, New Jersey, pp 451–474
Etkin A, Wager TD (2007) Functional neuroimaging of anxiety: a metaanalysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488
Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW (2000) Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton J (ed) The amygdala, 2nd edn. Oxford University Press, Oxford, pp 353–390
Eysenck HJ (1968) A theory of the incubation of anxiety/fear responses. Behav Res Ther 6:309–321
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP et al (2002) Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5:1242–1247
Hartley C, Phelps E (2010) Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35:136–146
Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A (2008) Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci 28:8074–8085
Heldt SA, Mou L, Ressler KJ (2012) In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Translat Psychiatry 2:e181. doi:10.1038/tp.2012.101
Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N (2006) Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24:261–269
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A (2010) Neuronal circuits of fear extinction. Eur J Neurosci 31:599–612
Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Orr SP, Cassidy BS et al (2009) Extinction memory is impaired in schizophrenia. Biol Psychiatry 65:455–463
Hong I, Song B, Lee S, Kim J, Kim J, Choi S (2009) Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. Eur J Neurosci 30:2089–2099
Ji J, Maren S (2007) Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 17:749–758
Konarski JZ, McIntyre RS, Soczynska JK, Kennedy SH (2007) Neuroimaging approaches in mood disorders: technique and clinical implications. Ann Clin Psychiatry 19:265–277
Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nat Rev Neurosci 5:844–852
Matas-Rico E, García-Díaz B, Llebrez-Zayas P, López-Barroso D, Santín L, Pedraza C et al (2008) Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci 39:342–355
Meyer-Lindenberg A, Buckholtz JW, Kolachana BR, Hariri A, Pezawas L, Blasi G et al (2006) Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103:6269–6274
Moustafa AA, Gilbertson MW, Orr SP, Herzallah MM, Servatius RJ, Myers CE (2013) A model of amygdala–hippocampal–prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn 81(1):29–43
Musazzi L, Di Daniel E, Maycox P, Racagni G, Popoli M (2011) Abnormalities in α/β-CaMKII and related mechanisms suggest synaptic dysfunction in hippocampus of LPA1 receptor knockout mice. Int J Neuropsychopharmacol 14:941–953
Ocaña M, Del Pozo E, Barrios M, Baeyens JM (1995) Subgroups among, μ-opioid receptor agonists distinguished by ATP-sensitive K+ channel-acting drugs. Br J Pharmacol 114:1296–1302
Otha H, Sato K, Murata A, Damirin A, Malchinkhuu E et al (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64:994–1005
Pare D, Duvarci S (2012) Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol 22:717–723
Quirk G, García R, González-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343
Quirk G, Paré D, Richardson R, Herry C, Monfils MH, Schiller D et al (2010) Erasing fear memories with extinction training. J Neurosci 30:14993–14997
Reznikov LR, Reagan LP, Fadel JR (2008) Activation of phenotypically distinct subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol 508:458–472
Rodríguez-Manzanares PA, Isoardi NA, Carrer HF, Molina VA (2005) Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci 25:8725–8734
Sandin B, Chorot P (1989) The incubation theory of fear/anxiety: experimental investigation in a human laboratory model of Pavlovian conditioning. Behav Res Ther 27:9–18
Santin LJ, Bilbao A, Pedraza C, Matas-Rico E, López-Barroso D, Castilla-Ortega E et al (2009) Behavioural phenotype of maLPA1-null mice: increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav 8:772–784
Schimanski LA, Wahlsten D, Nguyen PV (2002) Selective modification of short-term hippocampal synaptic plasticity and impaired memory extinction in mice with a congenitally reduced hippocampal commissure. J Neurosci 22:8277–8280
Shepard JD, Barron KW, Myers DA (2000) Corticosterone delivery to the amygdala increases corticotrophin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 861:288–295
Shepard JD, Barron KW, Myers DA (2003) Stereotaxic location of corticosterone to the amygdala enhance hypothalmo-pituitary-adrenal responses to behaviorial stress. Brain Res 963:203–213
Spampanato J, Polepalli J, Sah P (2011) Interneurons in the basolateral amygdala. Neuropharmacology 60:765–773
Stafford JM, Raybuck JD, Ryabinin A, Lattal KM (2012) Increasing histone acetylation in the hippocampus–infralimbic network enhances fear extinction. Biol Psychiatry 72:25–33
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R et al (2007) Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27:684–691
Yang RJ, Mazhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A (2008) Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology 33:2595–2604
Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kröner S, Lewis DA, Krimer LS (2005) Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15:1178–1186
Acknowledgments
We are grateful to Juan Gómez Repiso for his technical assistance, Elisa Matas-Rico for her contribution to the NeuN and calcium-binding proteins immunohistochemistry, Román Moreno and Marina Navarro for their contribution to stereological quantification of GABA+ cells, Jose Peral for his help in the pharmacological experiment, and Jose Ángel Aguirre Gómez for access to stereology. We thank the animal housing facilities of the University of Málaga for maintenance of the mice. This work was supported by grants from Spanish Ministry of Economy and Competitiveness (MEC SEJ2007-61187, co-funded by ERDF, MICINN PSI2010-16160, to L.J.S.; PI10/02514, co-funded by ERDF, to G.E-T; Red de Trastornos Adictivos RD06/001/0000, to F.R.F), Andalusian Ministries of Health and Economy, Innovation, Science and Employment (SEJ-4515, to L.J.S; CTS643 and Nicolás Monardes Programme, to G.E-T.; and SAF2010-20521, to F.R.F). E.C., J.S. and E.Z. were supported by an FPU Grant of the Spanish Ministry of Education (AP-2006-02582, AP-2007-03719, and AP-2010-2044, respectively) and University of Malaga (Ayuda para la actividad productiva del PIF, III Plan Propio) and Postdoctoral Fellowship ‘Sara Borrell’ of the National Institute of Health Carlos III to E.C.C.R. was supported by a FPU Grant of the Andalusian Ministry of Economy, Innovation, Science and Employment (FPDI 2010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pedraza, C., Sánchez-López, J., Castilla-Ortega, E. et al. Fear extinction and acute stress reactivity reveal a role of LPA1 receptor in regulating emotional-like behaviors. Brain Struct Funct 219, 1659–1672 (2014). https://doi.org/10.1007/s00429-013-0592-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0592-9