Abstract
The developing mammalian heart initiates spontaneous contractions shortly after the myocardium differentiates from the splanchnic mesoderm. The precise timing and mode of the onset of heartbeat, however, have not been statistically described in mice. We analyzed the patterns of contractive activity in video-recorded heart regions ranging from the pre-somite stage to day 10.5 (E10.5). The first sign was detected at the 3-somite stage (E8.25), when a few cardiac myocytes constituted small contracting groups on both sides of the heart tube. Fluctuations of the basal [Ca2+]i level were detected in dormant 3-somite-stage hearts, indicating the initiation of electrical activity before visible contractions. After weak and irregular contractions at the 3-somite stage, the contractions were almost coordinated as early as the 4-somite stage. Unidirectional blood flow through the atrioventricular canal was established around the 20-somite stage at E9.25, correlated with the development of the endocardial cushion. We propose that not only the endocardial cushion but also coordinated contractions are essential for unidirectional flow, because induced bradycardia due to short exposure at room temperature caused regurgitation at E10.5 when otherwise highly organized flow was observed. These findings complement and extend earlier observations on functional heart development, providing a reference for fundamental research on mammalian cardiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heart is the first organ that starts functioning in the early gastrulating embryo. Though the mouse has attracted much attention as a model of mammalian embryogenesis, there are many obstacles to the assessment of the extensive transformations undergone by the developing heart primordium (Rothenberg et al. 2004). As a result, even recent knowledge has not yet described in detail the mode and timing of the initial contractions of the mouse embryo heart.
We have created germline connexin45-deficient mice, cardiac-muscle-specific connexin45-deficient mice, and cardiac troponin T-deficient mice (Kumai et al. 2000; Nishii et al. 2003; our unpublished results). These gene products have specific functions in the early heart contractions, and video-recorded data showed that the mouse embryo cannot survive beyond E10, in other words can survive till E10, without a normal heartbeat. Through extensive study of the early heart contractions of these mutants, we also accumulated data on the normal heartbeat of wild-type embryos; these wild-type embryos revealed a tightly coordinated developmental program of the heart. Here, we present reproducible data indicating the stage when the mouse heart starts beating. We also describe our video microscopy analysis of the development of embryonic blood flow. In addition to the endocardial cushion, we show that a regulated contraction pattern is a key factor of unidirectional blood flow in the early-stage embryo.
Materials and methods
Mice
This experiment was reviewed by the Ethics Committee for Animal Experiments and carried out under the Guidelines for Animal Experiments of the Faculty of Medicine, Kyushu University and Law No. 105 and Notification No. 6 of the Japanese Government. The mice used in this study had a pure C57BL/6N or C57BL/6N-129/Sv/Ev hybrid background. The contribution of the 129/Sv/Ev strain was less than or equal to 25%. They were all wild-type mice except where otherwise specified.
Preparation of embryos
Pregnant mice were anesthetized with pentobarbital (Nembutal). The uterine horns were exteriorized, and only one decidual swelling was removed at a time, so that the circulation in the embryos could be maintained until the experiment. The embryos were dissected in M2 medium (Hogan et al. 1994) on a thermal plate at 37°C. Their extraembryonic membranes were left undamaged if they had not already ‘turned’, before the 10-somite stage or thereabouts. In more developed embryos, however, the extraembryonic membranes were removed to render their heart contractions clearly observable. This manipulation caused inevitable bleeding from the umbilical vessels, and often resulted in the cessation or weakening of the heart contractions. We kept the embryos in the medium for a few minutes, during which resumption of the contractions was confirmed in most cases. The data from embryos showing any sign of impairment were discarded in the present study. Images of the heart regions were recorded by a digital versatile disk recorder (TOSHIBA RD-XS30) via the NTSC output of a NIKON COOLPIX 4500 digital camera connected to an OLYMPUS SZX12 dissecting microscope. In this study, the hearts were recorded from the front side (up to about the 6-somite stage) or from the left side (in later embryos), because the tails of the embryos frequently concealed the right side of the hearts.
[Ca2+]i measurement
Immediately after the video recording, the embryonic hearts were isolated with a pair of fine forceps under the dissecting microscope. The hearts were kept on ice in 100 μl of M2 medium for less than 2 h. An equal volume of 1 μmol/l fura-2 AM was added to the M2 medium, which resulted in a final fura-2 AM concentration of 0.5 μmol/l. After 5–10 min of incubation on ice, the hearts were transferred into fresh M2 medium at 37°C. After 15–30 min of incubation, the hearts were transferred into a drop of M2 medium on thermostated slides at 37°C. Fura-2 fluorescence was detected as the ratio of fluorescence emitted at 510 nm with alternating excitations at 340 and 380 nm, as previously described (Egashira et al. 2004).
Results
Embryos ranging from the pre-somite E8.0 to the E10.5 stage were analyzed. Each stage of embryonic development was represented by the corresponding number of the embryo’s somite pairs, except at E10.5. Though some overlap of the state of heart development was observed, we adopted this staging system because it is still the most acceptable, and no other more appropriate criterion exists at present (Kaufman and Navaratnam 1981; Navaratnam et al. 1986). We noticed neither preferred implantation sites within the uterine horns nor differences in degrees of development among the embryos within the respective litters (data not shown). The embryos presented here were all wild type, except when otherwise specified. We obtained roughly equal numbers of data on heterozygous connexin43, connexin45, and cardiac troponin T mutants. Though not indicated, additional data on the heterozygous hearts showed no deviation from the presented figures. As an exception, the data of 3-somite heterozygous mutant embryos are included below, because we believe that it is worth increasing the number of embryos at this critical stage.
Onset of heartbeat: pre-somite- (E8.0) to 3-somite- (E8.25) stage embryos
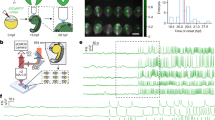
We detected no visible contractions of the cardiac myocytes at the 2-somite-stage or earlier (Fig. 1). We noticed variable heart development at the 3-somite stage (Figs. S1, S2), and about one-third of the embryos showed initial spontaneous contractions. The embryos with beating hearts had more apparent outward bulges of the myocardium than the embryos with no heartbeat. The contractions were limited and weak. Of 11 embryos at the 3-somite stage, four showed contractions (Fig. 1a). In addition, of eight heterozygous mutants at the 3-somite stage, four showed contractions. Small clusters of contracting cardiac myocytes constituted two groups on both sides of the heart tube in all embryos with beating hearts. Therefore, the period when only one side of the heart is contracting was presumably very short. The region that showed the earliest contraction in each contraction cycle was defined as the pacemaker region, which was detected on the left side (one wild type, two heterozygous), on the right side (one wild type, no heterozygous; Fig. S2), or on both sides (two wild types, two heterozygous; Fig. S1) of the bilateral heart tube, indicating that the pacemaker region is unsettled during early mouse heart development. When both sides acquired first pacemaking activity, they were contracting at their own paces, and so it is reasonable to assume that intercellular communication between the two groups was missing (Fig. S1). We were able to detect fluctuations in the [Ca2+]i level in the dormant 3-somite-stage heart (Fig. 2). This result indicates that electrical activity appears before visible contractions in the mouse heart, similar to the rat heart (Hirota et al. 1985).
Mouse hearts start beating at the 3-somite stage. a Embryos were examined in order to ascertain if their cardiac myocytes were contracting. The percentages of embryos showing spontaneous heart contractions are presented in a bar chart. Above the bars, the numbers of analyzed embryos are indicated (numbers of litters in parentheses). A total of 47 l were used in this study. b Pulse rates (mean±SD) of the embryos whose heart contractions were confirmed
Representative traces of fura-2 fluorescence in the hearts at the 3-, 4-, and 6-somite stage, indicating the [Ca2+]i level of each embryo. In the 3-somite-stage embryo shown here, we could not detect any visible contractions. However, irregular fluctuations of the fluorescence baseline were detected (arrowheads). This indicates that the initiation of electrical activity precedes the first contractions of the heart rudiment. The fluctuations soon become regular in the later stages, with frequencies and amplitudes increased
Establishment of unidirectional blood flow: 4-somite- (E8.25) to 24-somite- (E9.5) stage embryos
Unlike the contractions at the preceding stage, those at the 4-somite stage were fairly coordinated, generating peristaltic waves (Figs. 2, S3). Though we could observe a few blood cells floating within the lumen of some of the hearts at the 4-somite stage, blood cells were readily detected at the 8-somite stage (E8.5) and onward (Fig. S4), in agreement with a previous report (McGrath et al. 2003). Initial blood flow was immature. The blood cells were moving to and fro in response to the peristaltic heart contractions. However, they gradually flowed forward as a whole (Figs. 3a, S4). Physiological regurgitation through the atrioventricular canal was consistently observed before the 15-somite stage (E9.0; Figs. 3b, S5). After the intermediate period, unidirectional blood flow was established at around the 20-somite stage (E9.25; Fig. S6). The establishment of unidirectional blood flow was correlated with the development of the endocardial cushion (Nishii et al. 2001). At around the same 20-somite stage, the whole primitive ventricle began to contract almost simultaneously, replacing the former peristaltic contractions, probably in correlation with the emerging conduction system (Fig. S6) (Virágh and Challice 1977). This result is also consistent with the optical mapping data describing the emergence at E9.5 (Rentschler et al. 2002) and subsequent establishment (Rentschler et al. 2001) of a preferential conduction pathway in the primitive ventricle.
The establishment of unidirectional blood flow requires the development of the endocardial cushion, with the regulated contraction cycle of the myocardium as an additional factor. a The blood flow in the yolk sac vasculature of a 9-somite embryo was traced for 30 s. The positions of three randomly chosen blood cells are marked. The positions correspond to the positions at 0, 10, 20, and 30 s, respectively. The arrows are added to emphasize the direction of the flow. See the text and Fig. S4 for more details. b Blood flow in the heart of a 13-somite-stage embryo. Cells in the primitive atrium and exiting the atrioventricular canal are marked 1, at the diastolic stage; 2, at the atrial contraction stage; and 3, at the ventricular contraction stage. To-and-fro movement is evident. Some blood cells proceed to the next segments of the vascular system (3′). Figure S5 shows more details. c Blood flow during diastole of the E10.5 heart. Fairly coordinated contractions were visible in the control heart (see also Fig. S7). However, the same heart shows regurgitation through the atrioventricular canal (indicated by the arrow) in the diastolic phase of the contraction cycle during resumption after it was dissected at room temperature. See the text and Fig. S8 for more details. a, atrium; avc, atrioventricular canal; o, outflow; v, ventricle
Regurgitation through the atrioventricular canal due to induced bradycardia at E10.5
Technically, bleeding was inevitable with our surgical procedure, and it caused in many cases the cessation or weakening of the heart contractions. Therefore, the data at E10.5 were excluded from our statistics. However, we observed highly coordinated contractions and blood flow in many E10.5 embryos (Figs. 3c, S7). We found that bradycardia was induced when the embryos were dissected at room temperature. After the transfer to normal 37°C conditions, we were able to record a rapid resumption of contractions (Figs. 3c, S8). It took about a minute, during which we could observe that the atypical contractions were associated with regurgitation through the atrioventricular canal. Similar recordings were obtained in eight cases. This result indicates that the establishment of coordinated contractions is an essential factor required for unidirectional blood flow in the early stage embryo.
Discussion
Some investigators have noted the stage when the heart starts beating in mice. In the valuable report of the morphological analysis performed by Navaratnam et al. (1986), they have described that the twitching of heart rudiments can be observed at the 3–4-somite stage. Kumai et al. (2000) and Linask et al. (2001) have placed the first twitching at the 3-somite stage. Others have observed the first contractions at the 3–5-somite stage, as part of their original work. All of the previous studies, however, only state that contractions occurred at certain stages, and lack a statistical basis. This report presents a more detailed description of the first contractions of the mouse heart, thereby complementing and extending the earlier observations.
The mechanisms that underlie action potential generation around the first contraction stage are markedly different from those of adult hearts. Spontaneous action potentials of the early embryonic heart are dependent on the external Ca2+ concentration, but are insensitive to the Na+ channel blocker tetrodotoxin (reviewed in Kamino 1991). Ca2+ from the extracellular space plays a central role in excitation–contraction coupling of the early heart, in contrast to the adult heart where Ca2+ is also released from the sarcoplasmic reticulum. In support of this, neither the transverse tubule system nor the sarcoplasmic reticulum is differentiated in the early heart (Navaratnam et al. 1986). Patterns of expression of the myocardial genes (Franco et al. 1998; Linask et al. 2001) well explain why electrical activity precedes the initial contractions. The emergence of visible contractions, however, seems to coincide with the morphological myofibrillar development, which takes place thereafter (Navaratnam et al. 1986). At the 1–2 somite stage, the cardiac myocytes of the myocardial plate have only scattered microfilaments in the cytoplasm. However at the 3–4 somite stage, transverse striation begins to form, with immature Z lines regularly interspersed within fascicles of filaments. At the cellular level, the first contractions of the chick heart are very similar to those of the mouse heart (Kamino 1991). In mice, the initial contractions are seen before a complete heart tube has formed, while in chick embryos the tubular heart forms first and then begins to beat. The difference is notable.
Anesthesia is necessary to enable the acquisition of reproducible data. The anesthetic agent pentobarbital used in this study is a rather safe anesthetic (Huang and Linask 1998), though its effects on cardiac function in the early mouse embryo have not been fully understood. We have noticed that embryonic heart rates did not change significantly between the start and the end of experiments. This suggests that anesthesia level, which changes significantly over the examination period of 1 h, has little effect on heart rates of early embryos.
In a recent report, Ji et al. (2003) have placed the first contractions at the 5-somite stage, as a result of observing mouse embryos in utero using ultrasound biomicroscopy Doppler imaging. The method no doubt has an advantage in terms of the integrity of the embryos, but it probably lacks the spatial resolution sufficient for the detection of the very first contractions, as they discussed in their report. The present invasive method provides images in which even a single cell can be discriminated. In our experimental procedure, we observed hardly any impairment of the contractions in embryos before the 10-somite stage. Therefore, we conclude that the first contractions start at the well-developed 3-somite stage in mice, similarly to rats (Goss 1938; Hirota et al. 1985). Ji et al. (2003) have also provided data on the heart rates of early-stage embryos; the rates given by them seem to be two-fold higher than our data. This may be due to a difference in the medium conditions, damage to the embryos or other factors. It is notable, however, that just after the decidual walls were opened, we did not notice any change in the rhythm of the heartbeat caused by the experimental procedure in embryos before the 10-somite stage.
The endocardial cushion forms functional valves that enable proper circulation of the blood. Defective formation of this tissue results in the regurgitation of blood flow in embryos (Kumai et al. 2000; Phoon et al. 2004). Our data suggested that unidirectional blood flow was established around the 20-somite stage, as described previously (Nishii et al. 2001). However, we confirmed this hypothesis with extensive video recording. In addition, we propose here for the first time that coordinated contractions and the endocardial cushion are both responsible for the establishment of unidirectional blood flow. This novel finding casts further light onto the highly organized developmental program of the heart.
Finally, this report provides supplementary video movies that are essential to understand the mode of the first contractions. Together with these movies, we believe this is the first report that describes in detail the contraction patterns of the early mouse embryo heart.
References
Egashira K, Nishii K, Nakamura K, Kumai M, Morimoto S, Shibata Y (2004) Conduction abnormality in gap junction protein connexin45-deficient embryonic stem cell-derived cardiac myocytes. Anat Rec 280A:973–979
Franco D, Lamers WH, Moorman AFM (1998) Patterns of expression in the developing myocardium: towards a morphologically integrated transcriptional model. Cardiovasc Res 38:25–53
Goss CM (1938) The first contractions of the heart in rat embryos. Anat Rec 70:505–524
Hirota A, Kamino K, Komuro H, Sakai T, Yada T (1985) Early events in development of electrical activity and contraction in embryonic rat heart assessed by optical recording. J Physiol 369:209–227
Hogan B, Beddington R, Costantini F, Lacy E (1994) Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor, Cold Spring Harbor, New York
Huang GY, Linask KK (1998) Doppler echocardiographic analysis of effects of tribromoethanol anesthesia on cardiac function in the mouse embryo: a comparison with pentobarbital. Lab Anim Sci 48:206–209
Ji RP, Phoon CKL, Aristizábal O, McGrath KE, Palis J, Turnbull DH (2003) Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res 92:133–135
Kamino K (1991) Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol Rev 71:53–91
Kaufman MH, Navaratnam V (1981) Early differentiation of the heart in mouse embryos. J Anat 133:235–246
Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y (2000) Loss of connexin45 causes a cushion defect in early cardiogenesis. Development 127:3501–3512
Linask KK, Han MD, Artman M, Ludwig CA (2001) Sodium–calcium exchanger (NCX-1) and calcium modulation: NCX protein expression patterns and regulation of early heart development. Dev Dyn 221:249–264
McGrath KE, Koniski AD, Malik J, Palis J (2003) Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101:1669–1676
Navaratnam V, Kaufman MH, Skepper JN, Barton S, Guttridge KM (1986) Differentiation of the myocardial rudiment of mouse embryos: an ultrastructural study including freeze-fracture replication. J Anat 146:65–85
Nishii K, Kumai M, Shibata Y (2001) Regulation of the epithelial–mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc Med 11:213–218
Nishii K, Kumai M, Egashira K, Miwa T, Hashizume K, Miyano Y, Shibata Y (2003) Mice lacking connexin45 conditionally in cardiac myocytes display embryonic lethality similar to that of germline knockout mice without endocardial cushion defect. Cell Commun Adhesion 10:365–369
Phoon CKL, Ji RP, Aristizábal O, Worrad DM, Zhou B, Baldwin HS, Turnbull DH (2004) Embryonic heart failure in NFATc1 -/- mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circ Res 95:92–99
Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, Jalife J, Fishman GI (2001) Visualization and functional characterization of the developing murine cardiac conduction system. Development 128:1785–1792
Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI (2002) Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci USA 99:10464–10469
Rothenberg F, Efimov IR, Watanabe M (2004) Functional imaging of the embryonic pacemaking and cardiac conduction system over the past 150 years: technologies to overcome the challenges. Anat Rec 280A:980–989
Virágh S, Challice CE (1977) The development of the conduction system in the mouse embryo heart: I. The first embryonic A–V conduction pathway. Dev Biol 56:382–396
Acknowledgements
We thank Kanako Hashizume and Yumi Miyano for their technical assistance, Mamiko Kamizono and Takeyo Omura for their care of the experimental animals, and Dr. Sachio Morimoto (of the Department of Clinical Pharmacology) for allowing us to use the [Ca2+]i-recording equipment. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16790126 to K.N. and No. 17390052 to Y.S.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Nishii, K., Shibata, Y. Mode and determination of the initial contraction stage in the mouse embryo heart. Anat Embryol 211, 95–100 (2006). https://doi.org/10.1007/s00429-005-0065-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-005-0065-x