Abstract
The human cerebral cortex contains numerous myelinated fibres, many of which are concentrated in tangentially organized layers and radially oriented bundles. The spatial organization of these fibres is by no means homogeneous throughout the cortex. Local differences in the thickness and compactness of the fibre layers, and in the length and strength of the radial bundles renders it possible to recognize areas with a different myeloarchitecture. The neuroanatomical subdiscipline aimed at the identification and delineation of such areas is known as myeloarchitectonics. There is another, closely related neuroanatomical subdiscipline, named cytoarchitectonics. The aims and scope of this subdiscipline are the same as those of myeloarchitectonics, viz. parcellation. However, this subdiscipline focuses, as its name implies, on the size, shape and arrangement of the neuronal cell bodies in the cortex, rather than on the myelinated fibres. At the beginning of the twentieth century, two young investigators, Oskar and Cécile Vogt founded a centre for brain research, aimed to be devoted to the study of the (cyto + myelo) architecture of the cerebral cortex. The study of the cytoarchitecture was entrusted to their collaborator Korbinian Brodmann, who gained great fame with the creation of a cytoarchitectonic map of the human cerebral cortex. Here, we focus on the myeloarchitectonic studies on the cerebral cortex of the Vogt–Vogt school, because these studies are nearly forgotten in the present attempts to localize functional activations and to interprete findings in modern neuroimaging studies. Following introductory sections on the principles of myeloarchitectonics, and on the achievements of three myeloarchitectonic pioneers who did not belong to the Vogt–Vogt school, the pertinent literature is reviewed in some detail. These studies allow the conclusion that the human neocortex contains about 185 myeloarchitectonic areas, 70 frontal, 6 insular, 30 parietal, 19 occipital, and 60 temporal. It is emphasized that the data available, render it possible to compose a myeloarchitectonic map of the human neocortex, which is at least as reliable as any of the classic architectonic maps. During the realization of their myeloarchitectonic research program, in which numerous able collaborators were involved, the Vogts gradually developed a general concept of the organization of the cerebral cortex. The essence of this concept is that this structure is composed of about 200 distinct, juxtaposed ‘Rindenfelder’ or ‘topistische Einheiten’, which represent fundamental structural as well as functional entities. The second main part of this article is devoted to a discussion and evaluation of this ‘Vogt–Vogt concept’. It is concluded that there is converging quantitative cytoarchitectonic, receptor architectonic, myeloarchitectonic, hodological, and functional evidence, indicating that this concept is essentially correct. The third, and final part of this article deals with the problem of relating particular cortical functions, as determined with neuroimaging techniques, to particular cortical structures. At present, these ‘translation’ operations are generally based on adapted, three-dimensional versions of Brodmann’s famous map. However, it has become increasingly clear that these maps do not provide the neuroanatomical precision to match the considerable degree of functional segregation, suggested by neuroimaging studies. Therefore, we strongly recommend an attempt at combining and synthesizing the results of Brodmann’s cytoarchitectonic analysis, with those of the detailed myeloarchitectonic studies of the Vogt–Vogt school. These studies may also be of interest for the interpretation of the myeloarchitectonic features, visualized in modern in vivo mappings of the human cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1898, the young couple Oskar and Cécile Vogt (born in 1870 and 1875, respectively) took a remarkable initiative. They founded in Berlin a private centre, to be devoted entirely to the study of the structure and function of the central nervous system (CNS). This ‘Neurologisches Zentralstation’ grew out to one of the largest and most prestigious research institutes of Germany. In 1902, it was incorporated under the name ‘Neurobiologisches Laboratorium’ into Berlin University and in 1931 it was transformed into the ‘Kaiser Wilhelm Institut für Hirnforschung’, comprising a large research centre with 30 scientific and 70 technical collaborators, as well as a research clinic (Forschungsklinik) with 60 beds. This ‘KWIH’ was established in the Berlin suburb of Buch. In 1937, Oscar Vogt was forced to give up his directorship of the institute for political reasons. However, generous financial support of the steel tycoon and armaments manufacturer E. A. Krupp and the Rockefeller foundation enabled the Vogts to establish a new research facility in the small city of Neustadt in the Black Forest. In this Institute, which was named ‘Institut für Hirnforschung und allgemeine Biologie’, they remained scientifically active almost to their death. Oscar Vogt died on 31 July 1959, and Cécile followed him on 4 May 1962. Adolf Hopf succeeded Oscar Vogt as director of the Institute in Neustadt. In 1965, it was relocated to Düssseldorf, where it flourishes, under the name ‘C. & O. Vogt—Institut für Hirnforschung der Universität Düsseldorf’, up to the present as a centre for brain research. The historical data just briefly reviewed are derived from Hopf (1970a) and Klatzo (2002), to which the reader is referred for details.
The research program of the newly founded laboratory in Berlin was comprehensive and ambitious. It was decided that the emphasis would be laid on the ‘higher’ and more complex centres of the brain, particularly the cerebral cortex (Vogt 1903). As regards the latter structure, the Vogts had not to start from the very first beginning. At the turn of the twentieth century, it was known already that the cerebral cortex is composed of cells of many different kinds (Fig. 1a), that the somata of these cells are arranged in layers, and that the size, shape, density and arrangement of the somata in these layers could display considerable local differences (Fig. 1b, c). It was also known that the cerebral cortex contains numerous myelinated fibres, forming tangentially organized plexuses and radially arranged bundles, which, just like the neuronal somata, could show marked local differences (Fig. 1b, c). Finally, there was clinical and experimental evidence, indicating that the cortex harbours centres with clearly different functions (Fig. 2).
Lateral view of the human telencephalon. Areas, the functions of which were known at the end of the nineteenth century are marked. A motor speech centre of Broca, B the somatomotor and somatosensory areas, C auditory area, D centre concerned with writing, E Wernicke’s sensory speech centre, F visual area. Modified from Vogt and Vogt (1954)
The approach chosen by the Vogts was relatively simple and remained essentially unchanged throughout their long scientific career (Vogt 1903, 1943; Vogt and Vogt 1919, 1936, 1942, 1954, 1956). It included a systematic analysis of those structural features of the cerebral cortex that can be readily recognized with relatively weak magnifications, with a view at identifying and delimiting fundamental morphological units within that organ, assuming that the units or areas of distinct structure thus identified, would also proven to be organs of special function. The results of these architectonic studies were expected to provide an adequate basis for clinicopathological studies, as well as for the study of the brains of geniuses (‘Elitegehirne’), and feeble-minded people.
At the time that the Vogts began their studies there were two staining procedures for brain tissue, which yielded reliable and reproducible results, i.e. the Nissl stain for neuronal cell bodies, and the Weigert stain and its variants for myelinated nerve fibres. Thus, it became routine in the Neurologisches Zentralstation to prepare serial sections of human and animal brains, and to stain these series according to the two procedures mentioned. The systematic study of the material thus prepared led to the emergence of two new neuroanatomical subdisciplines, which were designated by Vogt (1903) as cytoarchitectonics and myeloarchitectonics.
The cytoarchitectonics of the cerebral cortex became the specialism of Korbinian Brodmann, who joined the Vogts in 1901 and remained attached to their laboratory until 1909. Brodmann studied the cellular structure of the cortex in a considerable number of mammals, including the hedgehog, the flying fox, the lemur, the guenon, and the human, resulting in an impressive series of publications (Brodmann 1903a, b, 1905a, b, 1906, 1908a, b), and in a summarizing monograph (Brodmann 1909). His famous map of the cytoarchitecture of the human cerebral cortex was first published in Brodmann (1908a), and its final version appeared in Brodmann (1914). It is of note that the practising of the cytoarchitectonics of the human cerebral cortex remained by no means confined to the institute of the Vogts. Von Economo and Koskinas (1925, Vienna), Bailey and Von Bonin (1951, Urbana, Illinois) and Sarkissov et al. 1955, Moscow) all published comprehensive works on the human cortex, resulting in complete cytoarchitectonic parcellations of that organ (see Braak 1980 and Zilles and Amunts 2010, for synopsis and further developments).
Study of the myeloarchitectonics of the cerebral cortex, on the other hand, remained largely concentrated in the laboratories of the Vogts, from its beginning, marked by the appearance of Vogt’s (1910a) preliminary note on the human frontal cortex, to its end, marked by the appearance of Hopf’s (1970b) study of the human parietal cortex. The comprehensive program included analyses of the myeloarchitecture of all parts of the human cortex, as well as the cortices of a number of mammals, including the hedgehog (Flores 1911), the mangabey (Mauss 1908), the orangutan (Mauss 1911) and the chimpanzee (Beck 1929; Strasburger 1937b; Gerhardt 1938).
The principles of myeloarchitectonics
Preparations stained with the Weigert method reveal that the neocortex contains numerous myelinated fibres, which show two principal orientations, tangential and radial. The tangential fibres tend to form local concentrations or bands, the most conspicuous of which can be clearly observed with the naked eye in unstained sections. The radial fibres are concentrated in bundles or radii. Vogt (1903) designed a basic plan (‘Grundschema’) of the myeloarchitectonic organization of the neocortex, which formed the point of departure of all myeloarchitectonic studies produced in his laboratory. This scheme is shown in Fig. 3, together with a comparable basic scheme of the cytoarchitectonic organization. In both schemes the neocortex is subdivided into six layers. The cytoarchitectonic layers are: (I) the cell-poor zonal layer, (II) the external granular layer, (III) the external pyramidal layer, (IV) the internal granular layer, (V) the internal pyramidal layer, and (VI) the multiform layer. The corresponding myeloarchitectonic layers are, to avoid confusion, designated with Arabic, rather than with Roman numerals.
Vogt’s (1903) basic schemes of the cytoarchitectonic layers (designated with Roman numbers), and the myeloarchitectonic layers (designated with Arabic numbers)
-
1.
The zonal layer is differentiated into four sublayers, the narrow sublayer 1°, which contains only very few fibres, and the external, intermediate and deep sublayers 1a, 1b and 1c, of which 1a contains clearly more fibres than 1b and 1c.
-
2.
The dysfibrous layer which contains, just like sublayer 1°, only very few fibres.
-
3.
The suprastriate layer has three sublayers, of which the superficial sublayer 3a1 is more rich in fibres than the remaining sublayers 3a2 and 3b. In certain cortical regions, sublayer 3a1 show a distinct concentration of fibres, known as the stripe of Kaes-Bechterew. Sublayer 3b is characterized by the presence of the end-segments of the radial bundles.
-
4.
The external stria or outer stripe of Baillarger forms a dark band of tightly packed, tangential fibres.
-
5a.
The intrastriate layer is generally relatively poor in tangential fibres, thus contrasting with the bordering stripes of Baillarger.
-
5b.
The internal stria or inner stripe of Baillarger is again a dense plexus of tightly packed tangentially oriented fibres.
-
6.
This layer is subdivided into the pale substriate lamina 6a1 and laminae 6a2, 6b1 and 6b2, which show an increasing wealth of tangentially oriented fibres. Sublayer 6b2 forms the zone of transition to the subcortical white matter.
Variations in the number and density of the tangential and radial fibres define the boundaries of the myeloarchitectonic areas. With regard to the lamination of the tangential fibres, Vogt (1910a, b, 1911) distinguished four principal types (Fig. 4).
The four principal types of myeloarchitectonic layering, according to Vogt (1910a). a Bistrate type, b unistriate type, c unitostriate type, d astriate type
-
(a)
A bistriate type, characterized by the presence of two distinct and clearly separated bands of Baillarger (Fig. 4a). It should be added that the situation in which the density of fibres in the outer stripe equals that in the inner stripe is designated as typus equodensus, and that the situations in which the fibres in the outer stripe are more densely or less densely arranged than those in the inner stripe, are designated as typus externodensior and internodensior, respectively.
-
(b)
A unistriate type, in which only the external stripe of Baillarger can be distinguished as a separate entity. The inner stripe, though present, cannot be delineated because of the high fibre content of the substriate lamina 6a1 (Fig. 4b, in which the substriate lamina is labelled 6aα).
-
(c)
A unitostriate type, in which the fibre-poor interstriate layer is lacking and the two stripes of Baillarger form a single plexus (Fig. 4c).
-
(d)
An astriate type, in which, due to the presence of an unusually large number of tangential fibres in intrastriate layer 5a and substriate lamina 6a1, layers 4–6 form a single dark and undivided fibre zone (Fig. 4d).
As regard the disposition of the bundles of radial fibre bundles, Vogt distinguished three types, euradiate, supraradiate and infraradiate. In the euradiate type, the radii do not extend beyond the level of the suprastriate layer (Fig. 4a, d); in the supraradiate type, the bundles traverse almost the entire width of the cortex and reach the zonal layer (Fig. 4c), whereas in the infraradiate type, the radii are very short and terminate already in the fifth layer at the level of the inner stripe of Baillarger (Fig. 4b).
The radii do not vary only in length, but also in breadth, number and calibre of their fibres. On the basis of differences in the breadth of radii, latoradiate, medioradiate and tenuiradiate types were distinguished. Differences in the number of radii led to distinction of densoradiate, modicoradiate and sparsoradiate types, whereas differences in the size of the fibres forming the radii found their expression in grossoradiate and finoradiate types.
Vogt codified many other myeloarchitectonic variations as types. Thus, he referred to an overall wealth of myelinated fibres in a given area as typus dives, whereas an overall scarcity of such fibres was characterized as typus pauper. The various tangential layers generally consist of a plexus of thin fibres of about equal size (‘Grundfasern’), in which individual fibres of larger size (‘Einzelfasern’) are embedded. Vogt designated layers, in which the coarse individual fibres are scarce, as representing a typus tenuifibrosus, and layers in which these fibres are numerous as representing a typus grossofibrosus.
It should be appreciated that the myeloarchitectonics of the cortex, as developed by Vogt, in spite of its extremely detailed and intricate typology, remained a purely descriptive and qualitative neuroanatomical subdiscipline. Several attempts at the development of a more quantitative and more objective myeloarchitectonics have been made. Thus, Braitenberg (1962) devised a method in which the light absorption, being directly proportional to the fibre density, is systematically measured in narrow strips of Weigert-stained sections of the cerebral cortex, passing from the pial surface to the white matter. With this method, he recorded the fibre density in sections taken from 14 different cortical regions. Representation of the results in graphic form yielded quite characteristic curves for most of the regions studied (Fig. 5a, b). Braitenberg also produced an interesting diagram, explaining the relationships between the basic functional wiring and the myeloarchitecture of the cortex, and the overall course of his photometric curves (Fig. 5c). Hopf (1966) developed a photometric method for determining the extent of myelinization in the cortex, which closely resembled that of Braitenberg. With the aid of this technique, he successfully explored the myeloarchitecture of the human frontal, temporal and parietal cortices (Hopf 1968a, b, 1969, 1970b).
Braitenberg’s (1962) photometric analysis of the myeloarchitecture of the human cerebral cortex. a, b Graphic representations of photometric analyses of strips of myelin-stained sections of the cortex, with distance from the surface of the cortex (in μm) on the abscissa, and fibre density (in arbitrary units) on the ordinate. The tangents of the curves show different slopes. The outer and inner stripes of Baillarger (Bo, Bi) produce local prominences over the tangents. a Cortex of middle frontal convolution, showing the presence of both stripes. b Area striata with the pronounced outer stripe of Baillarger (or line of Gennari, or line of Vicq d’Azyr), after which it is named. c Diagram, clarifying the relationships between the overall course of the photometric curves, the myeloarchitecture, and the basic functional wiring of the neocortex. The following hodological features are taken into account: (1) the number of specific afferent fibres in the cortex decreases if we ascend from the white matter; most of these fibres form their terminal ramifications in layers III and IV. (2) The number of efferent fibres increases if we descend to the white matter; practically all of these fibres are produced by pyramidal neurons, situated principally in layers III and V. These two features explain the steady increase in myelinization if we pass in the cortex from superficial to deep, and therewith the overall course of curve a. (3) The axons of the pyramidal neurons produce long, tangentially running collaterals, which tend to assemble at two different cortical levels. It is these concentrations of pyramidal collaterals that, forming the stripes of Baillarger, produce the local increases in fibre density shown in curve b
In what follows, the literature on the myeloarchitecture of the human cerebral cortex, as produced by the Vogts and their numerous disciples, will be reviewed first. Next, a general concept, concerning the organization of the cerebral cortex, which has been developed from the myelarchitectonic studies reviewed, will be discussed, and finally, some remarks will be made on the functional parcellation of neocortex. It is felt appropriate, however, to preface this long story with a few remarks on the work of three myeloarchitectonic pioneers, who did not belong to the Vogt–Vogt school.
Notes on the work of three myeloarchitectonic pioneers
Alfred Walter Campbell (1868–1937)
The pathologist Campbell published in 1905 a monograph, entitled: “Histological studies on the localization of cerebral functions”. The opening paragraph, which gives a clear view on the perspective in which the author placed his work, may be quoted in full: “The process leading to the accomplishment of functional localization in the cerebral cortex is such a complicated one, and involves so many side issues, that perfection cannot be attained or even hoped for until the fruits of investigation in a number of departments are thoroughly weighed, sifted and assorted. It is anticipated that the observations set forth in this research will help to establish the value of histological work as an auxiliary force in the final settlement of that functional subdivision of the cerebral cortex at which we aim” (l.c. p. XV).
The normal human material, on which the work is based, consisted of three cerebral hemispheres completely examined for both nerve cells and nerve fibres, and three additional hemispheres examined for fibres only. Campbell distinguished 16 cortical areas, which he designated either with topographical (such as: frontal, postcentral, and temporal), or with (provisional) functional names (such as: olfactory, visuo-sensory and visuo-psychic). All of these areas were described in some detail, and their cytoarchitecture and myeloarchitecture were recorded in beautiful drawings (Fig. 6c, d). The resultant map, which represents the only complete, combined cyto- and myeloarchitectonic map of the human cerebral cortex, produced thus far, is shown in Fig. 6a, b. It will be seen that in Campbell’s parcellation, the frontal cortex is unusually large; that the intermediate-central cortex, which roughly corresponds to Brodmann’s area 6, is not demarcated from Broca’s motor speech region (areas 44 and 45 of Brodmann), and that the temporal area does not only occupy the inferior and middle temporal convolutions, but rather extends over vast regions of the parietal lobe.
Some illustrations from Campbell’s (1905) monograph: “Histological Studies on the Localization of Cerebral Function”. Architectonic map of the human cerebral cortex; a lateral view, b medial view, drawings showing the structure of the precentral or motor area (c), and the postcentral or somatosensory area of the human cerebral cortex (d). In each of these figures the pattern of myelinated fibres is shown to the left, and the arrangement of the neuronal cell bodies to the right. Representative cell types are shown at a higher magnification to the far right. e, f Architectonic map of the cerebral cortex of the chimpanzee
Campbell did not confine himself to the human brain, but also studied and mapped the cortex of several mammals, including the cat, the orangutan and the chimpanzee (Fig. 6e, f).
Grafton Elliot Smith (1871–1936)
The anatomist Elliot Smith published in 1907 a detailed map of the human cerebral cortex, which, surprisingly, was exclusively based on macroscopic observations (Fig. 7a, b). He made fresh sections at many locations of the hemisphere, using differences in the width and distinctness of the stripes of Baillarger as the main criteria for his parcellation. In unstained preparations, these stripes can be recognized as whitish bands, contrasting with the darker hue of the cortical grey matter (Fig. 7c). With the aid of this simple technique, Elliot Smith was able to distinguish about 50 different cortical areas. He noticed that most of these areas have precise relations to various stable sulci. As regards the nature of the interareal boundaries, Elliot Smith (1907, p. 240) took a firm stand: “There is a very widespread belief that the characters of an area merge gradually and imperceptably into those of the neighbouring areas, but this is entirely mistaken. The changes in structure occur with the utmost abruptness, so that it is possible to determine with absolute precision the exact boundaries of each area.”
Elliot Smith’s (1907) ‘myeloarchitectonic’ analysis of the human cerebral cortex. a, b Architectonic map, based entirely on the study of fresh, unstained macroscopic sections of the cerebral cortex. c Pictures showing 28 of the about 50 areas, distinguished by Elliot Smith
Theodor Kaes (1852–1913)
Kaes was psychiatrist and prosector at the asylum Friedrichsburg near Hamburg. Between 1891 and 1904, he published a series of papers on the various techniques, used for the staining of myelinated fibres, and on the myeloarchitecture of the human cerebral cortex. He summarized his findings and views in his opus magnum (Kaes 1907), entitled: “Die Grosshirnrinde des Menschen in ihren Maszen und ihrem Fasergehalt. Ein gehirnanatomischer Atlas mit erläuterndem Text”, comprising, apart from a concise text, an atlas consisting of 90 large colour plates, showing the myeloarchitecture of 12 selected cortical regions in 45 individuals, and numerous tables and curves, documenting thousands of measurements of the total width of the cortex, and of its various layers and zones.
Kaes collected 45 human brains, ranging from 3 months postnatal to 97 years, among which several mentally retarded and criminal individuals. He selected 12 regions in both hemispheres of these brains for further analysis. These regions were designated as: (1) vordere Stirne, (2) hintere Stirne, (3) vordere Zentralwindung, (4) hintere Zentralwindung, (5) Operculum, (6) Insel, (7) vordere Schläfe, (8) hintere Schläfe, (9) oberer Scheitel, (10) unterer Scheitel, (11) Sehrinde, and (12) Gyrus fornicatus. Most unfortunately, any further indication concerning the exact location of the regions selected, is lacking in the work. Sections, taken from these 12 regions, were stained according to the Weigert–Wolters technique. The best-stained sections were carefully drawn and included in the atlas. Thus, in the atlas 24 sections of the cortex of each individual investigated were included, 12 from the left, and 12 from the right hemisphere (Fig. 8).
One of the 90 coloured plates, with which Kaes (1907) illustrated his great work on the human cerebral cortex, reproduced at half of the original size. The plate shows the structure of the cortex, as seen in preparations stained with Wolters’ variant of the Weigert technique, in 12 representative cortical areas. The numbers are specified in the text
Kaes used the material, thus selected, for a qualitative and quantitative analysis of the postnatal development of the cortex under normal and abnormal circumstances. In his quantitative studies, he divided the cortex into an external principal zone (‘äuszere Hauptzone’), encompassing layers I–III, and an internal principal zone (‘innere Hauptzone’), consisting of layers IV–VI (Fig. 3). A detailed discussion of the results of Kaes, falls outside the scope of the present review. Hence, I confine myself to some of his main conclusions: (1) Cortices, which are poor in myelinated fibres, are in general wider than cortices containing numerous fibres. (2) The internal principal zone attains the peak of its development around the 19th year of life; the external principal layer continues developing until the 45th year of life and beyond. (3) The findings on brains of mentally retarded individuals (‘Idiotengehirne’), confirm the rule, mentioned above under 1. (4) The brains of criminals show generally an abnormally low weight, and an infantile level of development.
Myeloarchitectonic parcellations of the human neocortex
The frontal lobe
The literature on the myeloarchitecture of the frontal lobes is voluminous and encompasses studies of Vogt (1910a, b), (Vogt and Vogt 1919), Strasburger (1937a, b, 1938), Braitenberg (1956), Hopf (1956, 1968a), and Sanides (1962, 1964). The myeloarchitectonic parcellation of this lobe, presented by Vogt (1910a), is complex (Fig. 9). He distinguished six regions, which were designated with Roman numerals. Each of these regions was subdivided into several (two to four) subregions, and these were, in their turn, further subdivided into divisions, and locally even still further into subdivisions. Finally, one or several areas were delineated within each of the (sub) divisions. In total, 66 myeloarchitectonic areas, designated with Arabic numerals, were distinguished within the frontal lobe. These numerals have nothing to do with the—also Arabic—numerals, used by Brodmann (1909) for his cytoarchitectonic areas. Each of the entities distinguished was designated with a full Latin name, referring to particular myeloarchitectural features characterizing that particular entity. A survey of Vogt’s nomenclature for region III and its subdivisions is presented in Table 1. Vogt does not specify the histological material he used in this study. Strasburger (1937b) mentioned that it was principally based on serial sections of a single hemisphere, designated as A 18r. Vogt’s (1910a) paper ends abruptly after the description of the last area (Fig. 10).
The end of Vogt’s (1910a) preliminary study on the myeloarchitectonic parcellation of the human frontal lobe
In a subsequent publication, Vogt (1910b) comments briefly on the findings just reviewed, in relation to the results of the cytoarchitectonic analyses of the cortex, published shortly before by Brodmann (1909). He points out that in general, the myeloarchitectonic approach is superior to the cytoarchitectonic one, because the number of cortical areas that can be delineated with the aid of the former, far exceeds that delineable with the aid of the latter. Unfortunately, he does not address the specific relationship between the results of his myeloarchitectonic, and Brodmann’s cytoarchitectonic parcellation of the frontal lobe. Vogt (1910b) also points out that the relation between the sulci, separating the various convolutions, and the myeloarchitectonic boundaries, is variable in the frontal lobe. Some of the sulci coincide with such boundaries, but many others do not.
(Vogt and Vogt 1919) published pictures of the myeloarchitecture of a number of frontal areas, some of which are reproduced in Fig. 11.
The myeloarchitecture of some frontal areas, as depicted by Vogt and Vogt (1919). a Area 17, which is situated in the rostrobasal part of the cingulate gyrus (Fig. 6c), is of the infraradiate type, because the radii terminate principally already at the level of the transition of layers 5a and 5b. Layer 1 is subdivided, just as in the ‘Grundschema’ (Fig. 3), into four sublayers, one of which (1b) contains numerous ‘Einzelfasern’. There is a paucity of both radial and tangential fibres in layers 2–4, which contrasts with the much higher fibre density seen in layers 5b and 6. Layer 5a occupies, with regard to the density of its fibres, an intermediate position between these two sets of layers. b Area 36 forms part of the regio unistriata euradiata grossofibrosa (Fig. 6a, c: III). Because of its rich endowment of fibres it is characterized as area dives. c Area 42 also forms part of regio III (Fig. 6a, c), and corresponds to a part of the cytoarchitectonic area gigantocellularis. The radii contain numerous coarse ‘Einzelfasern’. Vogt (1910a, p. 230) characterized this area as “absolut astriär”. Vogt and Vogt (1919) emphasized that the marking of the layers 4–6aβ in the figure is only based on the tracing of these various cortical levels into adjacent cortical areas with a more distinct lamination. d Another picture of the myeloarchitecture of area 42, based on a preparation subjected to a further differentiation (‘stärkerer Entfärbung’). It will be seen that distinct local differences in the density of the horizontal fibres become manifest after this procedure. e Area 63, forms part of the orbitofrontal sector of the regio unitostriata (Fig. 6b). Although the term ‘unitostriate’ refers to the situation in which both stripes of Baillarger form together a single broad band (Fig. 4c), in area 63 these two stripes, forming layers 4 and 5b, are nevertheless discernable as separate formations. Area 63 is designated as area pauper (Fig. 7), because of its extreme poverty in ‘Grundfasern’
Before turning to a discussion of the publications of Strasburger (1937a, b, 1938), brief attention should be paid to Mauss’ (1908, 1911) studies on the cortex of some non-human primates. This author analyzed the myeloarchitecture of the cortex in two ‘lower’ monkeys, the rhesus macaque Macaca mulatta and the mangabey Cercocebus fulginosus (Mauss 1908), and in two anthropomorph monkeys, the gibbon Hylobatus spec. and the orangutan Pongo pygmaeus (Mauss 1911). His principal results can be summarized as follows: (1) The results of the myeloarchtectonic analysis of the cortex of the mangabey (Fig. 12) closely resemble those of Brodmann’s (1905a) cytoarchitectonic analysis in the same species. (2) In the frontal lobe of the mangabey, 11 different myeloarchitectonic areas can be distinguished, nine of which (areae 4, 6, 8, 9, 10, 11, 12, and 24) can be readily equated to similarly numbered cytoarchitectonic areas. (3) The total number of delineable myeloarchitectonic cortical areas in the orangutan (Fig. 13) is larger than that in the mangabey: 41 versus 31. (4) Using similarity in structure and similarity in position as criteria, 26 of the 31 myeloarchitectonic areas, present in the cortex of the mangabey, can be readily homologized with (similarly numbered) areas in the cortex of the orangutan. (5) Although the areas numbered 8 in the maps of the mangabey and the orangutan differ considerably in extent, they should, nevertheless, be considered as homologous. (6) The cortex of the orangutan contains ten myeloarchitectonic areas, which could not be identified in the mangabey. (7) All of the 11 myeloarchitectonic areas, present in the frontal lobe of the mangabey, i.e. areas 4, 6, 8, 9, 10, 11, 12, 24, 31, 32 and 33, have (similarly numbered) homologues in the frontal lobes of the orangutan. (8) The frontal lobe of the orangutan contains a single new myeloarchitectonic area, which is numbered 37 (Figs. 12, 13).
Mauss’ (1911) myeloarchitectonic map of the cortex of the orangutan Pongo pygmaues; a lateral view, b medial view
Strasburger (1937a, b) thoroughly analyzed the myeloarchitecture of the frontal lobe in a human hemisphere (A 39r), and in hemispheres of two different chimpanzees (A 117l, A 118l). He compared the results of his analysis of the human frontal lobe (Fig. 14), with those of Vogt (1910a: Fig. 9), adopting the numbering system for the various areas of the latter; moreover, he compared the frontal lobes of the two chimpanzees studied (Fig. 16) with each other, as well as with that of the human (Fig. 14). Contrary to Vogt (1910a), Strasburger illustrated his descriptions of the various areas, with numerous large, very detailed drawings, some of which are reproduced (at half of their original size) in Fig. 15. He not only depicted individual areas, but laudably also complexes of two adjacent areas (e.g. Fig. 15b, c), enabling the reader to visualize the structural differences between these areas. The criteria for the establishment of homologies, used by Strasburger, were the same as those of Mauss, namely, (a) similarity in structure, and (b) similarity in position. If he was certain that a particular area in the human frontal lobe was homologous to an area delineated by Vogt (1910a), he adopted the number given to that area by the latter. However, if he considered the homology between a particular ‘Strasburger’ area and a particular ‘Vogt’ area as highly probable, but not entirely certain, he designated the area in question with the Roman equivalent of Vogt’s (Arabic) number of that area (cf. areas X, XI, XII and XXV in Fig. 14c, with areas 10, 11, 12 and 25 in Fig. 9c). A similar procedure was followed in the comparison of the parcellations of the frontal cortex of the two chimpanzees studied, with that of the human frontal cortex (see the numerous Roman numerals in Fig. 16b, d). In his characterization of the myeloarchitecture of the various areas, Strasburger paid particular attention to: (a) the disposition of the stripes of Baillarger (as regards the areas depicted in Fig. 15, he considered areas 51 and 61 as bistriate; areas 2, 21, 26, 30 and 50 as unistriate, and areas 64 and 65 as unitostriate); (b) the distinctness of the various sublayers in layer 1, and (c) the size and length of the radii.
Lateral (a), basal (b), and medial views (c) of the human frontal lobe, showing the myeloarchitectonic parcellation of Strasburger (1937a). All allocortex, cc central sulcus, Insel island of Reil, olf olfactory bulb, P parietal lobe
The myeloarchitecture of some areas in the human frontal lobes, as depicted by Strasburger (1937a)
Myeloarchitectonic parcellation of the frontal cortex of the chimpanzee, according to Strasburger (1937b). Lateral (a) and medial views (b) of the frontal lobe of specimen A 117l. Lateral (c) and medial views (d) of the frontal lobe of specimen A 118l. d–f The myeloarchitecture of some fields in the frontal cortex of specimen A 117l. For abbreviations, see Fig. 11. Reproduced from Strasburger (1937b)
The number of myeloarchitectonic areas, delineated by Strasburger in the human frontal cortex exceeds that of Vogt: 78 versus 66. If we disregard the slight doubts, expressed in the Roman numerals (see above), 58 of the areas distinguished by Strasburger appeared to be directly comparable to ‘Vogt areas’. Strasburger remained unable to identify homologues of the ‘Vogt areas’ 7, 40 and 46. He split each of the five ‘Vogt areas’ 41, 54, 55, 56 and 65, into two separate areas: 41a, 41b, 54a, 54b and so on. Finally, Strasburger distinguished ten new areas in the frontal cortex of the human specimen studied. He did not designate these areas with new numbers. Rather, he took the number of another, adjacent area, and added the letter a, or α to it (see, e.g. 49a and 59α in Fig. 14a).
As regards the comparison between the frontal lobes of the two chimpanzees (A 117l, A 118l), and the human studied (A 39r), Strasburger (1937b) reported that, in general, the fibre layers in the human (Fig. 15) are more distinct than in the chimpanzee (Fig. 16e–h), and that the number of areas in the human is larger: 78 versus 60 (in A 118l) or 65 (in A 117l). Some areas, including 14, 22, 26, 30, 30α, 44 and 45, present in the human frontal cortex (Fig. 14), could not be identified in the chimpanzee. Moreover, several sets of separate areas in the human frontal cortex, appeared to be represented by a single area (‘Sammelfeld’) in the chimpanzee (see, e.g. 1 + 4 in Fig. 16b, d; 37 + 38 in Fig. 16a, c, and 48 + 49 in Fig. 16a, c, h).
Strasburger (1937b, Table 6, p. 603) compared the results of his myeloarchitectonic parcellation of the frontal cortex of the chimpanzee (50–55 areas; Fig. 16a–d), with those of Mauss (1911), obtained from a similar study in the orangutan (12 areas; Fig. 13). He considered the areas 4, 6 and 12 of Mauss directly comparable to fields 42, 39 and 6, respectively, of his parcellation. The remaining nine areas of Mauss were homologized with smaller or larger sets of areas delineated by him. To give a single example: area 11 of Mauss corresponds, according to Strasburger, with ‘his’ areas 2 + 3, 1 + 4 + 8, 5, 9 and 61.
In a subsequent study, Strasburger (1938) presented a detailed myeloarchitectonic analysis of frontal areas 56–66, as distinguished by Vogt (1910a; Fig. 9a, c), in both hemispheres of six human brains (A 20, A 22, A 27, A 34, A 38, A 39). With a single exception (area 63 in A 201), all of the 12 areas could be identified in all of the 12 hemispheres investigated. The 12 fields showed only very slight left–right or interspeciminal structural and positional differences. The size (volume) of some areas showed considerable differences, however.
Braitenbergs’ (1956) myeloarchitectonic analysis of the human frontal cortex differs from that of Vogt (1910a) and Strasburger (1937a, b, 1938), in that he based his parcellation exclusively on structural differences that could be clearly observed in his Weigert-material, either with the naked eye, or with the magnifying glass. He also indicates to have included only those structural entities in his map (Fig. 17), that were clearly distinguishable in all of the series studied, though he does not mention on how many series his study was actually based. Another difference with the approach of Vogt and Strasburger is that he took the gross anatomy, i.e. the gyrification of the frontal lobe as point of departure. Thus, his frontal regions F1, F2, and F3 correspond (partly) to the superior, middle, and inferior frontal gyri, respectively, whereas his region F1 recta is centred around the straight gyrus (gyrus rectus), which forms the medial part of the basal surface of the frontal lobe. Braitenberg delineated the following myeloarchitectonic entities within the frontal lobe (Fig. 17):
-
1.
An unnamed elongated, astriate region, situated directly in front of the central sulcus. This region corresponds, according to Braitenberg, with area 4 of Brodmann, and with areas 42 and 43 of Vogt (in what follows, all area numbers correspond, unless otherwise stated, to those of the latter).
-
2.
The unistriate Regio Frontalis 1 (r. F1), which includes the rostral part of the precentral gyrus, most of the superior frontal gyrus, and the medial part of the basal surface of the frontal lobe. It can be divided into a dorsal part (r. F1 dors.), and a ‘straight’ part (r. F1 recta). r. F1 dors. includes parts of Vogts’ regions III and IV, and encompasses areas 33–41 (cf. Fig. 6). r. F1 recta corresponds roughly with Vogts’ region I, and with the stretch of cortex occupied by areas 1–14.
-
3.
The bistriate Regio Frontalis 2 (r. F2), which corresponds to region V, and to areas 46–55 of Vogt. This region is divisible into to dorsal and ventral moieties, designated as r. F2 dors. and r. F2 vent. or -polaris. The boundary between these two divisions of r. F2 is not sharp.
-
4.
A small unistriate area on the medial surface of the frontal lobe, corresponding to the area 2, manifesting itself, on the basis of its extraordinary wealth of fibres, clearly as a separate entity (Fig. 14a).
-
5.
The, mostly unitostriate, Regio Frontalis 3 (r. F3), which corresponds to region VI of Vogt, and which is clearly further differentiated into separate fields than r. F1 and r. F2. This region contains, according to Vogt, 11 different areas, which he numbered 56–65 (Fig. 9a, b). Reference to Fig. 17b, c shows that Braitenberg was able to identify all of these areas, except for 61, in his material. It is of note that Strasburger (1938), as we have seen, delineated the same areas in both hemispheres of six different brains.
-
6.
The Regio cinguli anterior (r. cingul. ant.), which corresponds to region II of Vogt, and which is characterized by very feebly developed stripes of Baillarger, and very short radii. Braitenberg indicates that the presence of subtle myeloarchitectonic differences, recognizable only with the aid of higher magnifications, render it possible to recognize the equivalents of areas 15–32 within the confines of this region.
Braitenberg emphasizes that the boundaries between all of the six myeloarchitectonic entities just discussed, are distinct, except for that between r. F2 dors., and r. F2 vent. However, he also points out to have observed the following changes, in passing from caudodorsal to rostroventral in r. F1 and r. F2: (1) A gradual decrease in the number of fibres; (2) a gradual decrease in the size (calibre) of the individual fibres, and (3) a gradual decrease in the width of the cortex. These three phenomena appeared to be correlated with (4), a cytoarchitectonic change, viz. a gradual increase in the number of granule cells in layer IV of the cortex. These observations are noteworthy, in relation to the fact that, within the orthodox Vogt–Vogt school (to which Braitenberg did not belong), the existence of gradual architectonic changes was categorically denied. Braitenberg made a somewhat infelicitous attempt, to indicate the gradual changes discussed, by including in his maps some borders according to fibre content (‘Grenzen nach Faserreichtum’), separating cortical compartments of ‘equal darkness’ (Fig. 17 A–E).
In summary, Braitenberg, studying series of Weigert sections through the human frontal lobe with a simple magnifier, divided the cortex of this lobe into six large regions, r. F1 dors., r. F1 recta, r. F2 dors., r. F2 vent., r. F3, and r. cingul. ant., and two independent smaller entities, one corresponding to the area 4 of Brodmann, the other to area 2 of Vogt. Within r. F3, 11 different areas could be delineated, almost all of which appeared to be directly comparable to myeloarchitectonic areas distinguished by Vogt. Passing from the central sulcus to the rostral pole of the frontal lobe, Braitenberg observed several correlated gradual structural changes in r. F1 and r. F2.
We now turn to the studies of Adolf Hopf, a collaborator of the Vogts in the Neustadt institute. Hopfs’ (1956, 1968a) contributions to the myeloarchitecture of the frontal cortex are threefold. Firstly, he performed a new myeloarchitectonic parcellation of this structure; secondly, he prepared maps, showing the main myeloarchitectonic features of the frontal cortex, and thirdly, he made an attempt at the objective registration of the myeloarchitecture of the frontal lobe, using the photometric technique already discussed in a previous section.
Hopfs’ (1956) renewed myeloarchitectonic parcellation of the human frontal cortex was based on serial sections of two brains, A 18, which had been previously studied by Vogt (1910a), and A 39, previously studied by Strasburger (1937a, b). Hopf remained, just like Strasburger, unable to distinguish areas 7 and 40, and to delimit area 45 from 46. Moreover, he regarded Strasburger’s areas 43a, 48a, 54b, 56b and 63° as inconspicuous and inconstant variants. The resultant myeloarchitectonic map, encompassing 69 areas, is shown in Fig. 18.
To visualize the distribution of the various myeloarchitectonic features over the frontal cortex, Hopf (1956) prepared separate maps, showing the overall density of fibres (Fig. 19), the density and size of ‘Einzelfasern’, the length of the radii, and the disposition of the stripes of Baillarger. This ‘feature-mapping’ showed, inter alia, that the precentral cortex possesses a high content of coarse fibres, and that the fibre content decreases in a step-like fashion with increasing distance from the central sulcus (Fig. 19).
Hopf’s (1968a) publication on the objective registration of myeloarchitectonic features in the human frontal cortex opens with a discussion of the six basic qualitative myeloarchitectonic types, occurring in this cortex (Fig. 20). He then presents the results of his registrations of the differential density of fibres, in stretches of cortex involving two different myeloarchitectonic areas. Two of these registrations are shown in Fig. 19. Hopf draws the following general conclusions from these registrations: (1) The relative fibre density of the two stripes of Baillarger in relation to each other and to the neighbouring sublayers, as well as the general content in myelinated fibres, play a dominant role in these registrations. (2) The existence and reliability of some myeloarchitectonic features can be objectively demonstrated with this new technique. (3) The existence of all of the six basic, qualitatively determined types of frontal cortex (Figs. 20, 21) could be confirmed. It is important to note that Hopf used his new myeloarchitectonic registration technique only to substantiate his qualitatively obtained maps (Fig. 18), and not for the creation of new, objective ‘supermaps’.
Semidiagrammatic representation of the myeloarchitectonic types of cortex, found in the human frontal lobe. a Unistrate type, in which only the outer stripe of Baillarger (in layer 4) is clearly visible. b Propeunisstriate (=nearly unistriate) type, in which the inner stripe of B. (in layer 5b) is somewhat denser than layer 6a. c Bistriate type, in which both stripes of B. are well demarcated. d Unitostriate type, in which the two stripes of B. are united by a dense fibre plexus in layer 5a. e Astriate type, showing a homogeneous fibre density throughout layers 4–7. f Propeastriate (=nearly astriate) type, in which the stripes of B. are inconspicuous. Reproduced from Hopf (1968a)
Photometric recordings of the fibre density in bistriate (a), and unistriate areas (b) of the human frontal cortex. The recordings in a are taken from area 59, shown in the lower photograph (curve A), and from area 56b, shown in the upper photograph (curve B). The recordings in b are taken from area 35, shown in the lower photograph (curve A), area 37 (curve B), area 38 (curve C), and area 39, shown in the upper photograph (curve D). Note that there is a clear increase in fibre content from A to D. Reproduced from Hopf (1968a)
The last study on the human frontal lobe, to be discussed here, is that of Sanides (1962, 1964), another pupil of the Vogts. This study was principally based on transversely cut serial sections through the left hemisphere of one brain, A43. The sections were stained alternately according to Nissl, for cell bodies, and according to Heidenhain–Woelcke, for myelinated fibres. Sections of other brains, including A43 and A63, were used for comparison. Sanides’ purpose was twofold: (1) To carry out a combined cytoarchitectectonic and myeloarchitectonic analysis of the frontal cortex, hence his choice of material, and (2) to work out a concept, briefly mentioned by Vogt and Vogt (1919, p. 396), according to which the architecture of the cortex shows ‘gradations’, i.e. discontinuous, stepwise changes of architectonic features.
The results of Sanides’ architectonic analysis are shown in Fig. 22. He distinguished eight different zones, which were designated as the frontomotor (FmZ), frontopercular (FoZ), frontopolar (FpZ), orbitomesial (OmZ), dorsal paralimbic (PlZd), ventral paralimbic (PlZv), paramotor (PmZ), and paropercular zones (PoZ). These zones are outlined in red in Fig. 22. It is important to note that Sanides excluded a considerable portion of the medial surface of the frontal lobe from his analysis (Fig. 22d: Pro = proisocortex). This portion corresponds to region II of Vogt (1910a, Fig. 9c), which encompasses the areas 14–33 of that author, and the rostral part of Vogt’s region III, containing areas 34 and 35. Thus, the portion of the frontal lobe, investigated by Sanides, is in Vogt’s parcellation occupied by 44 (66 minus 22) areas. Sanides delineated a considerable number of separate areas within each of his eight zones, their total number amounting to 62. He established that 35 of these areas are directly comparable to one of the 44 Vogt areas, and hence, designated them with the same numbers. Four of the Vogt areas, viz. 51, 52, 53, and 59, were divided into separate dorsal and ventral parts (Fig. 22b). Sanides also observed that in many places separate fields are located between adjacent Vogt areas. These intercalated areas, eight in number, were indicated with the numbers of the two areas involved, separated by a slash (see, e.g. 39/40 and 40/47 in Fig. 22a). Moreover, he delineated numerous new areas, which he specified by adding letters to the number of an adjacent area (see, e.g. 38l, 51p and 39z in Fig. 22b). Sanides failed, just like Strasburger and Hopf, to identify Vogt’s area 7, and finally, he disagreed with Vogt’s delineation of areas 3, 10 and 11, replacing them collectively by two concentric paralimbic areas, Pvl and Pvz (Fig. 22b). Sanides emphasized that all of the areas distinguished by him have an equal status, and that the boundaries of all of these areas coincide with changes in both cytoarchitecture and myeloarchitecture. He also emphasized that in the frontal lobe there is a close correlation between structural differentiation and gyrification. According to his observations, most zonal and areal boundaries are located in the depths of the intergyral sulci.
Dorsal (a), lateral (b), basal (c), and medial views (d) of the human frontal lobe, showing the cyto-myeloarchitectonic parcellation of the frontal cortex, according to Sanides (1962). The areal boundaries, which do not coincide with sulci, are indicated with dotted lines. The boundaries of zones are in red, just as the abbreviations of the names of these zones. The numbers indicating the areas correspond to those of Vogt (1910a). The arrows indicate the direction of the ‘gradations’, discussed in the text. ce central sulcus, cm callosomarginal sulcus, fs superior frontal sulcus, fm middle frontal sulcus, fi inferior frontal sulcus, o. tr transverse orbital sulcus, pc precentral sulcus, r. ant. hor. F. S. anterior horizontal ramus of fissura Sylvii, Tp temporal pole
The presence of ‘gradations’ in the frontal lobes (once again: streams or chains of structurally related, but discrete areas) could be confirmed (see the arrows in Fig. 22). Sanides distinguished three of such gradations in the frontal cortex and surmised that they reflect the directions of the evolutionary expansion of that cortex. It is noteworthy that the existence of such gradations has been recently reconfirmed in a study of Broca’s region (Amunts and Zilles 2012).
From the foregoing it appears that the four authors, Vogt, Strasburger, Hopf and Sanides have carried out detailed analyses of the myeloarchitecture of the human frontal cortex. The differences between their results appear to be limited. If we introduce a provisional rule, saying that the presence of a particular area in the frontal cortex is sufficiently secured, if it has been identified by at least three of the four authors involved, it appears that the presence of no less than 61 of the 66 areas, originally delineated by Vogt, has been confirmed by later investigations. There appeared to be no support for the presence of Vogt’s areas 7, 29 and 40, and for his delineation of area 45 from area 46. Accepting the suggestion of Hopf, that several of the areas distinguished by Strasburger represent inconspicuous and inconstant variants, the total number of myeloarchitectonic areas, present in the frontal lobe may be estimated at about 70. It is important to note that this outcome differs considerably from those of the myeloarchitectonic pioneers Campbell (1905) and Elliot Smith (1907). The former distinguished only five, the latter 17 areas in the human frontal cortex.
The insula
The human insular cortex forms a distinct, but entirely hidden lobe, situated in the depth of the Sylvian fissure. The insula is shaped like a triangle, the apex of which is directed basally. A distinct sulcus centralis insulae divides the insula into a larger lobulus anterior and a smaller lobulus posterior. The lobulus anterior is commonly composed of three short gyri, the gyrus brevis primus, -secundus, and -tertius (or -centralis anterior), which converge towards the apex. The lobulus posterior is generally incompletely divided into two gyri, known as the gyrus longus primus (or -centralis posterior) and the gyrus longus secundus. These gross anatomical relations are clearly visible in Fig. 23, although not all of the structures mentioned are labelled in this figure.
Lateral views of the left human insula. a Myeloarchitectonic parcellation according to Vogt and Vogt (1911). b Cyto- and myeloarchitectonic parcellation according to Brockhaus (1940). For explanation, see text. Brev. prim. gyrus brevis primus, Brev. sec. gyrus brevis secundus, Cent. ant. gyrus centralis anterior (=gyrus brevis tertius), Cent. post. prim. gyrus centralis posterior primus (=gyrus longus primus), Cent. post. sec. gyrus centralis posterior secundus (=gyrus longus secundus)
The myeloarchitecture of the human insular cortex has been studied by (Vogt and Vogt 1911) and by Brockhaus (1940). The preliminary study of the Vogts was based on a Weigert–Pal series of a single hemisphere, A 18l. They divided the insular cortex into a ventral allocortical zone, and a dorsal isocortical zone. Within the latter, they distinguished six, rostrocaudally arranged areas, which they designated as i1-i6. The bounderies of most of these areas appeared to coincide exactly with the sulci separating the various insular gyri (Fig. 23a). This is remarkable, because Vogt (1903, 1910a, 1923) has repeatedly emphasized that the relations between the cerebral sulci and the areal boundaries, are variable and inconstant.
The very detailed, combined cytoarchitectonic and myeloarchitectonic study of Brockhaus (1940), another collaborator of the Vogts in the Neustadt institute, was based on six different brains, A 18, A 39, A 40, A 61, A 65, and A66. Brockhaus regarded the presence of the claustrum, a thin sheet of grey matter, situated between the insula and the putamen, as the defining structural feature of the insula, hence he designated the cortex covering this region as claustrocortex. He distinguished three ventrodorsally arranged principal regions within the insula, which he designated as allocortex claustralis (Acl), mesocortex claustralis (Mcl), and isocortex claustralis (Icl), thus intercalating a transitional zone between the two zones of the Vogts (Fig. 23b). So far as the myeloarchitecture of the neocortical (or isocortical) zone of the insula is concerned, Brockhaus’ observations tallied with those of the Vogts, with the reservation that he felt justified to subdivide areas i4a, i5a and i6a, into several smaller entities (Fig. 23b). All in all, he distinguished 12 neocortical insular areas, within the boundaries of which, there was a complete match of cytoarchitecture and myeloachitecture. Given the fact that the surface of the insular lobe takes up <2 % of the total cortical surface area, we consider it likely that the subdivisions of i4a, i5a and i6a, introduced by Brockhaus, represent subareas, rather than areas.
The parietal lobe
Contributions to our knowledge of the myeloarchitecture of the human parietal cortex, were made by Vogt (1911), Gerhardt (1940), Batsch (1956), Hopf and Vitzthum (1957), and Hopf (1969, 1970b). Vogt’s study on the myeloarchitecture of the parietal cortex is again preliminary in character, and ends, just as the one on the frontal cortex, abruptly after the description of the last area (cf. Fig. 10). It is, however, contrary to that paper, well illustrated with beautiful drawings (Fig. 25). The myeloarchitecture of the parietal cortex presented Vogt with several problems, which he solved by extending his terminology. Thus, he introduced the terms eucingulate and dyscingulate. In eucingulate areas, layer 2 is, by a paucity of its constituent fibres, sharply demarcated from layer 3 (cf. Fig. 3, right panel); in dyscingulate areas, this sharp boundary does not exist. Vogt’s subdivision of the parietal cortex, which was based on the study of three hemispheres, A 18r, A 20l, and A 20r, is even more complex than that of the frontal cortex. Suffice it to mentioning that he distinguished two principal regions within this cortex, which he designated as the euradiate region and the supraradiate region, indicated as VIII and VII, respectively, in Fig. 24. The euradiate region was subdivided into eucingulate (VIIIα) and dyscingulate subregions (VIIIβ). Reference to Fig. 24 shows that all of these (sub)regions were subdivided into several areas, indicated with Arabic numbers. The numbering of these areas links up with that, used in the frontal cortex (Fig. 9). It should be emphasized once again that these numbers have nothing to do with those, used by Brodmann for his cytoarchitectonic areas. All in all, Vogt delineated 30 myeloarchitectonic areas in the parietal cortex, numbered 67–96, nine of which (67–75) were situated in subregion VIIIα, fifteen (76–90) in subregion VIIIβ, and six (91–96) in region VII (Fig. 24). The myeloarchitecture of some of these areas is shown in Fig. 25.
Lateral (a), dorsal (b), medial (c), and opercular views (d) of the human parietal lobe, showing the myeloarchitectonic parcellation according to Vogt (1911)
Drawings showing the myeloarchitecture of some parietal cortical areas. Reproduced from Vogt (1911)
The analysis of the human parietal cortex by Gerhardt (1940), who worked at the KWIH in Berlin-Buch, is primarily cytoarchitectonic in character. Gerhardt indicates, however, to have studied several series of which the sections were stained alternately for cell bodies (Nissl) and myelinated fibres (Heidenhain), and emphasizes on this account that the resultant map (Fig. 26) is cytoarchitectonic as well as myeloarchitectonic. This map was based on the analysis of a single hemisphere, A 61l. She took Vogt’s (1911) subdivision as point of departure, but had to deviate at several points from it, for the simple reason that her parcellation was, as she put it, more thorough (‘eingehender’) than that of Vogt. Thus, she subdivided many Vogt areas into two or more subareas (‘Unterfelder’). This holds in particular for the large areas 83, 85, 89 and 90. Within the area last mentioned, Gerhardt delineated no less than seven subareas, designated as 90ai, 90aip, 90am, 90p, 90t1, 90t1′ and 90t1′o (Fig. 26).
Lateral (a), and medial views (b), of the human parietal lobe, showing the cyto-myeloarchitectonic parcellation according to Gerhardt (1940)
It is noteworthy, that Gerhardt (1938) also studied the architecture of the parietal cortex in the chimpanzee. She mentioned that this cortex shows a striking resemblance to that in the human, and that only a few of the areas, delineated in the human parietal cortex by Vogt (1911), could not be identified with certainty in the chimpanzee.
The aim of the study of Batsch (1956) was to investigate whether the very detailed parcellation of the parietal cortex, presented by Gerhardt, which was based on the study of a single hemisphere, is applicable to other brains as well. To this end, Batsch, who worked at the Neustadt Institute, studied, apart from hemispheres A 37l and A 61l, ‘mehrere Hemisphären’, without further specification. Batsch subdivided the parietal cortex into the following eight subregions: (a) subregio postcentralis, (b) s. opercularis, (c) s. parietalis inferior, (d) s. parietalis intermedia, (e) s. parietalis superior-medialis, (f) s. parietalis paracingularis oralis, (g) s. parietalis cingularis caudalis, and (h) s. parietalis cingularis. The basic myeloarchitecture of these subregions is shown in Fig. 27. Batsch subdivided all of these eight subregions into a (varying) number of areas. All of these areas corresponded in a one-to-one fashion to the areas 67–96 of Vogt, and were numbered accordingly. Sixteen of the ‘Vogt areas’ remained undivided. Most of these are located in the parietal paracingular oral (77–82), and parietal cingular subregions (91–96). The remaining 14 ‘Vogt areas’ were further subdivided into subareas. Thus, area 67 was subdivided into subareas I–IV, area 75 into subareas if and sup, and area 89 into subareas a, ip, m, p and t. In total, Batsch delineated within the human parietal cortex, 45 subareas. The study of Batsch is well documented with photomicrographs, showing the myeloarchitecture of all of the areas and subareas distinguished.
Semidiagrammatic representation of the myeloarchitecture in the various subregions (a–h) of the parietal cortex. The names of these subregions are mentioned in the text. Reproduced from Batsch (1956)
Batsch indicates that his division of the parietal lobe into subregions differs from that of Gerhardt, and that his findings concerning the size and extent of some of the ‘Vogt areas’, including 71, 73 and 74, also deviate from those of Gerhardt. In general, it may be said that the parcellations of Batsch and Gerhardt correspond, at the area level, closely to each other, and to that of Vogt as well. However, although the total number of subareas distinguished by the two authors first mentioned is about the same, there appeared to be no close one-to-one correspondence at this level.
The last publications on the myeloarchitecture of the parietal cortex, to be discussed here, are those of Hopf and Vitzthum (1957) and Hopf (1969, 1970a, b). Hopf and Vitzthum (1957) visualized the distribution of the various myeloarchitectonic characteristics over the parietal lobe in a series of ‘feature maps’, whereas Hopf (1969, 1970a, b) registered the relative content of myelinated fibres in the various cortical layers, in each of the postcentral and parietal areas. The ‘feature maps’ were based on slightly modified versions of the (very complex) cortical maps of Batsch (1956). These ‘modified-Batsch-maps’ are reproduced in Fig. 28. The only differences with the originals are that the dorsal extent of subareas 70I and 71I is reduced, and that the anterior surface of the postcentral gyrus is folded anteriorly. By this transformation, areas 67 and 69, which are normally hidden in the central sulcus, are exposed. The studies of Hopf and Vitzthum (1957) and Hopf (1969, 1970a, b) showed that the most characteristic myeloarchitectonic features in the parietal lobe include the overall fibre density, the strength of the radial bundles, and the relation of the stripes of Baillarger to each other and to their neighbouring layers. The highest overall fibre content was found in the postcentral region, the lowest in the inferior parietal lobule. Hopf (1969, 1970a, b), who included eight different hemispheres in his registrations of the relative laminar fibre content in the parietal lobe, found only minor interindividual variations. It is of note that the paper of Hopf and Vitzthum (1957, pp. 98–102) contains a very useful comparison of the various subdivisions of the parietal cortex in tabular form.
Dorsolateral (a), medial (b), lateral (c), and opercular views (d), of the human parietal lobes, showing the myeloarchitectonic parcellation, according to Batsch (1956), slightly modified by Hopf and Vitzthum (1957). The arrows in a and c indicate the locations where areas 67 and 69 have been folded anteriorly to expose them
The occipital lobe
As is well known, Brodmann (1909) divided the human occipital lobe into three, concentrically arranged cytoarchitectonic areas, the area striata (17), -occipitalis (18), and -praeoccipitalis (19). These three areas have also been recognized by Vogt and Vogt (1919), in their preliminary reconnaissance of the myeloarchitecture of the human cortex. The boundary between the striate area and the occipital area is cytoarchitectonically, as well as myeloarchitectonically, by far the most distinct one in the entire neocortex. Reference to Fig. 29a, b shows that this boundary is marked by the sudden transition of cytoarchitectonic sublayers IVa–c in area 17, into the undivided layer IV in area 18, as well as by the equally sudden ending of the outer stripe of Baillarger (=line of Gennari; =line of Vicq d’Azyr). This striking myeloarchitectonic difference between the striate and occipital areas is also clearly seen in the more detailed pictures shown in Fig. 29c, d.
The architecture of the human occipital cortex. Cytoarchitecture (a) and myeloarchitecture (b) of the transition of the calcarine area into the occipital area. Note that a and b are each other’s mirror image. Reproduced from Brodmann (1914). c, d Detailed pictures of the myeloarchitecture of the calcarine and occipital areas, reproduced from Vogt and Vogt (1919). e, f Detailed pictures of the myeloarchitecture of subareas scet, sct and scd, situated within the preoccipital area, reproduced from Lungwitz (1937)
The only detailed myeloarchitectonic study on the human occipital cortex is that of Lungwitz (1937), who worked at the KWIH in Berlin-Buch. His study, which was based on sections through both hemispheres of brain A 37, stained according to Weigert–Kulschitzky, was confined to the preoccipital area, which he designated, following Vogt, as PrO. Lungwitz characterized his study as a “myeloarchitectonische Unterfelderung” of that area. He delineated 17 subareas within PrO, which he designated with combinations of two-to-four letters (Fig. 30). Although it is indicated in the text that these letter combinations refer to conceptualizations of Vogt, their significance is not fully clear (at least not to the present reviewer). All of the subfields are described in a peculiar sort of ‘myelo-shorthand’, and documented with detailed drawings. The pictures of three subareas, scet, sct and scd, are shown in Fig. 29e, f). To give the reader some idea of the style of the paper under discussion, the description of one subarea, scd, is shown in full in Fig. 31.
Lateral (a), medial (b), and basal views (c), of the human occipital lobe, showing the myeloarchitectonic parcellation of the preoccipital area (=area 19 of Brodmann), according to Lungwitz (1937). 17, 18, 19 areas of Brodmann
Description of subarea scd in Lungwitz (1937, p. 624)
At the end of his paper, Lungwitz puts forward the question whether PrO has to be considered a Regio, that is, a complex of areas, or rather as an area with numerous subareas. He then emphasizes that this question can only be adequately answered in light of knowledge concerning the physiological differentiation of the entity in question, and he adds that this knowledge is currently lacking: “Darüber wissen wir jedoch zur Zeit nichts sicheres” (Lungwitz 1937, p. 638). In this respect, the situation has changed considerably in the mean time. Physiological studies have shown that the preoccipital region harbours a large number of functional areas (see: Wandell et al. 2007). Taking these new data into consideration, it seems reasonable to assume that the myeloarchitectonic entities delineated by Lungwitz, represent areas, rather than subareas.
The temporal lobe
The myeloarchitecture of the human temporal lobe, has been studied by two authors, Beck (1925, 1928, 1930), and Hopf (1954b, 1955, 1968b).
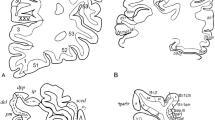
The very detailed studies of Beck are confined to the dorsal surface of the temporal lobe. His first paper (Beck 1925) is entitled (in translation): “On the exactness of the myeloarchitectonic parcellation of the cerebral cortex”. In this paper, he describes the results of an analysis of the dorsal temporal cortex, based on sections of a single hemisphere, A 27l, stained according to Weigert–Kultschitzky. This choice was intentional, because he knew that Vogt had previously studied the same region of the same brain, but had not published the results as yet. Beck was able to distinguish 28 sharply defined myeloarchitectonic areas in the region investigated (Fig. 32a). He also shows the results of Vogt’s unpublished parcellation (Fig. 32b), emphasizing to have worked completely independently from the latter. Beck observed that there is a striking resemblance between the results of the two studies. He points out that his map does not only contain all of the areas distinguished by Vogt, but that these areas also correspond in position and size, to such an extent (Beck 1925, p. 285): “daß man fast von einer mathematischen Exaktheit sprechen könnte.” And he continued: “Bei solchen Resultaten muß jeder Zweifel (on the reliability of the myeloarchitectonic method, R. N.) verstummen.” The fact that his map contained more areas than that of Vogt (28 vs. 20) is according to Beck, due to the fact that he divided some of Vogt’s areas into subareas.
Myeloarchitectonic parcellation of the cortex covering the dorsal surface of the human temporal lobe. Both maps are based on serial sections of one and the same hemisphere (A 27l) and prepared independently by Beck (a) and Vogt (b) (see text). Reproduced from Beck (1925)
In two subsequent papers, published in 1928 and 1930, Beck presents a detailed myeloachitectonic analysis of the same region, reportedly based on no less than 24 (non-specified) hemispheres, stained according to Kultschitzky–Wolters. The study is well documented with numerous photomicrographs, a part of one of which is reproduced in Fig. 34b. Beck found that the medial part of the dorsal temporal lobe contains several allocortical regions, including the Regio temporalis insularis (ti), R. praepiriformis (prpy), R. entorhinalis (e), and R. periamygdalaris (Pam). The remaining, neocortical superior temporal cortex appeared to be divisible into six subregions (Fig. 33a), each of which containing a number of parts, which in their turn could be further divided into a number of areas (Fig. 34b), as follows:
The myeloarchitecture of the cortex covering the dorsal surface of the temporal lobe. a Parcellation in the human, according to Beck (1928), based on hemisphere A 37l. b Transverse section through the caudal part of the temporal lobe of the same human hemisphere, reproduced from Beck (1930). c Parcellation in the chimpanzee, according to Beck (1929)
-
Subregio temporopolaris (tp), two parts (tpm, tpl), 17 areas;
-
S. temporalis superior (ts), two parts (tsm, tsl), 12 areas;
-
S. parainsularis (tpar), 3 areas;
-
S. temporalis transversa prima (ttrI), five parts (ttrIin, ttrIi, ttrIe, ttrIex, ttrIl), 24 areas;
-
S. temporalis transversa secunda (ttrII), two parts (ttrIIm, ttrIIl), 11 areas;
-
S temporalis transversa tertia (ttrIII), two parts (ttrIIIm, ttrIIIl), 7 areas.
So, Beck distinguished within the superior temporal neocortex, six subregions, with 13 parts, and 74 areas. If we add the 15 areas, delineated by him within the four allocortical regions analyzed (Fig. 34b), the total number of superior temporal areas distinguished by that author appears to amount to 89. This number is much higher than that found in his previous study (see above). Beck explains this difference from the fact that many of the 28 areas of his old scheme (Fig. 32a), appeared to be divisible on closer scrutiny into two or more subareas (Fig. 34a). He emphasized that the boundaries between all of the areas distinguished, were sharp and omnilaminar (i.e., involved all of the seven layers of the cortex), and that all of the areas could be recognized in all of the hemispheres investigated.
At the end of his last paper on the subject, Beck (1930, p. 258) addresses the question, whether it makes sense to delimit such a large number of areas in a relatively small cortical region. He answers this question in the positive, for the simple reason that all of these areas are true anatomical entities. He concludes the paper by stating (p. 259): “Alles zusammengenommen glauben wir, daß wir eher zu wenig als zu viel Felder abgegrenzt haben”.
It is noteworthy that Beck (1929) has devoted a separate study to the myeloarchitecture of the superior temporal cortex of the chimpanzee. A detailed discussion of this paper is beyond the scope of the present review. Suffice it to mention that Beck (1929, p. 406) found that the graphical reconstruction of this region of the chimpanzee and that of the human, shows an astounding similarity (“eine verblüffende Ähnlichkeit”), both at the subregional (Fig. 33a, b), and at the areal levels (Fig. 34a, c). The only notable difference between these two species was that in the human temporal cortex the total content of myelinated fibres (“Markfasergehalt’) was much higher than in the chimpanzee.
Hopf (1954b) has been the first, and so far only investigator, who subjected the entire human temporal cortex to a detailed myeloarchitectonic analysis. His study is reportedly based on serial sections of several brains, stained according to Weigert–Wolters. The graphical reconstructions, illustrating his results, were all based on a single brain, specified as MB 59.
Hopf divided the temporal cortex into seven regions, each of which showing a typical and characteristic myeloarchitecture (Fig. 35). Most of these regions appeared to be subdivisible into two or more subregions, and within each of these subregions a number of areas could be delineated. All of the entities distinguished received full Latin names. The names of the seven regions are presented below. The names of the subregions belonging to the first region will also be mentioned. As regards the areas, we confine ourselves to mentioning the names of those lying within the first subregion.
Semidiagrammatic representation of the myeloarchitecture of the seven regions of the human temporal lobe. The abbreviations are explained in the text. Reproduced From Hopf (1954b)
-
1.
Regio temporopolaris (tp), four subregions, 13 areas
-
Subregio medialis (tp.m).
-
Area medialis interna (tp.m.i).
-
Area medialis externa (tp.m.e).
-
Area medialis posterior (tp.m.p).
-
Area medialis postica (tp.m.pt).
-
Area medialis inferior (tp.m.if).
-
Übergangsfeld (tp/mti).
-
Übergangsfeld (tp/mtm).
-
-
Subregio ventralis (tp.v).
-
Subregio lateralis (tp.l).
-
Subregio dorsalis (tp.d).
-
-
2.
Regio temporalis separans (tsep), two subregions, six areas
-
3.
Regio temporalis parainsularis (tpari), one subregion, three areas
-
4.
Regio temporalis transversa (ttr), five subregions, 13 areas
-
5.
Regio temporalis paratransversa (tpartr), one subregion, four areas
-
6.
Regio temporalis magna (tmag), four subregions, 13 areas
-
7.
Regio temporalis limitans (tlim), three subregions, eight areas
Thus, according to Hopf (1954b), the cortex of the temporal lobe can be myeloarchitectonically subdivided into seven regions, 20 subregions, and 60 areas (Figs. 36, 37). Hopf pointed out that the various regions could be macroscopically distinguished in his preparations, and that the subregions and areas delimited were detectable in all of the series studied.
Dorsal (a), lateral (b) and basal views (c) of the human temporal lobe, showing the location of the various myeloarchitectonic regions and subregions. Reproduced from Hopf (1954b). The regions are as follows: tp regio temporopolaris, tsep regio temporalis separans, tpari regio temporalis parainsularis, ttr regio temporalis transversa, tpartr regio temporalis paratransversa, tmag regio temporalis magna, tlim regio temporalis limitans. The subregions are as follows: c caudal, d dorsal, l lateral, m medial, o oral, v ventral. The gyri: T1, T2, T3 superior, middle and inferior temporal gyri; T4 lateral occipitotemporal (fusiform) gyrus; Ttr1, Ttr2, Ttr3 first, second and third transverse gyri of Heschl
Dorsal (a), lateral (b) and basal views (c) of the temporal lobe, showing the myeloarchitectonic parcellation, according to Hopf (1954b)
The number of temporal areas distinguished by Hopf is much higher than that recognized in the pioneering myeloarchitectonic studies of Campbell (1905, Fig. 6a, b) and Elliot Smith (1907, Fig. 7a, b). The former delineated only two, the latter seven temporal areas. The areas delineated by Elliot Smith show so far as the lateral surface of the temporal lobe is concerned, a certain resemblance with the subregionalization of Hopf (cf. Fig. 7a with Fig. 36b). As regards the dorsal surface of the temporal lobe, the five regions distinguished here by Hopf correspond in a one-to-one fashion with the subregions delimited by Beck. The only difference at this level is that the Regio temporalis transversa (ttr) of Hopf is subdivided by Beck into three subregions, the subregio temporalis prima (ttrI), secunda (ttrII), and tertia (ttrIII) (cf. Fig. 33a with Fig. 36a). The 12 subregions which Hopf distinguished in the superior temporal cortex, correspond fairly well to the 13 parts of Beck. It is only at the level of the areas that the studies of Hopf and Beck differ considerably: 29 versus 73! Not surprisingly, Hopf ascribes this difference to the fact that Beck in some regions, particularly the regio temporalis polaris (tp) and the regio temporalis transversa (ttr) has descended with his parcellation to the subareal level (Figs. 34a, 37a).
Finally, it should be mentioned that Hopf, following the myeloarchitectonic mapping study, discussed above (Hopf 1954b), has devoted two additional publications to the temporal cortex, one to the distribution of the principal myeloarchitectonic features over this cortex (Hopf 1955), the other to a photometric analysis of the myeloarchitecture of the same cortex (Hopf 1968b). Special attention is paid in these studies to the total content of myelinated fibres in the various temporal regions, as well as to the density of individual fibres (‘Einzelfasern’), the presence of a stripe of Kaes-Bechterew, the disposition of the stripes of Baillarger and the differentiation of the bundles of radial fibres. It was found that the regio temporalis transversa (ttr), which covers the transverse gyri of Heschl (Fig. 37a: Ttr1, Ttr2), and which forms the end station of the auditory projection, shows by far the highest content of myelinated fibres of all temporal regions. There appeared to be a step-like decrease in the content of myelinated fibres and radial bundles, with increasing distance from the primary auditory cortex. The existence of the seven myeloarchitectonically different regions in the temporal cortex, delineated by microscopic inspection (Fig. 35), could be photometrically confirmed.
Summary and conclusions
From the foregoing, it appears that all parts of the neocortex have been the subject of one or more myeloarchitectonic studies. Most of these studies were based on an analysis of one or two hemispheres, but in some, notably those of Strasburger (1938), on the frontal lobe, and Beck (1928, 1930) on the temporal lobe, a much larger material was used.
The cortices of all five telencephalic lobes, frontal, insular, parietal, occipital and temporal, have been parcellated into number of separate myeloarchitectonic entities. In the frontal, parietal, and temporal lobes, hierarchical subdivisions into regions, subregions, (divisions, subdivisions), and areas were used.
The data available do not allow for a definitive assessment of the exact number of myeloarchitectonic areas in the neocortex, because of the following uncertainties: (1) The findings of the various authors concerning the number of areas, present in a given lobe, may show considerable differences. (2) Most authors label the smallest units in their parcellations areas, but some designate them as subareas. (3) Criteria for distinguishing areas from subareas are lacking. (4) The data available for some lobes are scant. Nevertheless, in light of the data and considerations presented above, we venture to estimate the total number of myeloarchitectonic areas in the human neocortex to be about 185: 70 frontal, 6 insular, 30 parietal, 19 occipital, and 60 temporal.
It should be emphasized that the data reviewed, are adequate and sufficient for the composition of a myeloarchitectonic map of the human neocortex, which would be at least as reliable as any of the classic architectonic maps, i.e. the maps of Campbell (1905), Elliot Smith (1907), Brodmann (1909, 1914), von Economo and Koskinas (1925), Bailey and von Bonin (1951) and Sarkissov et al. (1955).
A general concept of the architecture of the cerebral cortex
Retrospect and prospect
In the preceding pages, we reviewed the entire literature on the myeloarchitectonic parcellation of the human neocortex, in total 31 publications, no less than 27 of which stemming from the Vogts themselves or from their direct collaborators. Almost all of these publications, many of them of extraordinary length, appeared in the ‘home journals’ of the successive ‘Vogt–Vogt-Institutes’, i.e. the “Journal für Psychologie und Neurologie”, and the “Journal für Hirnforschung”. Surveying this body of literature, one cannot help but admire the patience and perseverance with which the authors concerned, and their technical assistants, have accomplished this enormous amount of work. These publications, and the companion cytoarchitectonic studies were, as we have seen, aimed at the division of the cerebral cortex into units, designated as fields or areas. A special noun, ‘Felderung’, and a special verb, ‘feldern, felderte, gefeldert’ (or even ‘durchfeldern’) were created to designate these special activities. It should be remembered that the results of these, extremely time-consuming, activities were not merely ‘contributions to the architectonics of the cerebral cortex’, but represented at the same time the realization of the research programme formulated by Vogt (1903), outlined in the introductory part of the present review. It is important to note that the Vogts did not only participate in the realization of this programme, but also developed, in light of the results obtained, a general concept of the structural and functional architecture of the cerebral cortex, and of the CNS in general. The present paper provides the opportunity to articulate for the first time, this general concept, the elements of which are dispersed over numerous publications of the pertinent authors. In what follows, an annotated synopsis of the ‘Vogt–Vogt concept’ will be presented. This synopsis is preceded, however, by a brief note on techniques.
Note on techniques
The research programme of the Vogts and their co-workers, and the resultant concept to be discussed below, were exclusively based on observations derived from two histological staining procedures, the Nissl- and Weigert techniques. The question arises whether these techniques are appropriate for an analysis of the architecture of the cortex. The answer to this question is: yes. The essence of the Nissl and Weigert techniques is that they reduce the baffling intricacy of the cortex (and of the CNS in general) to manageable, i.e., to analyzable proportions. The Nissl technique ‘strips’ the neurons from their processes, reducing them to spots, differing in size, shape and arrangement (Fig. 3, left panel), whereas the Weigert technique stains only the myelin sheaths of the axons, which are concentrated in radial bundles and tangential plexuses (Fig. 3, right panel). All of these are “leicht erkennbare Merkmale” (Vogt 1903, p. 161). A difficulty, inherent to the Weigert method is that slight variations in the time of differentiation may lead to considerable differences in the pattern of staining (Fig. 11c, d). Several other ‘architectonics’, based on other staining procedures, have been attempted, such as fibrillo-architectonics, based on silver-reduction techniques, glia-architectonics, based on glia stains, and angio-architectonics, based on the injection of dyes in blood vessels, but none of these procedures has been really successful. This does not hold true, however, for the more recently developed receptor architectonics, i.e., the study of the regional density and laminar distribution of different transmitter receptor types, as visualized by autoradiography or immunohistochemistry. The borders of cortical areas defined by cytoarchitecture, are generally perfectly matched by those derived from receptor mapping (Zilles and Amunts 2009, 2010; Zilles and Palomero-Gallagher 2001). Other complementary techniques, such as the histochemical staining for acetylcholinesterase, and the immunohistochemical stainings for calcium-binding proteins, have also been shown to be useful in architectonic studies (Carmichael and Price 1994; Öngür et al. 2003).
An annotated synopsis of the Vogt–Vogt concept of the organization of the cerebral cortex
In what follows, the views of the Vogts are summarized in ten theses. These theses, including the sources on which they are based, are printed in italics.
-
1.
The entire cerebral cortex is divisible into a number of juxtaposed cytoarchitectonic areas (Vogt 1906, p. 74; 1927, p. 251).
It is clear that Vogt relied here on the results of his collaborator Brodmann, who had successfully parcellated the human cerebral cortex, and that of a considerable number of other mammals as well. The reliability of the results of Brodmann, and those of classic cytoarchitectonics in general, has been challenged repeatedly, most ardently by Lashley and Clark (1946). These authors independently studied serial Nissl sections through the brains of two spider monkeys, both producing a map of the cerebral cortex. The two maps showed little agreement (Fig. 38). They then compared their two specimens and found that there was considerable variation in size and appearance of corresponding areas from brain to brain, and that some areas could be seen in the one brain, but not in the other. Moreover, they remained unable to locate most of the neocortical areas recognized by other investigators in this monkey and in the rhesus macaque. Lashley and Clark (1946, p. 231) concluded that standard cytoarchitectonic maps are unreliable and “represent little more than the whim of the individual student”. However, numerous recent studies, including Amunts et al. (1999, 2003), Caspers et al. (2006, 2008), Scheperjans et al. (2008a, b), using quantitative and observer-independent procedures, have confirmed the presence of many of the areas delineated by Brodmann, and detected additional, previously not described areas.
Parcellation of the cortex of two different specimens of the spider monkey Ateles geoffrroyi, made independently by Lashley (a) and Clark (b). Reproduced from Lashley and Clark (1946)
-
2.
The entire cerebral cortex is divisible into a number of juxtaposed myeloarchitectonic areas. (Vogt 1910b, p. 418).
The evidence for this thesis is presented in the first part of this review. The following points may be recalled:
-
(i)
Elliot Smith (1907; Fig. 7a, b), who practised a sort of myeloarchitectonics ‘avant la lettre’, was able to distinguish no less than 50 different, sharply delimited areas in the human cortex.
-
(ii)
Many experts maintain that most areal borders can be observed with the naked eye in their myelin-stained preparations. This is in line with the findings of Vogt (1928a, b, p. 467): “Die myeloarchitektonische Gliederung der Grisea ist viel augenfälliger als die cytoarchitektonische.”
-
(iii)
The fact that the results of Beck and Vogt, who mapped the human dorsal temporal cortex independently from each other, show a striking similarity (Fig. 32), pleads strongly for the reliability of the myeloarchitectonic approach. Their findings are in marked contrast with those of Lashley and Clark (1946, Fig. 38) on cytoarchitectonics.
-
(iv)
The presence of most of the cortical areas, delineated by Vogt (1910a, 1911) in his early myeloarchitectonic studies, has been confirmed by several later investigators.
-
(v)
The thorough myeloarchitectonic explorations of the human cerebral cortex by Adolf Hopf (1968a, b, 1969, 1970b), were not only based on visual inspection, but also on objective registrations of myeloarchitectonic features with a photometric technique.
-
(vi)
It remains enigmatic that the results of myeloarchitectonic parcellations of particular cortices, or parts thereof, may show considerable differences. The rather global subdivision of the cortex of the orangutan by Mauss (1911; Fig. 13), and the much more detailed parcellations of the frontal, parietal, and temporal cortices of the chimpanzee by Strasburger (1937b; Fig. 16), Gerhardt (1938), and Beck (1929; Fig. 34c), respectively, are striking cases in point. It is also highly remarkable that Beck (1925; Fig. 32a), who initially found 28 areas in the human dorsal temporal cortex, subdivided the same cortical region in a later study (Beck 1928; Fig. 34a) in no less than 89 areas!
-
(i)
-
3.
Because the boundaries of the cytoarchitectonic and the myeloarchitectonic cortical areas coincide completely, it is correct to designate these entities simply as architectonic areas (Vogt and Vogt 1919, p. 361; 1954, p. 16; 1956, p. 409).
The Vogts were deeply convinced of the correctness of this thesis. The discrepancy between the relatively low number of cytoarchitectonic areas in the human cortex, distinguished by Brodmann (1909), and the much higher number, resulting from their own myeloarchitectonic studies, was explained by claiming that Brodmann had missed numerous boundaries (Vogt 1918, p. VI). Vogt and Vogt (1919, p. 365) emphasized to have found cytoarchitectonic counterparts of all of their myeloarchitectonic cortical areas. It may be recalled in this context that Sanides (1962), Brockhaus (1940), and Gerhardt (1940), who carried out combined cytoarchitectonic and myeloarchitectonic analyses of the human frontal, insular, and parietal cortices, respectively, all reported complete concordance of the results obtained with the two techniques.
Combination of cytoarchitectonics with other types of ‘architectonics’ may also lead to concordant results. Thus, it has been shown that in the human cerebral cortex, receptor-architectonic borders quite often precisely match cytoarchitectonic borders. The finding that receptor mapping not only corroborates classical borders of cytoarchitectonic areas, but can also reveal more detailed subdivisions (Zilles and Amunts 2009), may well mark a shift from the ‘Brodmann’ to the ‘Vogt–Vogt-level of parcellation’ of the cortex (see below).
-
4.
The boundaries of the architectonic areas are hair-sharp and extend through all cortical layers (Vogt and Vogt 1919, p. 365; Vogt 1951, p. 117).
This is one of the most controversial points of the Vogt–Vogt concept. As for cytoarchitectonics, there can be no doubt that some boundaries, such as that between the primary motor and the somatosensory areas, and that between the calcarine and occipital areas (Fig. 29a), are very distinct, but this does not hold true for the borders of many other areas. Brodmann (1909) indicated that several of the boundaries in his map of the human cerebral cortex, particularly those between the various prefrontal and parietal areas, are not sharp. Von Economo and Koskinas (1925) found that in the human neocortex, gradual transitions, rather than distinct areal boundaries, are the rule. At this point, they were fiercely attacked by Marthe Vogt (1928a, b), the eldest daughter of the Vogts, who defended the existence of hair-sharp boundaries in the cortex with numerous examples. Von Economo (1928, p. 322) maintained, however, that the cortical areas and their borders do not constitute a totally rigid (“absolut starres”) system, and that the ‘limitrophic adaptations’, described by the Vogts (see below), point in the same direction.
Even among the direct collaborators of the Vogts, there was no unanimity as to the sharpness of the interareal boundaries, as the following example may show. Mauss (1911) analyzed the myeloarchitecture of the cortex of the orangutan, This study resulted in a beautiful map (Fig. 13), in which the cortex is parcellated into about 40 areas. Mauss (1911, p. 437) mentioned, that the preparation of this map had compelled him: “…mehr oder weniger scharfe Grenzen zu ziehen, wo es sich um allmähliche Übergänge, oft sogar nur relativ ausgedehnte Mischzonen handelt”. In 1938, Edith Gerhardt subjected the parietal cortex of the chimpanzee to a detailed cyto- and myeloarchitectonic analysis. She delineated a larger number of areas in this region, than Mauss had done in the entire cortex of the orangutan. At the end of her study, Gerhardt (1938, p. 385) compares her results with those of Mauss (1911): “Es ist interessant, dasz dort, wo Mauss von unscharfen Grenzen und Übergangsgebieten spricht, sich meist scharf gegeneinander abgesetzte kleinere Struktureinheiten finden lieszen,…”
Omnilaminarity, i.e., the phenomenon that the boundaries between architectonic units extend through all cortical layers, played an important role in the views of the Vogts. They believed that, within a given architectonic unit, all neurons, and hence all neuronal layers, are adapted to the central function of that particular unit (Vogt 1927, p. 251). The present author has always thought that this ‘omnilaminarity-claim’ does no justice to the cytoarchitectonic reality. Thus, I believed that the boundary between the striate and occipital cortical areas is essentially confined to the middle cortical layers, and that there is continuity in the superficial and deep layers (Fig. 39a). The analytical scheme of the situation, presented by Vogt and Vogt (1936, p. 266; Fig. 39b), indicates, however, that changes in all cortical layers mark the boundary between the two areas.
Fig. 39 The boundary (arrows) between the striate (Str) and occipital areas (O) in the human cortex. a Cytoarchitecture, based on a Nissl preparation. b Scheme, showing that this boundary is marked by cytoarchitectonic changes in all of the cortical layers. Reproduced from Vogt and Vogt (1936)
With regard to myeloarchitectonics, it may be recalled that Elliot Smith (1907) emphasized that the boundaries between his macroscopically observed myeloarchitectonic areas in the human cortex are very distinct. A similar conclusion was reached by the Vogts and their numerous collaborators, who studied the myeloarchitecture of the human cortex at the microscopic level. To enable the reader to form an opinion about these boundaries, we reproduced here a number of illustrations from the literature (Figs. 15b, c, 16h, 29b, f, 34b), showing the transition of two or more myeloarchitectonic areas into each other.
-
5.
Architectonic areas are, structurally, not always homogeneous throughout; their border zones may show ‘limitrophic adaptations’ to adjacent areas (Vogt and Vogt 1919, p. 369; 1928 , p. 468).
In their own words (Vogt and Vogt 1919, p. 369): “Es soll aber nicht verschwiegen werden daß die Architektonik in der ganzen Ausdehnung des Feldes durchaus nicht eine absolut gleiche ist. Speziell im Grenzgebiet des einzelnen Feldes tritt eine Annäherung an den jedesmaligen Bau des benachbarten Feldes auf”.
-
6.
The cortical architectonic units may be expected to have specific afferent and efferent connections (Vogt 1906, p. 74; Vogt and Vogt 1956, p. 408).
Vogt (1918, p. VI) referred in this context to the fact that focal cortical lesions always lead to sharply delimited thalamic degenerations. However, the hodological substantiation of their architectonic findings, has never formed part of the research programme of the Vogts. Several authors, including Lashley and Clark (1946), Bailey and von Bonin (1951), Le Gros Clark (1952), and Jones (1987, 2008), have indicated that in their opinion, the afferent and efferent connections form the most reliable basis for a rational subdivision of the cerebral cortex. Rose and Woolsey (1948), studying the structure and connections of the cingulate cortex in rabbit and cat, found that the cingulate cortex in these animals can be subdivided into three areas, and that each of these areas co-extends with the distribution field of a particular nucleus within the anterior thalamic nuclear group. Such close correlations between cytoarchitecture and the projection fields of individual thalamic nuclei were later also found in many other regions of the cerebral cortex (Rose and Woolsey 1949; Jones and Burton 1976; Seltzer and Pandya 1978). Using data collected in the macaque connectivity database CoCoMac (Stephan et al. 2001), Passingham et al. (2002) demonstrated that in this monkey, each cortical area has a unique pattern of cortico-cortical connections, a defining ‘connectional fingerprint’. Finally, it may be mentioned that recently, several authors, including Behrens and Johansen-Berg (2005), Johansen-Berg et al. (2004), and Anwander et al. (2007), using diffusion imaging, have presented evidence, suggesting that in the human cortex, particular areas have a distinct ‘connectional architecture’.
-
7.
The fact that all of the cortical areas distinguished display a specific structural organization indicates that all of these areas subserve a specific function (Vogt 1903, p. 160; Vogt and Vogt 1954, p. 116).
This categorical statement refers to an important motive for the architectural studies of the Vogts, which included, before all things, the performance of physiological preparatory work. “Vor allem wollen wir physiologische Vorarbeit liefern” (Vogt 1911, p. 379). We have seen that Campbell (1905) had precisely the same intention.
To substantiate their functional notions, the Vogts carried out extensive electrical stimulation studies in monkeys (Vogt and Vogt 1907, 1919, 1942). These studies, and parallel experiments of Foerster (1936), in patients who underwent brain operations, showed that areas from which particular motor responses can be elicited, generally match with particular architectonic areas. Vogt and Vogt (1928, p. 468) emphasized that only one single architectonic parcellation can be the physiologically correct one. They indicated that it would be the task of neurophysiologists to determine the precise functions of all of the architectonic units distinguished, and never speculated on the outcome of that inquiry.
Other authors have also advanced the hypothesis that the cerebral cortex is composed of units, which represent structural as well as functional units. Thus, Carmichael and Price (1994) carried out a detailed multiarchitectonic study of the orbital and medial prefrontal cortex (OMPFC) of the rhesus monkey. This study resulted in the identification of no less than 22 discrete areas in the OMPFC of this species. Subsequent experimental hodological studies (Carmichael and Price 1995a, b, 1996) revealed that all of the areas distinguished have specific connections. The authors mentioned concluded that each of the cortical areas delineated, represents a module with specific input–output relations, and a unique role in information processing. They considered it likely that much of the cortex consists of such discrete structural and functional modules. Roland and Zilles (1998) expressed the expectation that combining the results of quantitative architectonic studies of the cortex that apply objective, observer-independent procedures, with functional neuroimaging data, will lead to the detection of functional cortical fields. They advanced the hypothesis that the organization of the cortex is based on such functional fields, and speculated that all neurons and synapses within these fields perform a co-operative computation. Recently, Zilles and Amunts (2009) have pointed out that receptor mapping, particularly quantitative multireceptor mapping, may provide important clues to both the structural and the functional organization of the cerebral cortex.
-
8.
The fact that each of the cortical architectonic units subserves a specific function indicates that these units are to be considered as separate organs, and that the cortex as a whole represents a complex of organs (“Organkomplex”) (Vogt and Vogt 1922 , p. 163).
This view, which was strongly supported by Brodmann (1909, p. 237), goes back, as we shall see, to the phrenologists Gall and Spurzheim.
-
9.
Cortical architectonic units may show a specific vulnerability for particular pathological processes. These are manifestations of a general phenomenon to be designated as topical pathoclisis (Vogt and Vogt 1922 , p. 163; 1936 , p. 452; 1942 , p. 368; 1956 , p. 405).
This component of the topistic approach to the cortex of the Vogts has met with little response. Von Economo (2009, p. 178) noted that amyotrophic lateral sclerosis, at least initially, specifically affects the primary motor area, but that he was not acquainted with any other disease of the CNS showing a similar areal circumscription.
-
10.
The total number of cortical fields (“Rindenfelder”) or topistic units (“topistische Einheiten”) in the human cerebral cortex amounts to about 200 (Vogt and Vogt 1919 , p. 364; Vogt 1951 , p. 117).
This large number of areas, has been experienced by numerous previous authors, including Bailey and von Bonin (1951, pp. VII, 231), Le Gros Clark (1952, p. 104), and Jones (2003, p. 19), as being out of all proportions. It was felt that Brodmann’s subdivision into some 40 areas, comes somewhere near what seems reasonable. It is felt appropriate to sum up here the evidence indicating that, according to the present state of knowledge, the estimation of the Vogts was absolutely realistic.
-
(i)
Vogt and Vogt (1919, p. 365) maintained that Brodmann has missed numerous cytoarchitectonic borders, and that they had found cytoarchitectonic counterparts of all of their numerous myeloarchitectonic areas.
-
(ii)
The analyses of the human cerebral cortex of Von Economo and Koskinas (1925) and Sarkissov et al. (1955) resulted in a much larger number of cytoarchitectonic areas than that of Brodmann.
-
(iii)
The review of the myeloarchitectonic literature, presented in the first section of this paper, led us to the conclusion that the human neocortex contains about 185 myeloarchitectonic areas. If we take into consideration that the Vogts included, apart from neocortical areas, also allocortical areas in their estimation, their number appears to be practically identical to ours.
-
(iv)
Nieuwenhuys et al. (2008, p. 535) plotted the results of a detailed multiarchitectonic study of the OMPFC (Öngür et al. (2003), and of a number of functional imaging studies on the localization of visuotopic areas (Hadjikani et al. 1998; Press et al. 2001; Van Essen 2006), on a flattened version of Brodmann’s map (Van Essen 2006). It appeared that the 30 areas delineated and plotted, together occupy 20 % of the neocortical surface. Extrapolating from these data, it was estimated that that the neocortex is composed of some 150 areas or units.
-
(v)
During the last decades, the C. & O. Vogt Institute of Brain Research in Düsseldorf and the Institute of Neuroscience and Medicine, Research Centre Jülich, have produced a large number of detailed studies on the architecture of the human cerebral cortex. These studies, in which the results of quantitative cytoarchitectonic analyses are generally combined with receptor-architectonic data, have shown that many of the cytoarchitectonic areas, distinguished by Brodmann (1909, 1914), are divisible into two or more smaller units. In what follows, the results of a number of these studies are recorded in a very condensed form, as for instance: prefrontal cortex, 3 |BAs 9, 10, 11| 1, 7, which means in full: The pertinent authors investigated a sector of the prefrontal cortex, occupied by three Brodmann areas (BAs), namely: BAs 9, 10 and 11; they confirmed the presence of one BA, and subdivided the remaining BAs in this sector into seven smaller units.
-
Geyer et al. (1996, primary motor cortex: 1 |BA 4| 0, 2)
-
Amunts et al. (2010, Broca’s region: 2 |BAs 44, 45| 0, 4)
-
Uylings et al. (2010, orbitofrontal cortex: 2 |BAs 11,46| 1, 5)
-
Palomero-Gallagher et al. (2008, anterior cingulate cortex: 3 |BAs 24, 25, 32| 0, 10)
-
Kurth et al. (2010, posterior insular cortex: 1 |BA J.post| 0, 5)
-
Scheperjans et al. (2008a, superior parietal cortex: 2 |BAs 5, 7| 0, 12)
-
Caspers et al. (2006, inferior parietal cortex: 2 |BAs 39, 40| 0, 7)
-
Eickhoff et al. (2008, visual cortex: 3 |BAs 17,18, 19| 0, 8)
These data suggest that there may well be almost four times as many architectonic areas in the human neocortex as Brodmann indicated. Jones (2008, p. 2231) noted about the recent efforts of the C. & O. Vogt Institute that their numbers, though not yet finished, “seem well on the way to approximating those of the Vogts”.
-
-
(vi)
Physiological studies, using single cell recordings, have shown that monkeys have more than 30 cortical areas for processing visual information, at least 15 for somatosensory information, and some 20 for auditory information (Kaas 2002). Extrapolation of these numbers to the human points to the presence of a much larger number of cortical areas than the 44 of Brodmann’s parcellation scheme.
-
(vii)
Most recently, Glasser and van Essen (2011) estimated the total number of architectonic areas per hemisphere in humans at 150–200, on the basis of as yet unpublished observations of Van Essen, Glasser, Dierker, Harwell, and Coalson.
-
(i)
Before closing this section, some additional remarks on the Vogt–Vogt concept should be made.
-
1.
The Vogts believed that their concept does not hold only for the cortex, but is also applicable to other parts of the brain, such as the striatum and the thalamus (Vogt and Vogt 1922, p. 23).
-
2.
One of the plans of the Vogts that never has been realized was the implementation of an “Individualanatomie” of the cortex, based on a large collection of brains of geniuses (‘Elitegehirne’) and of mentally retarded people. It was expected that in the brains of highly gifted individuals, such as great musicians or great mathematicians, particular cortical areas would be strongly developed, whereas in feeble-minded people, particular areas would be underdeveloped or even rudimentary (Vogt 1910b, p. 419). It is noteworthy that Vogt and Vogt (1956, p. 426) emphasized on the last page of their last publication that, within the context of such an ‘Individualanatomie’, the brains of asocials would also deserve examination. We have seen that Kaes (1907) pursued a similar goal.
-
3.
Vogt and Vogt (1922, p. 12; 1929, p. 154) expected that their topistic analyses of the cortex could be conducive to a pathological–anatomical classification of mental diseases, whereas Brodmann (1913) considered knowledge on the segregation of the cortex into architectonic units of great potential importance for the tackling of anthropological problems: “Gibt es im besonderen am Gehirn verschiedener Menschenrassen lokalisatorische Tatsachen, die sich als Zeichen eines primitiveren Zustandes deuten und somit vielleicht auch für das Rassenproblem fruchtbar machen Lassen?” (Brodmann 1913, p. 202).
-
4.
Finally, it may be mentioned that not all of the collaborators of the Vogts agreed with all of the details of their concept. Thus, Brodmann (1909, p. 306) emphasized that some of the boundaries of some neocortical areas are not sharp. He also believed that higher cortical functions result from the conjoint activities of a large number of areas distributed more or less widely over the cortical surface. Hopf (1954a, p. 464) warned in a general paper on the architectonics of the cortex, for making the Vogt–Vogt concept absolute: “In jeder Area ein ganz isoliertes Organ mit einer einzigen bestimmten Funktion zu sehen, ist natürlich völlig unsinnig”.
Summary
-
1.
During their long scientific career, the Vogts gradually developed a general concept of the organization of the human cerebral cortex.
-
2.
This concept may be epitomized as follows: The human cerebral cortex is segregated into around 200 discrete, juxtaposed structural and functional modules or units.
-
3.
There is converging quantitative cytoarchitectonic, receptorarchitectonic, myeloarchitectonic, hodological, as well as functional evidence, indicating that this concept is essentially correct.
The myeloarchitectonic studies of the Vogt–Vogt school, and the current explorations of the functional organization of the cerebral cortex with neuroimaging techniques
We have just seen that the extensive architectonic studies of the Vogts and their collaborators, have resulted in the concept that the human cerebral cortex is composed of about 200 structural and functional units. The Vogts and their associates knew, of course that some of these units are involved in particular sensory or motor functions, but in general they refrained from speculating on the specific functions of the remaining units. Other authors have been less reluctant in this respect. Thus, Franz Joseph Gall (1758–1828) and Johann Spurzheim (1758–1832) maintained, as early as the beginning of the nineteenth century, that the cerebral cortex is composed of discrete organs or regions that represent different mental faculties, and that there are as many such organs as there are mental faculties. They distinguished some 35 of these localized faculties, including arithmetic, hope, conscientiousness, language, constructiveness, destructiveness and parental love. They based their theory on the examination of the skulls of a great variety of people, from eminent and highly gifted to lunatics and criminals, assuming: (1) that the intellectual and moral abilities are differentially developed in each individual; (2) that these differences are reflected in the size of the cortical organs related to these abilities, and (3) that the size of the pertinent cortical organs is in their turn reflected in prominences of the overlying skull, i.e., in cranial bumps. The highly speculative and dubious branch of science founded by Gall and Spurzheim is now generally known as phrenology (from Gr. phrèn = mind).
About a 100 years later, the neurologist Karl Kleist (1879–1960) made another attempt at a far going localization of functions in the human cerebral cortex. He had a vast amount of clinical material at his disposal, including, apart from numerous regular clinical cases, about 300 persons who had sustained local brain injuries during World War I (Kleist 1934). He summarized his findings in a map, shown here in Fig. 40. It will be seen that Kleist subdivided the cortex according to Brodmann (1909), and that he provided almost all of the 44 cytoarchitectonic areas distinguished by the latter with a functional label. The overall functions of the primary sensory and motor areas are correctly indicated. However, Kleist went far beyond that by attributing all sorts of higher cognitive and mental functions and faculties to many other areas. Thus, he associated temporal area 21 with auditory awareness (“akustische Aufmerksamkeit”), prefrontal area 10 with motor skill (“motorische Handlungsfolgen”), and orbitofrontal area 11 with personal and social ego (“Selbst- und Gemeinschafts-Ich”). Because of this detailed localization of psychic functions, many of his colleagues disposed of Kleist’s map as ‘brain mythology’ (cf. Creutzfeldt 1983). Uttal (2001, p. 109) qualified this map as “a modern manifestation of phrenological thinking.” It is ironic that Brodman, n.b. the creator of the map used by Kleist, as we mentioned already, did not believe that higher cognitive functions can be related to individual cortical areas.
From Kleist to the present! It is no exaggeration to say that the modern imaging techniques, such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have revolutionized our capacity to localize functions and functional complexes in the cerebral cortex. As eloquently put into words by Mesulam (2012, p. 2): “We are now in the midst of yet another revolution, a revolution powered by spectacularly successful methods for the non-invasive functional and structural imaging of the human brain. Impressive advances in signal acquisition, data analysis, and task design have collectively empowered a multidisciplinary army of investigators to map the cerebral cartography of vision, language, love, lust, greed, altruism, empathy, conflict, and virtually any other mental faculty that can be delineated.” Mesulam used this passage to introduce a pressing call to renewed and refined studies of human cortical connectivity. The present author is of the opinion that this activity, however, significant, should be preceded by renewed and refined studies on the problem as to how to establish the morphological identity of the entities, which form the edges, nodes and hubs in this connectivity, i.e., the foci of cortical activity, observed in functional neuroimaging studies.
At present, modified versions of Brodmann’s map are commonly used for the structural interpretation of neuroimaging data. However, it has become increasingly clear that these “Brodmann” maps do not provide the neuroanatomical precision and accuracy for an adequate mapping of fMRI data (Geyer et al. 2011). Brodmann’s map is a pioneering work, but necessarily contains false delineations (e.g. his area 19 does not match any of the extrastriate areas shown by retinotopic mapping), missing delineations (most of the intrasulcal cortex was never mapped by him) and various other problems (for review see Zilles and Amunts 2010). The data, reviewed in the present paper, have shown that there is converging evidence, indicating that the human cerebral cortex is divisible into some 200 structural and functional units, and that the maps, resulting from the meticulous myeloarchitectonic parcellations of the Vogts and their associates, have yielded similar results. It is for these reasons that we strongly recommend an attempt at combining and synthesizing the results of cytoarchitectonic mapping studies of Brodmann on the human cerebral cortex, with those of the Vogts. The resultant ‘supermap’ would not only mark the consummation of the enterprise on which these eminent scientists embarked, more than a century ago, but would probably also yield an optimal frame of reference for the localization of functions, as revealed by neuroimaging studies.
It stands to reason that, because of the considerable interindividual variability of the areal borders in the human cortex, interpretations based on the futuristic ‘supermap’ just referred to, could only be performed probabilistically.
The problems of functional localization in the human cortex would be greatly reduced if it were possible to map the specific structural correlates of functional activity, in a non-invasive way, directly in each individual living brain under study. Recent studies, using high-resolution MRI, have shown that such direct correlations between cortical architecture and function in living brains are now within our range. Such in vivo explorations of the histology of the cortex have revealed that local differences in the total fibre content (Glasser and Van Essen 2011), and in the laminar patterns of the myelinated fibres (Geyer et al. 2011) give excellent MRI contrast. This being so, it may be expected that the meticulous myeloarchitectonic studies of the Vogt–Vogt school, will play a prominent role in the interpretation of the results of these new in vivo mappings.
References
Amunts K, Zilles K (2012) Architecture and organizational principles of Broca’s region. Trends Cogn Sci 16(8):418–426. doi:10.1016/j.tics.2012.06.005
Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412(2):319–341. doi:10.1002/(SICI)1096-9861(19990920)412:2<319::AID-CNE10>3.0.CO;2-7
Amunts K, Schleicher A, Ditterich A, Zilles K (2003) Broca’s region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol 465(1):72–89. doi:10.1002/cne.10829
Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, Zilles K (2010) Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol 8(9):e1000489. doi:10.1371/journal.pbio.1000489
Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR (2007) Connectivity-based parcellation of Broca’s area. Cereb Cortex 17(4):816–825. doi:10.1093/cercor/bhk034
Bailey P, von Bonin G (1951) The isocortex of man. University of Illinois Press, Urbana
Batsch EG (1956) Die myeloarchitektonische Untergliederung des Isocortex parietalis beim Menschen. J Hirnforsch 2:225–258
Beck E (1925) Zur Exaktheit der myeloarchitektonischen Felderung des Cortex cerebri. J Psychol Neurol 31:281–288
Beck E (1928) Die myeloarchitektonische Felderung des in der Sylvischen Furche gelegenen Teils des menschlichen Schläfenlappens. J Psychol Neurol 36:1–21
Beck E (1929) Der myeloarchitektonische Bau des in der Sylvischen Furche gelegenen Teiles des Schläfenlappens beim Schimpansen (Troglodytes niger). J Psychol Neurol 38:309–420
Beck E (1930) Die Myeloarchotektonik der dorsalen Schläfenlappenrinde beim Menschen. J Psychol Neurol 41:129–262
Behrens TE, Johansen-Berg H (2005) Relating connectional architecture to grey matter function using diffusion imaging. Philos Trans R Soc Lond B Biol Sci 360(1457):903–911. doi:10.1098/rstb.2005.1640
Braak H (1980) Architectonics of the human telencephalic cortex. Springer, Berlin
Braitenberg V (1956) Die Gliederung der Stirnhirnrinde auf Grund ihres Markfaserbaus (Myeloarchitektonik). In: Rehwald E (ed) Das Hirntrauma. Thieme, Stuttgart, pp 183–203
Braitenberg V (1962) A note on myeloarchitectonics. J Comp Neurol 118:141–156
Brockhaus H (1940) Die Cyto-und Myeloarchitektonik des Cortex claustralis und des Claustrum beim Menschen. J Psychol Neurol 49:249–348
Brodmann K (1903a) Beiträge zur histologischen Lokalisation der Grosshirnrinde: regio Rolandica. J Psychol Neurol 2:79–107
Brodmann K (1903b) Beiträge zur histologischen Lokalisation der Grosshirnrinde. Zweite Mitteilung: der Calcarinatypus. J Psychol Neurol 2:133–159
Brodmann K (1905a) Beiträge zur histologischen Lokalisation der Grosshirnrinde. Dritte Mitteilung: die Rindenfelder der niederen Affen. J Psychol Neurol 4:177–226
Brodmann K (1905b) Beiträge zur histologischen Lokalisation der Grosshirnrinde. IV. Mitteilung: der Riesenpyramidentypus und sein Verhalten zu den Furchen bei den Karnivoren. J Psychol Neurol 6:108–120
Brodmann K (1906) Beiträge zur histologischen Lokalisation der Grosshirnrinde: fünfte Mitteilung: Über den allgemeinen Bauplan des Cortex Pallii bei den Mammaliern und zwei homologe Rindenfelder im besonderen. Zugleich ein Beitrag zur Furchenlehre. J Psychol Neurol 6:275–400
Brodmann K (1908a) Beiträge zur histologischen Lokalisation der Grosshirnrinde. Sechste Mitteilung: die Cortexgliederung des Menschen. J Psychol Neurol 10:231–246
Brodmann K (1908b) Beiträge zur histologischen Lokalisation der Grosshirnrinde. VII. Mitteilung: die cytoarchitektonische Cortexgliederung der Halbaffen (Lemuriden). J Psychol Neurol 10:287–334
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. J. A. Barth, Leipzig
Brodmann K (1913) Neue Forschungsergebnisse der Großhirnrindenanatomie mit besonderer Berücksichtigung anthropologischer Fragen. Gesellschaft deutscher Naturforscher und Ärtze 85:200–240
Brodmann K (1914) Physiologie des Gehirns. In: Von Bruns P (ed) Neue deutsche Chirurgie, vol 11 Pt. 1. Enke, Stuttgart, pp 85–426
Cajal SR (1894) The Croonian Lecture : the fine structure of the nerve centres. Proc R Soc Lond 55:444–468
Campbell AW (1905) Histological studies on the localisation of cerebral function. Cambridge University Press, Cambridge
Carmichael ST, Price JL (1994) Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol 346(3):366–402. doi:10.1002/cne.903460305
Carmichael ST, Price JL (1995a) Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363(4):615–641. doi:10.1002/cne.903630408
Carmichael ST, Price JL (1995b) Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 363(4):642–664. doi:10.1002/cne.903630409
Carmichael ST, Price JL (1996) Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371(2):179–207. doi:10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#
Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006) The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 33(2):430–448. doi:10.1016/j.neuroimage.2006.06.054
Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K (2008) The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212(6):481–495. doi:10.1007/s00429-008-0195-z
Creutzfeldt OD (1983) Cortex cerebri. Leistung, strukturelle und funktionelle Organisation der Hirnrinde. Springer, Berlin
Eickhoff SB, Rottschy C, Kujovic M, Palomero-Gallagher N, Zilles K (2008) Organizational principles of human visual cortex revealed by receptor mapping. Cereb Cortex 18(11):2637–2645. doi:10.1093/cercor/bhn024
Elliot Smith G (1907) A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J Anat Physiol 41(Pt 4):237–254
Flores A (1911) Die Myeloarchitektonik und die Myelogenic des Cortex Cerebri beim Igel. J Psychol Neurol 17:215–247
Foerster O (1936) Motorische Felder und Bahnen. Sensible cortical Felder. In: Bumke O, Foerster O (eds) Handbuch der Neurologie, vol 6. Springer, Berlin, pp 1–448
Gerhardt E (1938) Der lsocortex parietalis beim Schimpanzen. J Psychol Neurol 48:329–386
Gerhardt E (1940) Die Cytoarchitektonik des Isocortex parietalis beim Menschen. J Psychol Neurol 49:367–419
Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE (1996) Two different areas within the primary motor cortex of man. Nature 382(6594):805–807. doi:10.1038/382805a0
Geyer S, Weiss M, Reimann K, Lohmann G, Turner R (2011) Microstructural parcellation of the human cerebral cortex—from Brodmann’s post-mortem map to in vivo mapping with high-field magnetic resonance imaging. Front Hum Neurosci 5:19. doi:10.3389/fnhum.2011.00019
Glasser MF, Van Essen DC (2011) Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci 31(32):11597–11616. doi:10.1523/JNEUROSCI.2180-11.2011
Hadjikani N, Liu AK, Dale AM, Cavavanagh P, Tootell RB (1998) Retinotopy and color sensitivity in human visual cortical area V8. Nat Neurosci 1:235–241
Hopf A (1954a) Die Myeloarchitektonik des Isocortex temporalis beim Menschen. J Hirnforsch 1:208–279
Hopf A (1954b) Die Myeloarchitektonik des Isocortex temporalis beim Menschen. J Hirnforsch 1:443–496
Hopf A (1955) Über die Verteilung myeloarchitektonischer Merkmale in der isokortikalen Schläfenlappenrinde beim Menschen. J Hirnforsch 2:36–54
Hopf A (1956) Über die Verteilung myeloarchitektonischer Merkmale in der Stirnhirnrinde beim Menschen. J Hirnforsch 2(4):311–333
Hopf A (1966) Über eine Methode zur objektiven Registrierung der Myeloarchitektonik der Hirnrinde. J Hirnforsch 8(4):301–313
Hopf A (1968a) Photometric studies on the myeloarchitecture of the human temporal lobe. J Hirnforsch 10(4):285–297
Hopf A (1968b) Registration of the myeloarchitecture of the human frontal lobe with an extinction method. J Hirnforsch 10(3):259–269
Hopf A (1969) Photometric studies on the myeloarchitecture of the human parietal lobe. I. Parietal region. J Hirnforsch 11(4):253–265
Hopf A (1970a) Oskar Vogt. 100th anniversary of his birthday. J Hirnforsch 12(1):1–10
Hopf A (1970b) Photometric studies on the myeloarchitecture of the human parietal lobe. II. Postcentral region. J Hirnforsch 12(1):135–141
Hopf A, Vitzthum HG (1957) Uber die Verteilung myeloarchitektonischer Merkmale in der Scheitellappenrinde beim Menschen. J Hirnforsch 3(2–3):79–104
Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM (2004) Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101(36):13335–13340. doi:10.1073/pnas.0403743101
Jones EG (1987) Brodmann’s areas. In: Adelman G (ed) Encyclopedia of neurosciences, vol 1., BirkhäuserBoston, Basel, pp 180–181
Jones EG (2003) Two minds. Nature 421(6918):19–20
Jones EG (2008) Cortical maps and modern phrenology. Brain 131(8):2227–2233
Jones EG, Burton H (1976) Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol 168(2):197–247. doi:10.1002/cne.901680203
Kaas JH (2002) Neocortex. In: Ramachandran VS (ed) Encyclopedia of the human brain, vol 3. Academic Press, Amsterdam, pp 291–303
Kaes T (1907) Die grosshirnrinde des menschen in ihren Massen und in ihrem Fasergehalt. Ein gehirnanatomischer Atlas. G. Fischer, Jena
Klatzo I (2002) Cécile and Oskar Vogt: the visionaries of modern neuroscience. Springer, Wien
Kleist K (1934) Gehirnpathologie. J.A. Barth, Leipzig
Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K (2010) Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex 20(6):1448–1461. doi:10.1093/cercor/bhp208
Lashley KS, Clark G (1946) The cytoarchitecture of the cerebral cortex of Ateles; a critical examination of architectonic studies. J Comp Neurol 85(2):223–305
Le Gros Clark WE (1952) A note on cortical cyto-architectonics. Brain 75(1):96–104
Lungwitz W (1937) Zur myeloarchitektonischen Untergliederung der menschlichen Area praeoccipitalis (Area 19 Brodmann). J Psychol Neurol 47:607–639
Mauss F (1908) Die faserarchitektonische Gliederung der Grosshirnrinde. J Psychol Neurol 13:263–325
Mauss F (1911) Die faserarchitektonische Gliederung des Cortex cerebri der anthropomorphen Affen. J Psychol Neurol 18:410–467
Mesulam M (2012) The evolving landscape of human cortical connectivity: facts and inferences. Neuroimage. doi:10.1016/j.neuroimage.2011.12.033 (online 22 Dec 2011)
Meynert T (1884) Psychiatrie: Klinik der Erkrankungen des Vorderhirns begründet auf dessen Bau, Leistungen und Ernährung. W. Braumüller, Wein
Nieuwenhuys R, Voogd J, van Huijzen C (2008) The human central nervous system. Springer, Heidelberg
Öngür D, Ferry AT, Price JL (2003) Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460(3):425–449. doi:10.1002/cne.10609
Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B (2008) Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 508(6):906–926. doi:10.1002/cne.21684
Passingham RE, Stephan KE, Kotter R (2002) The anatomical basis of functional localization in the cortex. Nat Rev Neurosci 3(8):606–616. doi:10.1038/nrn893
Press WA, Brewer AA, Dougherty RF, Wade AR, Wandell BA (2001) Visual areas and spatial summation in human visual cortex. Vis Res 41(10–11):1321–1332
Roland PE, Zilles K (1998) Structural divisions and functional fields in the human cerebral cortex. Brain Res Brain Res Rev 26(2–3):87–105
Rose JE, Woolsey CN (1948) Structure and relations of limbic cortex and anterior thalamic nuclei in rabbit and cat. J Comp Neurol 89(3):279–347. doi:10.1002/cne.900890307
Rose JE, Woolsey CN (1949) The relations of thalamic connections, cellular structure and evocable electrical activity in the auditory region of the cat. J Comp Neurol 91(3):441–466
Sanides F (1962) Die Architektonik des menschlichen Stirnhirns. In: Müller M, Spatz H, Vogel P (eds) Monographien aus dem Gesamtgebiete der Neurologie und Psychiatrie, vol 98. Springer, Berlin
Sanides F (1964) The cyto-myeloarchitecture of the human frontal lobe and its relation to phylogenetic differentiation of the cerebral cortex. J Hirnforsch 47:269–282
Sarkissov S, Filimonoff I, Kononowa E, Preobraschenskaja I, Kukuew L (1955) Atlas of the cytoarchitectonics of the human cerebral cortex. Medgiz 20, Moscow
Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K (2008a) Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18(9):2141–2157. doi:10.1093/cercor/bhm241
Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008b) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18(4):846–867. doi:10.1093/cercor/bhm116
Seltzer B, Pandya DN (1978) Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res 149(1):1–24
Stephan KE, Kamper L, Bozkurt A, Burns GA, Young MP, Kötter R (2001) Advanced database methodology for the Collation of Connectivity data on the Macaque brain (CoCoMac). Philos Trans R Soc Lond B Biol Sci 356(1412):1159–1186. doi:10.1098/rstb.2001.0908
Strasburger EH (1937a) Die myeloarchitektonische Gliederung des Stirnhirns beim Menschen und Schimpansen—I. J Psychol Neurol 47:460–491
Strasburger EH (1937b) Die myeloarchitektonische Gliederung des Stirnhirns beim Menschen und Schimpansen—II. J Psychol Neurol 47:565–606
Strasburger EH (1938) Vergleichende myeloarchitektonische Studien an der erweiterten Brocaschen Region des Menschen. J Psychol Neurol 48:477–511
Uttal WR (2001) The new phrenology: the limits of localizing cognitive processes in the brain. MIT Press, Cambridge
Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G (2010) 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Res 183(1):1–20. doi:10.1016/j.pscychresns.2010.04.012
Van Essen DC (2006) SumsDB (2006). http://sumsdb.wustl.edu:8081/sums/index.jsp
Vogt O (1903) Zur anatomischen Gliederung des Cortex cerebri. J Psychol Neurol 2:160–180
Vogt O (1906) Über strukturelle Hirnzentra mit besonderer Berücksichtingung der strukturellen Felder des Cortex pallii. Anat Anz 29:74–114
Vogt O (1910a) Die myeloarchitektonische Felderung des Menschlichen Stirnhirns. J Psychol Neurol 15:221–232
Vogt O (1910b) Considerations generales sur la myelo-architecture du lobe frontal. Rev Neurol 19:405–420
Vogt O (1911) Die myeloarchitektonik des isocortex parietalis. J Psychol Neurol 18:379–390
Vogt O (1918) Korbinian Brodmann. J Psychol Neurol 24:I–X
Vogt O (1923) Furchenbildung und Architectonische Rindenfelderung. J Psychol Neurol 29:438–439
Vogt O (1927) Architektonik der menschlichen Hirnrinde. Jahresversammlung d. deutschen Verein fuer Psychiatrie Düsseldorf, 23./24.9.1926. Allg Z Psychiat 86:247–266
Vogt M (1928a) Über omnilaminaire Strukturdifferenzen und lineare Grenzen der architektonischen Felder der hinteren Zentralwindung des Menschen. J Psychol Neurol 35:177–193
Vogt M (1928b) Erwiderung zu dem vorstehenden Aufsatz von Economos. J Psychol Neurol 36:320–322
Vogt O (1943) Der heutigen Stand der cerebralen Organologie und die zukünftige Hirnforschung. Anat Anz 94:49–73
Vogt O (1951) Die anatomische Vertiefung der menschlichen Hirnlokalisation. Klin Wochenschr 29(7–8):111–125
Vogt C, Vogt O (1907) Zur Kenntnis der elektrisch erregbaren Hirnrindengebiete bei den Säugetieren. J Psychol Neurol 8:277–456
Vogt C, Vogt O (1911) Nouvelle contribution à l’étude de la myéloarchitecture de l’écorce cérébrale. XX. Congres des médecins aliénistes et neurologistes de France, Brüssel
Vogt C, Vogt O (1919) Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol 25:279–468
Vogt O, Vogt C (1922) Erkrankungen der Grosshirnrinde im Lichte der Topistik, Pathoklise und Pathoarchitektonik. J Psychol Neurol 28:8–171
Vogt C, Vogt O (1928) Die Grundlagen und die Teildisziplinen der mikroskopischen Anatomie des Zentralnervensystems. In: Handbuch des mikroskopischen Anatomie des Menschen, vol 4 Teil 1. Springer, Berlin, pp 448–477
Vogt C, Vogt O (1929) Űber die Neuheit und den Wert des Pathoklisen begriffes. J Psychol Neurol 38:147–154
Vogt C, Vogt O (1936) Sitz und Wesen der Krankheiten im Lichte der topistischen Hirnforschung und des Variierens der Tiere. J Psychol Neurol 47:237–457
Vogt C, Vogt O (1942) Morphologische Gestaltungen unter normalen und pathogenen Bedingüngen. J Psychol Neurol 50:161–524
Vogt C, Vogt O (1954) Gestaltung der topistischen Hirnforschung und ihre Forderung durch den Hirnbau und seine Anomalien. J Hirnforsch 1:1–46
Vogt C, Vogt O (1956) Weitere Ausführungen zum Arbeitsprogramm des Hirnforschungsinstitutes in Neustadt/Schwarzwald. J Hirnforsch 2:403–427
Von Economo C (1928) Bemerkungen zu dem Aufsatz von Marthe Vogt. J Psychol Neurol 36:320–322
Von Economo C (2009) Cellular structure of the human cerebral cortex. Triarhou, L.C. (translator) edn. Karger, Basel
Von Economo C, Koskinas GN (1925) Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Springer, Wien
Wandell BA, Dumoulin SO, Brewer AA (2007) Visual field maps in human cortex. Neuron 56(2):366–383. doi:10.1016/j.neuron.2007.10.012
Zilles K, Amunts K (2009) Receptor mapping: architecture of the human cerebral cortex. Curr Opin Neurol 22(4):331–339. doi:10.1097/WCO.0b013e32832d95db
Zilles K, Amunts K (2010) Centenary of Brodmann’s map conception and fate. Nat Rev Neurosci 11:139–145. doi:10.1038/nrn2776
Zilles K, Palomero-Gallagher N (2001) Cyto-, myelo- and receptor architectonics of the human parietal cortex. NeuroImage 14:8–20
Acknowledgments
The author thanks Drs. Bob Turner and Karl Zilles for critically reading an earlier version of this paper, Mr. Ton Put for help with the illustrations, and Suzanne Bakker M.Sc. for moral support and reference management. Finally, the author wants to acknowledge especially the invaluable and continuous assistance of Dr. Jenneke Kruisbrink, the librarian of our Institute. Without her help, this article would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nieuwenhuys, R. The myeloarchitectonic studies on the human cerebral cortex of the Vogt–Vogt school, and their significance for the interpretation of functional neuroimaging data. Brain Struct Funct 218, 303–352 (2013). https://doi.org/10.1007/s00429-012-0460-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0460-z