Abstract
Over the past few years multiple studies have attempted to uncover molecular signatures of memory reconsolidation when compared to consolidation. In the present study we used immunocytochemical detection of the MAPK/ERK1/2 pathway, to track activated neuronal circuits in the hippocampus and amygdala recruited during the consolidation and reconsolidation of a contextual fear conditioning (CFC) memory. We report selective differences in magnitude and temporal dynamics of activated ERK1/2 signalling in different subregions of these two structures between the post-training and post-retrieval periods, except in the dentate gyrus, where the patterns of activation were similar. We then focused on this brain area to dissect out the patterns of downstream ERK1/2 signalling components, including the phosphorylation of MSK-1 and histone H3 on ser10, along with the induction of the Immediate Early Genes (IEGs) Arc/Arg3.1, c-Fos and Zif268/Egr1 following CFC training and retrieval. We found that the completion of the nucleosomal response as well as the induction of IEGs shorter during the reconsolidation period as compared to consolidation. Our results shed new light on the cellular mechanisms underlying the consolidation and reconsolidation processes engaged following CFC training and retrieval and further extend the notion that memory reconsolidation is not mechanistically a repetition of consolidation. In addition, we provide evidence that the strength of a previously established CFC memory is characterized by distinct patterns of ERK1/2 activation in different hippocampal and amygdalar subfields upon CFC memory recall. Our results emphasize the differences between consolidation and reconsolidation processes in relation to contextual fear memories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fear conditioning is a commonly used paradigm to study learning and memory. This task can be designed to tax spatial/contextual (contextual fear conditioning—CFC) or non-spatial fear memory (cued or Pavlovian associative fear conditioning) where performance is motivated by emotions. The neuroanatomical substrate of fear memory formation has been extensively studied over the last two decades (Maren and Fanselow 1996; Fanselow and LeDoux 1999; Fendt and Fanselow 1999; Fanselow 2000; Maren and Quirk 2004; LeDoux 2000). Memory consolidation refers to the time-dependent stabilization process that leads to long-term storage of a newly acquired memory (McGaugh 2000). Compelling evidence also now suggests that reactivation of memory during retrieval can trigger the destabilization of the memory trace (Nader and Hardt 2009), a process that has been hypothesized to allow the updating of reactivated memories through the so-called reconsolidation process (Lee 2009). The degree of similarity between the events present at memory recall and the past learning experience is thought to gate the competition between new learning involving consolidation processes and memory updating involving reconsolidation processes (Besnard 2012; Besnard et al. 2012; Osan et al. 2011). Several lines of evidence now support the idea that reconsolidation recruits specific mechanisms that are not crucially involved in the consolidation process (Tronson and Taylor 2007).
At the cellular level, it is now well established that the learning of a new event increases the activity of several protein kinases, among which ERK1/2 is a major player (Thomas and Huganir 2004). For example, both contextual and cued fear learning are associated with a rapid and transient hyperphosphorylation of ERK1/2 in different subfields of the hippocampus (Sananbenesi et al. 2002; Trifilieff et al. 2006) and amygdala (Schafe et al. 2000; Radwanska et al. 2002; Trifilieff et al. 2007). Moreover, ERK1/2 activity in neurons is required for fear-motivated learning (Atkins et al. 1998; Selcher et al. 1999). MEK inhibitors that prevent ERK1/2 activation indeed impair cued (Schafe et al. 2000) and contextual (Athos et al. 2002) fear memory consolidation when infused into the lateral amygdala (LA) and hippocampus, respectively. These results are recapitulated in mutant mice expressing a dominant negative of MEK-1 (Kelleher et al. 2004; Shalin et al. 2004). Similarly, MEK inhibitors impair reconsolidation of cued fear memory when infused into the LA before recall (Duvarci et al. 2005; Doyere et al. 2007). However, whether ERK1/2 follows the same dynamics of activation during consolidation and reconsolidation remains to be established. Furthermore, present models of signalling in neurons suggest that ERK1/2 controls transcriptional events required for memory stabilization, but most ERK1/2 downstream molecular events involved, and their dynamics of activation in consolidation and reconsolidation, have as yet been poorly investigated.
Phosphorylation events are temporally limited (seconds to minutes) as opposed to the long-lasting expression of immediate early genes (IEGs) (minutes to hours). In this regard, cellular imaging of ERK1/2 phosphorylation has been previously used to explore the time-course of cellular activation in the hippocampus and amygdala following contextual and cued fear conditioning (Trifilieff et al. 2006, 2007). Such cellular imaging technique can also be applied to examine the post-translational modifications of histones underlying chromatin remodelling. The analysis of H3 (ser10) phosphorylation is therefore well suited for imaging intermediate events at the interface between the activation of ERK1/2 and the transcriptional programs leading to the induction of Arc/Arg3.1, c-Fos and Zif268 proteins via chromatin decompaction in response to mitogenic signalling (Brami-Cherrier et al. 2009).
In the present study, we first compared following CFC training and retrieval the temporal dynamic of ERK1/2 phosphorylation in different subregions of the hippocampus and amygdala. Second, we focused on the dentate gyrus (DG), a brain region in which the activation of a sparse but specific ensemble of neurons that contribute to a fear memory engram is sufficient for the recall of that memory (Liu et al. 2012) recently evidenced as an important hippocampal subfield involved in CFC consolidation and reconsolidation (Sekeres et al. 2012). In this hippocampal subfield, in association with the patterns of ERK1/2 signalling activity following CFC training and retrieval, we analysed MSK-1 phosphorylation, a direct downstream target of ERK1/2, H3 (ser10) phosphorylation, a landmark of chromatin remodelling, and the expression of the IEGs Arc/Arg3.1, c-Fos and Zif268. Finally, we examined the relationship between the strength of a previously established CFC memory and the pattern of ERK1/2 phosphorylation in the hippocampus and amygdala upon recall. We report a substantial difference in the magnitude and temporal dynamic of ERK1/2 signalling activity at the anatomical and molecular levels following CFC training and retrieval. Our results emphasize the differences between consolidation and reconsolidation processes and further raise the question as to whether this specific observation can be extended to cued fear memory and to other forms of memory.
Materials and methods
Mice
All experiments were conducted in accordance with the standard ethical guidelines (European Communities Guidelines on the Care and Use of Laboratory Animals: 86/608/EEC). Pharmacological and immunohistochemistry experiments were carried out in (10 week old) C57BL/6j male mice (Janvier, Le Genest St. Isle, France). Mice were maintained in a 12 h light/dark cycle in stable conditions of temperature (22 °C) and humidity (60 %), with food and water ad libitum. Testing was performed during the light phase of the cycle. Three days before the experiments, mice were briefly handled each day.
Contextual fear conditioning (CFC)
General procedure
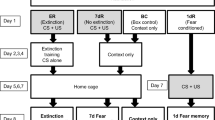
Mice were trained in conditioning chambers (17.5 × 17.5 × 15 cm) that had stainless steel rod floor through which scrambled footshocks could be delivered. Training consisted of placing mice in the chamber and delivering an unsignalled footshock (2 s duration 0.7 mA) 150 s later. Mice were returned to the home cage 30 s after the footshock. Memory was assessed as the percentage of time mice spent freezing when replaced in the training context. Freezing behaviour (defined as complete lack of movement, except for respiration) was assessed at 5 s intervals over a 300-s period (Fanselow 1980).
In the first set of experiments (Figs. 1, 2, 3, 4, 5, 6), 37 mice were used. Nine groups of mice were trained and one control group was perfused without being trained (No training control group, n = 3). Four groups were perfused at different time-points (0 min, n = 3; 15 min, n = 4; 30 min, n = 4 and 60 min, n = 4) following training (Training groups). On subsequent day, one of the five remaining groups was perfused without being submitted to a retrieval test (No retrieval control group, n = 3). The last four groups were perfused at different time-points (0 min, n = 4; 15 min, n = 4; 30 min, n = 4 and 60 min, n = 4) following a 5-min re-exposure session to the shock-paired context (Retrieval groups).
Time-course of ERK1/2 phosphorylation throughout the hippocampus and amygdala following CFC training. a ERK1/2 phosphorylation (P-ERK1/2) immunoreactive cells were analysed in the hippocampus and amygdala as indicated by grey areas on mouse brain coronal sections at various bregma coordinates. b We analysed the pattern of P-ERK1/2 at different time-points (0, 15, 30 and 60 min) following CFC training. Naive mice that did not undergo CFC training were used as control. c In the DG, the number of P-ERK1/2-positive cells was significantly increased immediately after training and returned to basal level within 15 min. d In the dorsal hippocampal CA3, the number of P-ERK1/2-positive cells was slightly increased immediately after training and returned to basal level within 60 min. e In the dorsal hippocampal CA1, the number of P-ERK1/2-positive cells was strongly increased immediately after training and progressively returned to basal level within 60 min. f In the LA and BLA the number of P-ERK1/2-positive cells was increased immediately after training and progressively returned to basal level within 60 min. In the CeA the number of P-ERK1/2-positive cells was increased 30 min and a second wave of ERK1/2 phosphorylation was detected 60 min after training. g Quantifications of P-ERK1/2 immunoreactive cells in the DG, CA3, CA1, LA, BLA and CeA at different time-points following CFC training. Data (mean ± SEM; n = 3–4 mice per group) were analysed using one-way ANOVA (between subjects) for DG (F (4,13) = 50.20, p < 0.001); for CA3 (F (4,13) = 1.34, NS); for CA1 (F (4,13) = 15.59, p < 0.001); for LA (F (4,13) = 2.67, NS); for BLA (F (4,13) = 3.41, p < 0.05); for CeA (F (4,13) = 7.27, p < 0.01); followed by post hoc comparisons (Dunett’s multiple test). *p < 0.05, **p < 0.01, ***p < 0.001, present time-point versus control. Scale bars 100 μm
Time-course of ERK1/2 phosphorylation throughout the hippocampus and amygdala following CFC retrieval. a P-ERK1/2 immunoreactive cells were analysed in the hippocampus and amygdala as indicated by grey areas on mouse brain coronal sections at various bregma coordinates. b We analysed the pattern of P-ERK1/2 at different time-points (0, 15, 30 and 60 min) following CFC retrieval. Trained mice that did not undergo CFC retrieval were used as control. c In the DG, the number of P-ERK1/2-positive cells was significantly increased immediately after retrieval and returned to basal level within 15 min. d In the dorsal hippocampal CA3, the number of P-ERK1/2-positive cells was slightly increased immediately after retrieval. e In the dorsal hippocampal CA1, the number of P-ERK1/2-positive cells was strongly increased immediately after retrieval and progressively returned to basal level within 60 min. f In the LA, BLA and CeA, the number of P-ERK1/2-positive cells was increased 30 min after retrieval and returned to basal level within 60 min. g Quantifications of P-ERK1/2 immunoreactive cells in the DG, CA3, CA1, LA, BLA and CeA at different time-points following CFC retrieval. Data (mean ± SEM; n = 3–4 mice per group) were analysed using one-way ANOVA (between subjects) for DG (F (4,14) = 12.99, p < 0.001); for CA3 (F (4,14) = 1.26, NS); for CA1 (F (4,14) = 3.12, p < 0.05); for LA (F (4,14) = 2.67, NS); for BLA (F (4,14) = 5.33, p < 0.01); for CeA (F (4,14) = 4.52, p < 0.05); followed by post hoc comparisons (Dunett’s multiple test). *p < 0.05, **p < 0.01, present time-point versus control. Scale bars 100 μm
Comparative patterns of ERK1/2 phosphorylation throughout the hippocampus and amygdala following CFC training and retrieval. a We compared the pattern of ERK1/2 phosphorylation at different time-points (0, 15, 30 and 60 min) following CFC training (black circles) and retrieval (grey circles). b The number of P-ERK1/2 immunoreactive cells was normalized to controls. In the DG the percent increase of P-ERK1/2-positive cells and the temporal profile of activation were similar following training and retrieval. In the dorsal hippocampal CA3 the percent increase of P-ERK1/2 was not significantly different following CFC training and retrieval. In the dorsal hippocampal CA1 the percent increase of P-ERK1/2-positive cells was significantly higher following CFC training than retrieval. In the LA, BLA and CeA the percent increase of P-ERK1/2 immunoreactive cells was significantly different following CFC training and retrieval. Whereas the peak of increase in P-ERK1/2 immunoreactive cells was observed immediately following CFC training, it was delayed following CFC retrieval (30 min). Data (mean ± SEM; n = 3–4 mice per group) were analysed using two-way ANOVA: for DG: effect of time F (4,27) = 48.22, p < 0.001; effect of procedure, F (1,27) = 1.85, NS; interaction, F (4,27) = 1.97, NS; for CA3: effect of time F (4,27) = 1.66, NS; effect of procedure, F (1,27) = 3.47, NS; interaction, F (4,27) = 0.91, NS; for CA1: effect of time F (4,27) = 14.64, p < 0.001; effect of procedure, F (1,27) = 16.69, p < 0.001; interaction, F (4,27) = 4.86, p < 0.01; for LA: effect of time F (4,27) = 2.04, NS; effect of procedure, F (1,27) = 7.03, p < 0.05; interaction, F (4,27) = 3.39, p < 0.05; for BLA: effect of time F (4,27) = 3.46, p < 0.05; effect of procedure, F (1,27) = 11.91, p < 0.01; interaction, F (4,27) = 4.9, p < 0.01; for CeA: effect of time F (4,27) = 5.26, p < 0.01; effect of procedure, F (1,27) = 1.39, NS; interaction, F (4,27) = 5.71, p < 0.01; followed by post hoc comparisons (Bonferroni test). # p < 0.05, ### p < 0.001, training versus retrieval
Time-course of MSK-1 and H3 (ser10) phosphorylation and Arc/Arg3.1, c-Fos and Zif268 expression throughout the DG following CFC training. a Immunoreactive cells were analysed in the DG as indicated by grey areas on mouse brain coronal sections at indicated bregma coordinates. b We analysed the pattern of MSK-1 and H3 (ser10) phosphorylation as well as Arc/Arg3.1, c-Fos and Zif268 expression at different time-points (0, 15, 30 and 60 min) following CFC training. Naive mice that did not undergo CFC training were used as control. c As illustrated, the maximal number of P-MSK-1 immunoreactive cells (white arrows) was detected immediately after training and returned to basal level in 15 min. d In contrast, the number of P-H3 (ser10) immunoreactive cells progressively increased over the first 30 min following training and returned to basal level in 60 min. e–f The number of Arc/Arg3.1 and c-Fos immunoreactive cells was maximal 30 min following training and was maintained up to at least 60 min. g Finally, the number of Zif268 immunoreactive cells progressively increased over the first 60 min following training. h Quantifications of P-MSK-1, P-H3 (ser10), c-Fos, Arc/Arg3.1 and Zif268 immunoreactive cells in the DG at different time-points following CFC training. The number of P-ERK1/2 immunoreactive cells from Fig. 1 is presented for comparison. Data (mean ± SEM; n = 3–4 mice per group) were analysed using one-way ANOVA (between subjects) for P-MSK-1 (F (4,13) = 13.63, p < 0.001); for P-H3 (ser10) (F (4,13) = 6.48, p < 0.01); for Arc/Arg3.1 (F (4,13) = 12.88, p < 0.001); for c-Fos (F (4,13) = 31.15, p < 0.001); for Zif268 (F (4,13) = 9.21, p < 0.001); followed by post hoc comparisons (Dunett’s multiple test). *p < 0.05, **p < 0.01, ***p < 0.001, present time-point versus control. Scale bars 100 μm
Time-course of MSK-1 and H3 (ser10) phosphorylation and Arc/Arg3.1, c-Fos and Zif268 expression throughout the DG following CFC retrieval. a Immunoreactive cells were analysed in the DG as indicated by grey areas on mouse brain coronal sections at indicated bregma coordinates. b We analysed the pattern of MSK-1 and H3 (ser10) phosphorylation as well as Arc/Arg3.1, c-Fos and Zif268 expression at different time-points (0, 15, 30 and 60 min) following CFC retrieval. Trained mice that did not undergo CFC retrieval were used as control. c As illustrated, the maximal number of P-MSK-1 immunoreactive cells (white arrows) was detected immediately after retrieval and returned to basal level in 15 min. d In contrast, the number of P-H3 (ser10) immunoreactive cells increased over the first 15 min following retrieval and returned to basal level in 60 min. e–f The number of Arc/Arg3.1 and c-Fos immunoreactive cells was maximal at 30 min following retrieval and returned to basal level in 60 min. g Finally, the number of Zif268 immunoreactive cells progressively increased over the first 60 min following retrieval. h Quantifications of P-MSK-1, P-H3 (ser10), c-Fos, Arc/Arg3.1 and Zif268 immunoreactive cells in the DG at different time-points following CFC retrieval. The number of P-ERK1/2 immunoreactive cells from Fig. 2 is presented for comparison. Data (mean ± SEM; n = 3–4 mice per group) were analysed using one-way ANOVA (between subjects) for P-MSK-1 (F (4,14) = 12. 31, p < 0.001); for P-H3 (ser10) (F (4,14) = 11.43, p < 0.001); for Arc/Arg3.1 (F (4,14) = 3.37, p < 0.05); for c-Fos (F (4,14) = 4.59, p < 0.05); for Zif268 (F (4,14) = 14.80, p < 0.001); followed by post hoc comparisons (Dunett’s multiple test). *p < 0.05, **p < 0.01, ***p < 0.001, present time-point versus control. Scale bars 100 μm
Comparative time-course of MSK-1 and H3 (ser10) phosphorylation and Arc/Arg3.1, c-Fos and Zif268 expression throughout the DG following CFC training and retrieval. a We compared the pattern of MSK-1 and H3 (ser10) phosphorylation as well as Arc/Arg3.1, c-Fos and Zif268 expression in the DG at different time-points (0, 15, 30 and 60 min) following CFC training (black circles) and retrieval (grey circles). b The number of immunoreactive cells was normalized to controls. The percent increase in P-ERK1/2 and P-MSK-1-positive cells was similar following training and retrieval when compared to the control groups. In contrast, the increase in P-H3 (ser10), Arc/Arg3.1, c-Fos and Zif268-positive cells was of a higher magnitude and longer duration following CFC training compared to retrieval. Data (mean ± SEM; n = 3–4 mice per group) were analysed using two-way ANOVA: for P-MSK-1: effect of time F (4,27) = 26.67, p < 0.001; effect of procedure, F (1,27) = 2.61, NS; interaction, F (4,27) = 0.82, NS; for P-H3 (ser10): effect of time F (4,27) = 13.87, p < 0.001; effect of procedure, F (1,27) = 12.45, p < 0.01; interaction, F (4,27) = 1.52, NS; for Arc/Arg3.1: effect of time F (4,27) = 13.47, p < 0.001; effect of procedure, F (1,27) = 7.09, p < 0.05; interaction, F (4,27) = 4.21, p < 0.01; for c-Fos: effect of time F (4,27) = 17.50, p < 0.001; effect of procedure, F (1,27) = 6.59, p < 0.05; interaction, F (4,27) = 5.33, p < 0.01; for Zif268: effect of time F (4,27) = 19.82, p < 0.001; effect of procedure, F (1,27) = 13.96, p < 0.001; interaction, F (4,27) = 1.36, NS; followed by post hoc comparisons (Bonferroni test). ### p < 0.001, training versus retrieval
In the second set of experiments (Fig. 7b, c), 24 mice were used. Three groups of mice were trained with either 0 (n = 8), 1 (n = 8) or 3 (n = 8) footshocks during the training session. The next day, freezing behaviour was assessed by re-exposing the three groups to the shock-paired context.
Effect of memory strength on ERK1/2 activation throughout the hippocampus and amygdala following CFC retrieval. a P-ERK1/2 immunoreactive cells were analysed in the hippocampus and amygdala as indicated by grey areas on mouse brain coronal sections at various bregma coordinates. b We analysed the pattern of ERK1/2 phosphorylation (P-ERK1/2) immediately (0 min) after CFC retrieval in mice previously trained with either 0, 1 or 3 footshocks. A naive group of mice was used as control. c Freezing performance elicited by the different training procedures with 0, 1 or 3 footshocks. d In the DG, the number of P-ERK1/2 immunoreactive cells was significantly increased in the groups submitted to retrieval. Note that the pseudo-trained group (0 footshock) exhibited the maximal number of P-ERK1/2-positive cells. e In the dorsal hippocampal CA3, the number of P-ERK1/2-positive cells activated after retrieval increased in correlation with memory strength, with a maximal number of P-ERK1/2 cells in the group that received three footshocks during training. f In the dorsal hippocampal CA1, the number of P-ERK1/2 immunoreactive cells was significantly increased following retrieval. No correlation was found between the number of P-ERK1/2-positive cells and memory strength. g In the LA, BLA and CeA, the number of P-ERK1/2-positive cells was not increased immediately after retrieval. c Data (mean ± SEM; n = 8 mice per group) were analysed using one-way ANOVA (between subjects) (F (2,20) = 149.7, p < 0.001); followed by post hoc comparisons (Newman–Keuls test). ***p < 0.001, 1 or 3 versus 0 shock-pairing; ### p < 0.001, 3 versus 1 shock-pairing. h Quantifications of P-ERK1/2 immunoreactive cells in the DG, CA3, CA1, LA, BLA and CeA at immediately following CFC retrieval in mice previously trained with either 0, 1 or 3 footshocks. Naive mice were used as control. Data (mean ± SEM; n = 4 mice per group) were analysed using one-way ANOVA (between subjects) for DG (F (3,12) = 12.19, p < 0.001); for CA3 (F (3,12) = 8.16, p < 0.01); for CA1 (F (3,12) = 7.38, p < 0.01); for LA (F (3,12) = 0.46, NS); for BLA (F (3,12) = 0.6, NS); for CeA (F (3,12) = 0.21, NS); followed by post hoc comparisons (Newman–Keuls test). *p < 0.05, **p < 0.01, ***p < 0.001, retrieval groups versus control; # p < 0.05, 1 or 3 versus 0 shock-pairing. Scale bars 100 μm
In the third set of experiments (Fig. 7d–h), 16 mice were used. Three groups of mice were trained with either 0 (n = 4), 1 (n = 4) or 3 (n = 4) footshocks during the training session. The next day, the three groups were perfused immediately following a 5-min re-exposure session to the shock-paired context. A group of naive mice was used as control (n = 4).
Tissue preparation and immunohistochemistry
Mice were anaesthetized immediately after the 300 s retrieval session with pentobarbital (500 mg/kg, i.p.; Sanofi-Aventis, Paris, France) and perfused transcardially with a fixative solution containing 4 % paraformaldehyde (PFA) (w/v) in 0.1 M Na2HPO4/Na2HPO4 buffer, pH 7.5 (4 °C), delivered via a peristaltic pump at 20 ml/min for 5 min. Brains were post-fixed overnight in the same solution and stored at 4 °C. Sections (30 μm thick) were cut with a vibratome (Leica, Nussloch, Germany) and kept at −20 °C in solution containing 30 % ethylene glycol (v/v), 30 % glycerol (v/v), and 0.1 M phosphate buffer. Sections were then processed as follows. Day 1, free-floating sections were rinsed three times for 10 min in Tris-buffered saline (TBS; 25 mM Tris–Cl, 150 mM NaCl, pH 7.5), followed by a permeabilization step 15 min in 0.2 % Triton X-100 in TBS. Note that for P-H3 immunostaining, sections were incubated for 5 min in TBS containing 3 % H2O2 and 10 % methanol at the very beginning of the experiment. For the detection of all phosphorylated proteins, 50 mM NaF was added in buffers and incubation solutions, as described previously (Sgambato et al. 1998). After three rinses in TBS the sections were incubated overnight at 4 °C with the primary antibodies. Day 2, sections were rinsed three times for 10 min in TBS and incubated for 90 min with a goat anti-rabbit Cy3-coupled secondary antibody (1:500; GE Healthcare, Piscataway, NJ). Sections were rinsed three times for 10 min in TBS and three times for 10 min in Tris Buffer (0.25 M Tris) before mounting in Vectashield (Vector Laboratories).
Primary antibodies
Cellular imaging of phosphorylated proteins was conducted using rabbit polyclonal antibodies against diphospho-Thr-202/Tyr-204-ERK1/2 (1:400; Cell Signaling Technology), phospho-Thr-581-MSK-1 (1:750; Cell Signaling Technology) and phospho-Ser-10-H3 (1:500; Millipore). The expression of IEGs was analysed using rabbit polyclonal antibodies against Arc/Arg3.1 (1:500; Synaptic Systems), c-Fos (1:800; Santa Cruz Biotechnology) and Zif268 (1:800; Santa Cruz Biotechnology).
Image analysis
Images were acquired bilaterally (20× objective) in each region of interest as indicated by grey areas on mouse brain coronal sections at various Bregma coordinates along the rostrocaudal axis (Franklin and Paxinos 2007). High-resolution reconstruction of the different hippocampal and amygdalar subfields was achieved by combining multiple images with overlapping fields of view using image editor software (Photoshop, Adobe, San Jose, CA). Quantifications were performed using image analyser software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD), taking into account cells with nuclear immunofluorescence above background.
Statistics
Data are presented as the mean ± SEM. Time-courses following CFC training and retrieval were analysed using a one-way ANOVA followed by Dunett’s post hoc test for specific comparisons (Figs. 1, 2, 4, 5). Comparison of time-courses were analysed using a non-repeated measures two-way ANOVA with the between-subjects factor of procedure and the within-subjects factor of time, followed by Bonferroni post hoc test for specific comparisons (Figs. 3, 6). The effect of the number of footshocks on freezing behaviour and ERK1/2 phosphorylation was analysed using a one-way ANOVA followed by Newman–Keuls post hoc test for specific comparisons (Fig. 7). Two-way ANOVA was followed by post hoc test only when the interaction between factors was statistically significant (Nieuwenhuis et al. 2011). In all cases, significance threshold was set at p < 0.05.
Results
Distinct patterns of ERK1/2 activity in hippocampal and amygdala subfields during consolidation and reconsolidation of CFC memory
We first analysed the pattern of ERK1/2 phosphorylation on Thr202 and Tyr204 residues (noted P-ERK1/2 since ERK1 and ERK2 cannot be discriminated in our immunohistochemical conditions), throughout the hippocampus and amygdala (Figs. 1a, 2a) at different time-points (0, 15, 30 and 60 min) following CFC training (Fig. 1b) and retrieval (Fig. 2b). In the DG the number of P-ERK1/2-positive cells was significantly increased immediately after training (Fig. 1c, g) and retrieval (Fig. 2c, g) and returned to basal levels within 15 min. In this hippocampal subfield, the pattern of ERK1/2 phosphorylation was similar following CFC training and retrieval (Fig. 3b: effect of time, p < 0.001; effect of procedure, NS; interaction, NS). In the dorsal hippocampal CA3, the number of P-ERK1/2-positive cells was not significantly altered after training (Fig. 1d, g) or retrieval (Fig. 2d, g), thus showing no difference when comparing the post-training and post-retrieval periods (Fig. 3b: effect of time, NS; effect of procedure, NS; interaction, NS). In the dorsal hippocampal CA1 region, the number of P-ERK1/2-positive cells was significantly increased immediately after training (Fig. 1e, g), returning towards basal levels within 60 min. The number of P-ERK1/2-positive cells was also increased after retrieval, peaking 30 min after the test (Fig. 2e, g). Comparison of the two conditions revealed that the pattern of ERK1/2 phosphorylation in CA1 was significantly higher following CFC training than following retrieval (Fig. 3b: effect of time, p < 0.001; effect of procedure, p < 0.001; interaction, p < 0.01). In the LA and BLA, the number of P-ERK1/2-positive cells was significantly increased immediately (0 and 15 min) after training (Fig. 1f, g) and in a delayed manner (30 min) after retrieval (Fig. 2f, g), returning towards basal level within 60 min. The pattern of phosphorylation was therefore significantly higher after training than after retrieval at the two early time-points of 0 and 15 min (Fig. 3b: LA: effect of time, NS; effect of procedure, p < 0.05; interaction, p < 0.05; BLA: effect of time, p < 0.05; effect of procedure, p < 0.01; interaction, p < 0.01). The pattern of activation was different in the central amygdala (CeA), where the number of P-ERK1/2-positive cells progressively increased during the first 60 min following training (Fig. 1f, g) and 30 min after retrieval (Fig. 2f, g), returning to basal level within 60 min, with again a significant difference between the patterns of activation in the two conditions (Fig. 3b: effect of time, p < 0.01; effect of procedure, NS; interaction, p < 0.01).
Distinct patterns of IEGs expression during consolidation and reconsolidation of CFC memory
The IEGs c-Fos, Zif268 and Arc/Arg3.1 are commonly used as activity markers for the mapping of behaviourally activated circuits for reviews (Guzowski et al. 2005; Tzingounis and Nicoll 2006; Kubik et al. 2007). Extracellular stimuli can trigger the rapid activation of c-Fos, Zif268 and Arc/Arg3.1 through different responsive elements present in their promoter regions such as the CRE consensus sequence (Changelian et al. 1989; Herrera et al. 1997; Kawashima et al. 2009). Recent evidence indicates that increasing CRE-dependent transcription in the DG results in the enhancement of CFC consolidation and reconsolidation (Sekeres et al. 2012). The neurons activated upon CFC learning seem to be involved in the allocation of the memory engram. For instance, optogenetic reactivation of a sparse but specific ensemble of neurons in the DG that previously expressed c-Fos during CFC training was shown to be sufficient to induce freezing behaviour (Liu et al. 2012). Having established the temporal pattern of ERK1/2 activation following CFC learning and retrieval, we therefore analysed the components of ERK signalling pathway along with IEGs expression. Since ERK1/2 was rapidly but transiently activated in the DG, we selected this specific hippocampal subfield to obtain relevant information on the magnitude and temporal window of expression of these downstream events when ERK1/2 phosphorylation, the initial step in the cascade, is terminated.
We first analysed MSK-1 and histone H3 (ser10) phosphorylation, two well-established nuclear targets of ERK1/2 that are implicated in chromatin remodelling. ERK1/2 activity controls the phosphorylation state of histone H3 at ser10 residue via the nuclear protein kinase MSK-1 (Brami-Cherrier et al. 2009). This molecular event is considered a landmark of chromatin remodelling since it triggers the induction of chromatin decompaction in response to mitogenic signalling (Bode and Dong 2005). Such involvement of ERK1/2 signalling in the nucleosomal response is thought to participate in synaptic plasticity, learning and memory (Day and Sweatt 2011). Consistent with this, CFC-mediated ERK1/2 activation in CA1 subfield was demonstrated to control MSK-1 (Sindreu et al. 2007) and histone H3 (ser10) (Chwang et al. 2006) phosphorylation depending on MSK-1 activity (Chwang et al. 2007).
Here, we found that the number of phospho-MSK-1 (Thr-581 residue, noted P-MSK-1) positive cells was significantly increased in the DG (Figs. 4a, 5a) immediately after training (Fig. 4c, h) and retrieval (Fig. 5c, h) and returned to basal levels within 15 min and the pattern of MSK-1 phosphorylation was similar following CFC training and retrieval (Fig. 6b: effect of time, p < 0.001; effect of procedure, NS; interaction, NS). Interestingly, in the DG the pattern of MSK-1 phosphorylation strongly matched that of ERK1/2 following CFC training and retrieval (Fig. 6b). In contrast, the number of P-H3 (ser10) positive cells progressively increased over the first 30 min following CFC training (Fig. 4d) and the first 15 min following CFC retrieval (Fig. 5d), returning to basal levels within 60 min in both cases. The pattern of H3 (ser10) phosphorylation was thus significantly different following CFC training and retrieval (Fig. 6b: effect of time, p < 0.001; effect of procedure, p < 0.01; interaction, NS).
Analyses of IEGs expression in this hippocampal subfield showed that the number of cells expressing Arc/Arg3.1 and c-Fos rapidly increased following training, reaching maximal levels at around 30 min, and was maintained up to at least 60 min (Fig. 4e, f, h). In contrast, following CFC retrieval the number of Arc/Arg3.1 and c-Fos immunoreactive cells was maximal at 30 min but returned to basal levels in 60 min (Fig. 5e, f, h). These patterns of expression of Arc/Arg3.1 and c-Fos were significantly different following CFC training and retrieval (Fig. 6b: effect of time, p < 0.001; effect of procedure, p < 0.05; interaction, p < 0.01). Finally, the number of Zif268 immunoreactive cells progressively increased over the first 60 min following CFC training (Fig. 4g, h) and retrieval (Fig. 5g, h), with a similar temporal pattern but a significant difference in magnitude between training and retrieval (Fig. 6b: effect of time, p < 0.001; effect of procedure, p < 0.001; interaction, NS).
Relationship between the strength of a previously established CFC memory and neuronal activity upon recall
In order to examine the relationship between the strength of a previously established CFC memory and neuronal activity throughout the hippocampus and amygdala (Fig. 7a), ERK1/2 phosphorylation was analysed immediately after a retrieval session conducted 24 h after a 0, 1 or 3 footshocks training session (Fig. 7b). Re-exposure to the training context elicited robust freezing in the one-shock group compared to the unshocked group (Fig. 7c). Freezing, however, was significantly increased in the 3-footshock group (Fig. 7c). In the DG, the number of P-ERK1/2 immunoreactive cells was significantly increased in the previously trained groups, as compared to naive controls (Fig. 7d, h). Unexpectedly, the number of P-ERK1/2 immunoreactive cells was even higher in the pseudo-trained animals (0 footshock) compared to the animals trained with 1 or 3 footshocks (Fig. 7d, h). In dorsal CA3, the number of P-ERK1/2 immunoreactive cells was significantly increased after retrieval compared to the control group, with a maximal number of P-ERK1/2 immunoreactive cells in the 3-footshock trained group (Fig. 7e, h). In dorsal CA1, the number of P-ERK1/2 immunoreactive cells was also significantly increased following retrieval in the one- and three-shock trained groups, compared to the naive control group, this effect was not influenced by the intensity of training (Fig. 7f, h). Finally, we did not detect any increase in the number of P-ERK1/2 immunoreactive cells in the different nuclei of the amygdala at this early time-point (Fig. 7g, h), in accordance with our findings showing a delayed activation of ERK1/2 in the amygdala following recall (30 min) (Fig. 2f). Altogether, these data indicate that the number of footshocks administrated during training and the number of P-ERK1/2 immunoreactive cells are positively correlated in CA3 but not in the DG or CA1. These data also confirm that upon CFC recall, ERK1/2 is immediately activated in the hippocampus, but not in the amygdala.
Discussion
The main aim of this study was to precisely analyse and compare the amplitude–time course of ERK1/2 activity following acquisition and recall of a contextual fear memory in key brain regions involved in this type of memory, and to explore some of the downstream molecular events controlled by ERK1/2 that are implicated in the transcriptional regulation believed to play a crucial role in memory stabilization. For this purpose, we used the Pavlovian fear conditioning paradigm that offers two different conditioning versions promoting either an elemental association between a conditioned tone stimulus (CS) and unconditioned stimulus (US footshock) (foreground/cued fear conditioning) or a contextual conditioning by systematically minimizing CS/US contingency (background/contextual fear conditioning) (Kim and Fanselow 1992). Interestingly, both types of conditioning induce fear to the context, but they are known to result in distinct contextual processing (background vs. foreground contextual conditioning) (Phillips and LeDoux 1994). Several studies have investigated the temporal dynamic of ERK1/2 phosphorylation following contextual (Sananbenesi et al. 2002; Trifilieff et al. 2006, 2007; Sindreu et al. 2007) and cued fear conditioning (Trifilieff et al. 2007; Paul et al. 2007; Di Benedetto et al. 2009; Schafe et al. 2000; Bergstrom et al. 2011) and retrieval (Chen et al. 2005). Here, following a similar strategy, we analysed the temporal dynamics of ERK1/2 activity in hippocampal subregions and amygdalar nuclei after learning and retrieval in the same experiment. Following learning, our results recapitulate those obtained by Trifilieff et al. (2007) showing a rapid activation of ERK1/2 in the DG, areas CA3 and CA1 and in the lateral and basolateral nuclei of the amygdala and they extend these findings by showing that ERK1/2 is also activated after retrieval, as rapidly in the hippocampus, but in a much delayed manner in the amygdala. In addition, we also confirm previous reports of an increase in ERK1/2 activity in CA1 following CFC training (Sindreu et al. 2007; Trifilieff et al. 2007).
Interestingly, our data in contextual fear conditioning show rapid and preferential activation of ERK1/2 in the BLA, whereas others using cued fear conditioning reported delayed ERK1/2 activation in the LA occurring 60 min following training (Schafe et al. 2000; Trifilieff et al. 2007; Paul et al. 2007; Di Benedetto et al. 2009; Bergstrom et al. 2011), suggesting that distinct types of contextual processing, engaging background versus foreground contextual processing (Kim and Fanselow 1992), may differentially engage the ERK cascade in specific amygdala nuclei. Consistent with this idea, Trifilieff et al. (2007) directly assessed this question and found preferential recruitment of ERK1/2 activation in the LA 60 min following cued, but not contextual fear conditioning (Trifilieff et al. 2007). It should be noted that the vast majority of the aforementioned studies, including ours, used the same antibody raised against diphospho-Thr-202/Tyr-204-ERK1/2; therefore, suggesting that this discrepancy is more likely to rely on a procedural rather than methodological distinction.
In the hippocampus, CFC memory retrieval was associated with the strongest activation in the DG, which strictly corresponded in magnitude and time course to the pattern observed after CFC training, supporting the idea that ERK1/2 phosphorylation in the DG may be highly dependent on contextual exposure, including in the training phase. Conversely, the pattern of ERK1/2 phosphorylation in CA1 departs between learning and retrieval, with a much smaller magnitude of activation after retrieval than after training. In the amygdala the pattern of ERK1/2 phosphorylation was delayed and culminated 30 min following retrieval, as opposed to the immediate activation observed following training. The differences in magnitude and kinetics of ERK1/2 activation observed in CA1 and amygdala can be explained in part by the nature of the stimuli presented during CFC training (footshock unconditioned stimulus associated with the context) and CFC retrieval (re-exposure to the context in the absence of the footshock). Although the extent of ERK1/2 activation we report in CA1 following recall is lower than that following training, it is likely to be of functional importance for CFC memory retrieval since micro-infusion of a MEK inhibitor into the dorsal hippocampus was shown to impair freezing performance upon recall (Chen et al. 2005). Whether the delayed activation in the amygdala plays a functional role in reconsolidation but not recall of CFC memory as it does in cued fear memory reconsolidation (Duvarci et al. 2005; Doyere et al. 2007) would need to be addressed using the same pharmacological approach.
We next examined the activation of ERK1/2 downstream events, focusing on the DG as the activation of a sparse but specific ensemble of neurons that previously expressed c-Fos during CFC training in this hippocampal subfield was shown to be sufficient to induce freezing behaviour (Liu et al. 2012). Moreover, extracellular stimuli can trigger the rapid activation of c-Fos, Zif268 and Arc/Arg3.1 through different responsive elements present in their promoter such as the CRE consensus sequence (Changelian et al. 1989; Herrera et al. 1997; Kawashima et al. 2009). Recent evidence indicates that increasing CRE-dependent transcription in the DG results in the enhancement of CFC consolidation and reconsolidation (Sekeres et al. 2012). Having established the temporal pattern of ERK1/2 activation following CFC learning and retrieval, we sought to analyse the components of ERK signalling pathway along with IEGs expression. Since ERK1/2 was rapidly and transiently activated in the DG, we hypothesized that focusing on this specific hippocampal subfield would provide relevant information on the magnitude and temporal window of these downstream events. We specifically compared the patterns of phosphorylation of MSK-1 and H3, along with the induction of IEGs Arc/Arg3.1, c-Fos and Zif268, following CFC training and retrieval. Our results show that while MSK-1 phosphorylation is rapid and transient both after training and recall, a pattern of activation identical to that of ERK1/2, H3 (ser10) phosphorylation along with IEGs induction was higher in magnitude and more sustained following CFC training than following retrieval. Again, the nature of the stimuli presented during learning (footshock in context) and recall (context alone) could well account for this difference. In any case, our observation that IEGs induction is more transient during the reconsolidation period is in accordance with several findings suggesting that memory reconsolidation occurs in a more limited time window than consolidation (Anokhin et al. 2002; Gordon 1977; Gordon and Spear 1973; Languille et al. 2009; Cheval et al. 2011). Hence it is tempting to speculate that the lower magnitude and duration of gene induction during CFC reconsolidation can explain its increased sensitivity to interfering treatments, such as hypothermia (Mactutus et al. 1979) or pharmacological manipulations (Anokhin et al. 2002; Bustos et al. 2006; Debiec and Ledoux 2004; Przybyslawski et al. 1999).
Our results clearly show that activation of ERK1/2, along with downstream molecular events, occurs rapidly and transiently in the DG. By contrast, several reports failed to detect these signalling events in this hippocampal area. Most of these studies, however, were conducted at late time-points compared to ours. For example, H3 (ser10) phosphorylation was analysed at 60 min (Lubin and Sweatt 2007), Arc/Arg3.1 induction at 90 min (Mamiya et al. 2009) and c-Fos at 60 min (Hall et al. 2001) post-retrieval. Conversely, together with previous findings showing an increase in Zif268 expression levels up to 2 h following CFC retrieval (Lee et al. 2004), our data showing a rapid accumulation of Zif268 protein, remaining elevated for at least 60 min following retrieval indicate that Zif268 expression is more prolonged than that of Arc/Arg3.1 or c-Fos in the DG. In conclusion, our data provide the first evidence that ERK1/2 activation and IEG (c-Fos, Arc/Arg3.1) expression are induced after CFC retrieval in the DG, supporting the idea that targeting ERK1/2 in this hippocampal area may provide important insights into the role of this signalling cascade in the DG in CFC reconsolidation.
Finally, we examined the relationship between the strength of a previously established CFC memory and the pattern of ERK1/2 phosphorylation in the hippocampus and amygdala upon recall. In our hands, CFC training conducted with three footshocks resulted in a 40 % increase in freezing performance at the recall test compared to the 1-shock condition, thus confirming previous findings (Selcher et al. 1999). In the DG, the number of P-ERK1/2 immunoreactive cells following recall was significantly increased compared to controls; however, there was no difference depending on whether one or three footshocks were given during learning. Interestingly, the maximal number of P-ERK1/2 immunoreactive cells was observed during the recall test in the pseudo-trained group (no footshock). Thus, freezing performance was not correlated with the number of P-ERK1/2-positive cells in this hippocampal subfield. This observation suggests that the presentation of a neutral context triggers the activation of an extended dentate granule cell population. If this represents a “background” activity in response to the first re-exposure to a previously explored, but non-reinforced environment, then the smaller number of activated cells found after CFC retrieval suggests refinement of the neural ensemble activated when contextual exposure evokes a previously encoded fear memory (i.e. shock-associated). In support of this interpretation, a recent study in which dentate granule cells activated during CFC training were labelled with channelrhodopsin-2 (ChR2) revealed that a twofold reduction in background expression of ChR2 potently increased freezing elicited by light stimulation (Liu et al. 2012). Further experiments are needed to elucidate whether this phenomenon reflects a specific contribution of the DG to fear memory. In area CA1, the number of P-ERK1/2 immunoreactive cells was significantly increased in the three groups submitted to a retrieval test compared to controls, thus independently of whether they received no shock, one or three shocks, suggesting that ERK1/2 activation in CA1 is not correlated with freezing performance and can even be observed in response to contextual exposure only. In contrast, in area CA3 the number of P-ERK1/2 immunoreactive cells was increased only in the groups that have been trained with one or three footshocks and the number of ERK1/2-positive cells correlated positively with freezing performance. Since in the CFC paradigm, performance is motivated by emotions, we cannot rule out the possibility that such pattern of ERK activity reflects the consequence of arousal induced by the fear response. This correlation with memory strength, and the extent of neuronal activation in the CA3, echoes the recent demonstration that the activation of CA3 pyramidal neurons is necessary for CFC memory precision (Ruediger et al. 2011). Moreover, the finding of independent patterns of ERK1/2 phosphorylation in CA3 and CA1 upon CFC retrieval is in accordance with previous findings that contextual representations emerge independently in CA3 and CA1 (Leutgeb et al. 2004).
Collectively, these data point to substantial differences in the magnitude and temporal dynamic of ERK1/2 signalling activity between consolidation and reconsolidation of CFC memory, both in terms of brain structures involved as well as molecular events recruited. Our results are therefore in line with the growing body of literature supporting the notion that memory reconsolidation is not a simple recapitulation of consolidation. In addition, we provide the demonstration that the strength of a previously established CFC memory is characterized by distinct patterns of neuronal activity in the different hippocampal and amygdalar subfields upon recall.
Future studies might usefully extend these analyses to assess whether or not the differential engagement of the ERK cascade and downstream events observed here between consolidation and reconsolidation of contextual fear memory can be extended to other types of fear memory, and more generally, to other forms of memory.
Abbreviations
- BLA:
-
Basolateral amygdala
- CeA:
-
Central amygdala
- CFC:
-
Contextual fear conditioning
- ChR2:
-
Channelrhodopsin-2
- CRE:
-
cAMP response element
- DG:
-
Dentate gyrus
- ERK:
-
Extracellular-signal Regulated Kinase
- H3:
-
Histone H3
- IEG:
-
Immediate early gene
- LA:
-
Lateral amygdala
- MAPK:
-
Mitogen-activated protein Kinase
- MEK:
-
MAPK ERK kinase
- MSK:
-
Mitogen and Stressed-activated protein Kinase
References
Anokhin KV, Tiunova AA, Rose SP (2002) Reminder effects—reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur J Neurosci 15(11):1759–1765
Athos J, Impey S, Pineda VV, Chen X, Storm DR (2002) Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci 5(11):1119–1120
Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD (1998) The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1(7):602–609
Bergstrom HC, McDonald CG, Johnson LR (2011) Pavlovian fear conditioning activates a common pattern of neurons in the lateral amygdala of individual brains. PLoS ONE 6(1):e15698
Besnard A (2012) A model of hippocampal competition between new learning and memory updating. J Neurosci 32(10):3281–3283
Besnard A, Caboche J, Laroche S (2012) Reconsolidation of memory: a decade of debate. Prog Neurobiol 99(1):61–80
Bode AM, Dong Z (2005) Inducible covalent posttranslational modification of histone H3. Sci STKE 2005(281):re4
Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J (2009) Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem 108(6):1323–1335
Bustos SG, Maldonado H, Molina VA (2006) Midazolam disrupts fear memory reconsolidation. Neuroscience 139(3):831–842
Changelian PS, Feng P, King TC, Milbrandt J (1989) Structure of the NGFI-A gene and detection of upstream sequences responsible for its transcriptional induction by nerve growth factor. Proc Natl Acad Sci USA 86(1):377–381
Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR (2005) PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci 8(7):925–931
Cheval H, Chagneau C, Levasseur G, Veyrac A, Faucon-Biguet N, Laroche S, Davis S (2011) Distinctive features of Egr transcription factor regulation and DNA binding activity in CA1 of the hippocampus in synaptic plasticity and consolidation and reconsolidation of fear memory. Hippocampus. doi:10.1002/hipo.20926
Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD (2006) ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem 13(3):322–328
Chwang WB, Arthur JS, Schumacher A, Sweatt JD (2007) The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci 27(46):12732–12742
Day JJ, Sweatt JD (2011) Epigenetic mechanisms in cognition. Neuron 70(5):813–829
Debiec J, Ledoux JE (2004) Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129(2):267–272
Di Benedetto B, Kallnik M, Weisenhorn DM, Falls WA, Wurst W, Holter SM (2009) Activation of ERK/MAPK in the lateral amygdala of the mouse is required for acquisition of a fear-potentiated startle response. Neuropsychopharmacology 34(2):356–366
Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE (2007) Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci 10(4):414–416
Duvarci S, Nader K, LeDoux JE (2005) Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci 21(1):283–289
Fanselow MS (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15(4):177–182
Fanselow MS (2000) Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res 110(1–2):73–81
Fanselow MS, LeDoux JE (1999) Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23(2):229–232
Franklin K, Paxinos G (2007) The mouse brain in stereotaxic coordinates, 3rd edn. Academic Press, San Diego
Fendt M, Fanselow MS (1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23(5):743–760
Gordon WC (1977) Susceptibility of a reactivated memory to the effects of strychnine: a time-dependent phenomenon. Physiol Behav 18(1):95–99
Gordon WC, Spear NE (1973) The effects of strychnine on recently acquired and reactivated passive avoidance memories. Physiol Behav 10(6):1071–1075
Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA (2005) Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol 15(5):599–606
Hall J, Thomas KL, Everitt BJ (2001) Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci 13(7):1453–1458
Herrera RE, Nordheim A, Stewart AF (1997) Chromatin structure analysis of the human c-fos promoter reveals a centrally positioned nucleosome. Chromosoma 106(5):284–292
Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H (2009) Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci USA 106(1):316–321
Kelleher RJ 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S (2004) Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116(3):467–479
Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256(5057):675–677
Kubik S, Miyashita T, Guzowski JF (2007) Using immediate-early genes to map hippocampal subregional functions. Learn Mem 14(11):758–770
Languille S, Davis S, Richer P, Alcacer C, Laroche S, Hars B (2009) Extracellular signal-regulated kinase activation is required for consolidation and reconsolidation of memory at an early stage of ontogenesis. Eur J Neurosci 30(10):1923–1930
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Lee JL (2009) Reconsolidation: maintaining memory relevance. Trends Neurosci 32(8):413–420
Lee JL, Everitt BJ, Thomas KL (2004) Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304(5672):839–843
Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI (2004) Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305(5688):1295–1298
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484(7394):381–385
Lubin FD, Sweatt JD (2007) The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron 55(6):942–957
Mactutus CF, Riccio DC, Ferek JM (1979) Retrograde amnesia for old (reactivated) memory: some anomalous characteristics. Science 204(4399):1319–1320
Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S (2009) Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci 29(2):402–413
Maren S, Fanselow MS (1996) The amygdala and fear conditioning: has the nut been cracked? Neuron 16(2):237–240
Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nat Rev Neurosci 5(11):844–852
McGaugh JL (2000) Memory–a century of consolidation. Science 287(5451):248–251
Nader K, Hardt O (2009) A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10(3):224–234
Nieuwenhuis S, Forstmann BU, Wagenmakers EJ (2011) Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14(9):1105–1107
Osan R, Tort AB, Amaral OB (2011) A mismatch-based model for memory reconsolidation and extinction in attractor networks. PLoS ONE 6(8):e23113
Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ (2007) The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry 61(9):1049–1061
Phillips RG, LeDoux JE (1994) Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem 1(1):34–44
Przybyslawski J, Roullet P, Sara SJ (1999) Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci 19(15):6623–6628
Radwanska K, Nikolaev E, Knapska E, Kaczmarek L (2002) Differential response of two subdivisions of lateral amygdala to aversive conditioning as revealed by c-Fos and P-ERK mapping. NeuroReport 13(17):2241–2246
Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, Sacchetti B, Caroni P (2011) Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature 473(7348):514–518
Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J (2002) Phosphorylation of hippocampal Erk-1/2, Elk-1, and p90-Rsk-1 during contextual fear conditioning: interactions between Erk-1/2 and Elk-1. Mol Cell Neurosci 21(3):463–476
Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE (2000) Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci 20(21):8177–8187
Sekeres MJ, Mercaldo V, Richards B, Sargin D, Mahadevan V, Woodin MA, Frankland PW, Josselyn SA (2012) Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci 32(49):17857–17868
Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD (1999) A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem 6(5):478–490
Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J (1998) Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci 18(21):8814–8825
Shalin SC, Zirrgiebel U, Honsa KJ, Julien JP, Miller FD, Kaplan DR, Sweatt JD (2004) Neuronal MEK is important for normal fear conditioning in mice. J Neurosci Res 75(6):760–770
Sindreu CB, Scheiner ZS, Storm DR (2007) Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron 53(1):79–89
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5(3):173–183
Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J (2006) Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem 13(3):349–358
Trifilieff P, Calandreau L, Herry C, Mons N, Micheau J (2007) Biphasic ERK1/2 activation in both the hippocampus and amygdala may reveal a system consolidation of contextual fear memory. Neurobiol Learn Mem 88(4):424–434
Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8(4):262–275
Tzingounis AV, Nicoll RA (2006) Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron 52(3):403–407
Acknowledgments
This work has been supported by the “Centre National de la Recherche Scientifique” (CNRS), l’ “Agence Nationale pour la Recherche” (ANR-08-BLAN) and the “Fondation Jerôme Lejeune”. A.B. has been supported by the Edmond Rothschild Chemical Dependency Institute Beth Israël Medical center and Fondation pour la Recherche Médicale.
Conflict of interest
All authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antoine, B., Serge, L. & Jocelyne, C. Comparative dynamics of MAPK/ERK signalling components and immediate early genes in the hippocampus and amygdala following contextual fear conditioning and retrieval. Brain Struct Funct 219, 415–430 (2014). https://doi.org/10.1007/s00429-013-0505-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0505-y