Abstract
Primary cutaneous follicle center lymphoma (PCFCL) is an indolent variant of follicular lymphoma (FL) with limited information available on the genetic background of the disease. The genetic hallmark of nodal FL, the t(14;18) translocation, affecting the BCL2 gene, is rare in PCFCL. Loss of 1p36, the most common secondary chromosomal abnormality in nodal FL, has been recently reported in 16.7% of PCFCL cases. In order to further characterize PCFCL, 21 cases were analyzed using interphase fluorescence in situ hybridization with BCL2 break apart and 1p36/1q25 dual color probes. Sanger sequencing was used to investigate TNFRSF14 and EZH2 mutations and immunohistochemistry to assess BCL2, EZH2 protein expressions.
1p36 deletion occurred in 22% (5/21), BCL2 gene break in 10% (2/20) of the PCFCL cases. Mutations of the candidate tumor suppressor gene of the 1p36 region, TNFRSF14 mutations were detected in 4/17 (23.5%) cases with 2 cases presenting with concurrent 1p36 deletion. EZH2 hotspot mutations at Y641, A682, and A692 were not found. High EZH2 protein expression associated with a BCL2 negative phenotype was observed in 43% (9/21) of the cases. BCL2 gene break or 1p36 deletion did not impact the prognosis; however, they showed association with advanced stages at diagnosis (p = 0.016) and a tendency with shorter event free survival (p = 0.052).
In conclusion, 1p36 deletion co-occurs with acquired TNFRSF14 mutations, suggesting a role of this tumor suppressor gene in the development of a subgroup of PCFCL. High EZH2 protein expression associated with BCL2 negative phenotype is common and might represent an ideal therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary cutaneous follicle center lymphoma (PCFCL) is an indolent variant of follicular lymphoma with > 95% 5-year survival [1]. At diagnosis, solitary or multiple lesions, papules, plaques, or nodules are found, most often in a single region, particularly in the head and neck region or involving the trunk. Systemic dissemination occurs rarely; however, the disease often relapses. Local treatment, surgical excision, and low dose radiotherapy is highly effective with a complete remission rate approaching 100% [2]. Histologically, nodular, diffuse, or mixed growth pattern composed of centrocytes mixed with scattered centroblasts is seen [3]. Early studies emphasized the lack of BCL2 protein expression and BCL2/IgH translocation in PCFCL as an important diagnostic tool to distinguish it from nodal follicular lymphoma (NFL), as it occurs in almost 90% of the systemic, nodal cases [4, 5]. Further studies observed higher frequencies of BCL2 protein expression (10–57%) and associated BCL2 translocation (10–41%) in several series of PCFCL without prognostic significance [6,7,8,9]. Recently, deletion involving the 1p36 region (del 1p36) was identified as the most frequent cytogenetic abnormality affecting 16.7% of the PCFCL cases [10]. The candidate tumor suppressor gene of this region, TNFRSF14, belonging to the tumor necrosis factor receptor (TNFR) superfamily, is frequently mutated in NFL; however, TNFRSF14 mutation analysis has not been performed in PCFCL to date [11, 12]. Similarly, the mutation status and expression of the epigenetic regulator, EZH2, an attractive therapeutic target being involved in the pathogenesis of NFL, is unknown in PCFCL [13].
The aim of our study was to perform a cytogenetic, molecular, and immunohistochemical analysis, including mutation analysis of the previously unreported mutation targets TNFRSF14 and EZH2 in a cohort of 21 PCFCL cases and assess the clinical relevance of these findings.
Materials and methods
Patient selection
We collected and reevaluated 21 cases of PCFCL diagnosed at the Semmelweis University, Budapest between 2009 and 2017. Clinical data were collected from the medical records in the university database retrospectively. Clinical examinations included thorough physical, routine laboratory examinations (complete blood cell count, serum chemistry studies), chest X-ray, and CT scan. Peripheral blood and bone marrow involvement was investigated by flow cytometry and histology. Staging was based on the International Society for Cutaneous Lymphomas EORTC staging proposal for nonmycosis fungoides/Sezary syndrome cutaneous lymphomas. The male/female ratio was surprisingly low in our cohort, it could be due to the small number of cases, no other reason is identified.
Histology and immunohistochemistry
Histological analysis of the skin biopsies was performed on formalin-fixed, paraffin-embedded (FFPE) tissues. Twenty-nine samples of 21 patients were examined; in case of 7 patients, more than one biopsy was taken at different time points. The histological reevaluation was performed by two hematopathologist (CsJ, SZA) according to the revised WHO classification and EORTC classification [3, 14]. The main histological differential diagnosis of PCFCL are reactive follicular hyperplasia, primary cutaneous marginal zone lymphoma (PCMZL), and diffuse large B-cell lymphoma (DLBCL) with GCB phenotype or leg type. The main pathological findings differentiating PCFCL from reactive lymphoid proliferations were dense, deep dermal, or subcutaneous infiltration of CD20 positive B-cells, clonal expression of IgG light chains, or clonal IgH rearrangement; CD10 and BCL6 expressions for the vast majority of the tumor cells together with centrocytic cytology excluded PCMZL; nodularity with expanded presence of FDC network and MUM1 negativity were the main criteria to exclude DLBCL.
The LEICA Bond Max fully automatized staining system was used for immunohistochemistry. For antigen localization, DAB polymer (DAKO Denmark) was used. The antibodies employed recognized the following antigens: CD3 (clone QBEnd10), CD20 (clone L-26), Ki67 (clone MIB-1), BCL2 (clone 124), BCL6 (clone PG.B6p), MUM1 (clone M7259), (DAKO, Glostrup, Denmark), CD21 (clone 2G9, Leica, Saint-Petersburg, Russia), and EZH2 (clone 11/EZH2, BD Transduction Lab, San Jose, USA), tonsil samples were utilized as controls.
Stained slides were scanned at × 20 magnification using a Panoramic scan instrument (3D Histech, Budapest, Hungary) equipped with a Carl Zeiss objective (NA = 0.83; Carl Zeiss MicroImaging Inc., Jena, Germany). Ki67 expression was quantified with CaseViewer 2.0 (3D Histech, Budapest, Hungary), extended with QuantCenter 2.0 software. EZH2 protein expression was determined in the neoplastic follicles using a semiquantitative method, cases were considered positive if there was moderate to high intensity (2+ or 3+) staining in > 20% of the neoplastic cells. EZH2 positivity was considered high if there were > 50% positive cells in the neoplastic follicles. The cut off value for BCL2 positivity was 80%, for CD10, BCL6, and MUM1 30%.
IgH gene rearrangement analysis was carried out for clonality assessment using the BIOMED −2 Concerted Action protocol [15].
Interphase fluorescence in situ hibridization (FISH)
Interphase FISH was performed on 3-μm-thick tissue sections using split signal FISH DNA probe for BCL2/18q21 (Vysis LSI BCL2 Dual Color Break Apart rearrangement probe, ABBOTT Molecular diagnostic, Rungis, France) and dual-color probe for 1p36/1q25 (Vysis LSI 1p36 SpectrumOrange/Vysis LSI 1q25 SpectrumGreen Dual-Color Probe, ABBOTT Molecular Diagnostic, Rungis, France) according to the manufacturer’s instructions and as described before [16]. The FISH signals were visualized using a Nikon Eclipse E600 epifluorescence microscope. Image analysis was performed by using Lucia Cytogenetics image acquisition system (Laboratory Imaging, Republic of Czech). The cut-off for BCl2 split positivity and for del 1p36 assessment, lack of one orange (1p36) signal, was set at 20% counting 200 tumor cell nuclei.
Mutation analysis
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) skin biopsy specimens with tumor tissue presence exceeding 80% using the QIAamp DNA FFPE Tissue Kit (Qiagen) according to the manufacturer’s instructions. Bidirectional Sanger sequencing of Y641 (exon 16), A682, and A692 (exon 18) hotspots of the EZH2 gene and the whole coding sequence of the TNFRSF14 gene were carried out using the primers listed in Supplementary Table 1.
Response criteria and statistical analysis
The association between the different clinicopathological and immune parameters was estimated and compared using the Mann-Whitney U test for continuous variables. Categorical data were compared using Fisher’s exact test or Mann-Whitney statistics. A p value of < 0.05 was considered as statistically significant. The Kaplan Meier method was used for the comparison of event free survival (EFS). Time was defined as the time elapsed from the date of diagnosis to the date of first disease recurrence or death. Time was censored for patients who had not experienced disease relapse or had not died at the time of last follow-up. Potential prognostic factors examined in our study were extent of cutaneous involvement according to the TNM classification (T1 vs T2/T3), site of cutaneous lesions, proliferation rate determined by Ki67 expression, BCL2 expression, EZH2 expression, presence of cytogenetic abnormalities (deletion of 1p36 or BCL2 break), and presence of TNFRSF14 mutation [17]. All statistical analyses were performed using the SPSS 13.0 software).
Results
Clinical findings and follow-up
The clinical findings are summarized in Table 1. In our series of 21 patients with PCFCL, the male-to-female ratio was 4:17, with patients being exclusively Caucasian. The median age at diagnosis was 63 years (range 30–82). The clinical presentation was described as erythematous papules, plaques, or nodules. Solitary lesion (stages: T1a, b) was found in 57% of the patients (12/21), multiple lesions were present in 43% (9/21) of the patients (stages: T2a-T3b) especially on the head and neck region (11/21), and on the trunk and upper extremities (8/21) or both (2/21). The lower extremities were not involved (Fig. 1a). The patients did not have prior diagnosis of lymphoproliferative disease and any associated systemic symptoms (fever, weight loss, or night sweating). No extracutaneous disease, lymph node, or peripheral blood involvement were found at initial and within 6 months of diagnosis. Treatments included surgical excision, radiotherapy or both, and chemotherapy for two patients. Fifteen patients responded initially with complete remission (CR) (Fig. 1b), 3 with partial remission (PR). Three patients had multiple lesions at diagnosis and surgical excision was partial. They were followed without any treatment, spontaneous remission occurred in one of them, the other two have stable disease (SD). Relapse occurred in 10 cases, extracutaneous spreading to lymph node was found in one case, who responded with CR after chemotherapy (RCHOP). At the time of the last follow-up, one patient died of pulmonary embolization after 193 months, while in CR, unrelated to the cutaneous lymphoma. All other patients are alive with a median follow-up time of 45 months (7–93), (Table 1).
Histological and immunohistochemical findings
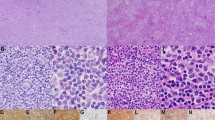
Pure follicular infiltration pattern was observed in most of the cases (17/21), (Fig. 2a, b) Four cases presented with mixed, follicular/diffuse infiltration pattern. The predominance of small/medium sized centrocytes were seen with few mixed scattered centroblasts in 11/21 cases (grades 1 and 2) while large centrocytes and centroblasts dominated > 50% of the cells (grade 3) in 10/21 cases (Fig. 2 c, d). Results of the immunohistochemistry are summarized in Table 2. High proliferation rates above 40% were found in 10/21 cases assessed with Ki67 immunohistochemistry (Fig. 2e, f). BCL2 protein expression was observed in 10/21 cases (Fig. 2 g, h). The BCL2 positive cases tended to have lower proliferation rate than the BCL2 negative ones, but the difference was not significant (p = 0.198) (Table 3). BCL6 protein was expressed by the follicular B-cells in all but one case, in which BCL6 negative tumor cells were positive for CD10. EZH2 protein expression was detected in the neoplastic nodules in all BCL6 positive cases (n = 20) with moderate to high intensity (Fig. 2i, j). High EZH2 protein expression (˃ 50%) was found in 11/21 cases in the neoplastic nodules and was associated with BCL2 negative phenotype (p = 0.009), (Table 3). BCL2 and EZH2 expressions were independent of clinical features and outcome.
Histopathologic features of PCFCLs with high-grade and low-grade morphology exhibiting different levels of Ki67, BCL2 and EZH2 protein expression. a, Hematoxilin-eosin (H&E) staining of the lesion (case 15) showing nodular growth pattern and high-grade morphology. b H&E staining of the lesion (case 2) showing nodular growth pattern and low-grade. c Predominance of large centroblasts and presence of scattered tingible body macrophages with apoptotic bodies in the neoplastic nodule (case 15). d Predominance of smaller centrocytes (case 2). e Immunohistochemistry (IHC) showing high Ki67 expression (> 40%) of the tumor cells (case 15). f IHC showing low Ki67 expression (< 40%) of the tumor cells (case 2). g IHC showing BCL2 negative tumor cells in the center of the nodule (case 15). h BCL2 protein expression of the tumor cells (case 2). i High EZH2 protein expression of the B-cells in the neoplastic nodule (case 15, EZH2 > 50%). j Low EZH2 protein expression of the B-cells in the neoplastic nodule (case 2, EZH2 < 50%)
Association of the FISH findings with clinical and morphological data
Using FISH, BCL2 gene break was found in 2/20 cases (10%, Fig. 3a), while 1p36 deletion was detected in 5/21 cases (22%, Fig. 3b). Cytogenetic abnormalities were strongly associated with stage at diagnosis: more advanced clinical stages with multiple lesions (> T1) were determined at diagnosis in 6/7 (86%) cases with cytogenetic abnormalities compared to cases without BCL2 gene break or 1p36 deletion (3/14, 21%), (p = 0,016, Table 4). Other clinical features, including response to therapy and relapse rate did not show any difference between the two groups. Only event-free survival (EFS) showed a tendency to be shorter in the group with cytogenetic abnormalities; however, it did not reach statistical significance (p = 0.052). Ki67 expression tended to be higher in cases with cytogenetic aberrations compared to the negative ones (5/7, 71% vs 5/14, 36%, p = 0.183), with similar BCL2 protein expression in the two groups (Table 3).
FISH images of representative cases demonstrating BCL2 break and 1p36 deletion. a PCFCL showing BCL2 rearrangement using BCL2 Dual Color Break Apart rearrangement probe, with isolated green and red signals. b PCFCL demonstrating 1p36 deletion using dual-color probe for 1p36/1q25, seen with unique red signals (1p36), whereas two green signals (1q25 control) are observed in tumor cell nuclei
Mutation analysis and association with clinical data, morphology, and FISH results
We assessed the mutation status of TNFRSF14 by Sanger sequencing in 17 patients with available DNA samples. TNFRSF14 mutations were detected in 4/17 (23.5%) patients with three non-sense mutations (c.35G > A; p.Trp12*) previously reported in NFL and one previously unreported missense variant (c.157 T > G; p.Cys53Gly) targeting the extracellular domain which is likely to interfere with the ligand binding activity of TNFRSF14 [18]. Variants previously reported in the dbSNP and 1000 Genomes databases were not considered. Two cases with TNFRSF14 mutations harbored concomitant 1p36 deletions resulting in complete loss of functional TNFRSF14. Significant impact of mutations on clinical features and disease outcome was not observed. Regarding the phenotypic features, 3/4 of the mutant cases were characterized by BCL2 negativity and 3/4 demonstrated high EZH2 protein expression. (Table 2). The presence of gain of function mutations affecting the methyltransferase EZH2 gene were also assessed at three recurrent mutation hotspots (Y646, A682, and A692), and no mutations were detected in our PCFCL cohort.
Discussion
The aim of the present study was to better understand the genetic background of PCFCL by performing FISH analysis of the BCL2 break and deletion of 1p36, and mutation analysis of the TNFRSF14 and EZH2 genes. We report for the first time mutations of the TNFRSF14 gene occurring simultaneously with 1p36 deletion resulting in loss of functional copy of the gene. High EZH2 protein expression was found in about half of the PCFCL cases, while EZH2 hotspot mutations was not detected in our cohort. We identified 1p36 deletion in 5/21 (22%) cases. Szablewski et al. were the first to report one case of PCFCL with 1p36 deletion in a cohort of 25 cases with 19 (76%) being BCL2 positive and 12 (48%) harboring BCL2 gene break [19]. A more recent manuscript from the same group described 4 cases out of 29 (16.7%) positive for 1p36 deletion, but this cohort included only 14 (48%) BCL2 positive cases and 3 cases (11%) with BCL2 gene break [10]. Our results are consistent with their findings, as our cohort included 10 (47%) cases positive for BCL2, and we identified BCL2 gene break in 2 out of 20 cases (10%). In both cohorts, 1p36 deletion was more common than BCL2 break: 22% compared to 10 and 16.7% compared to 11%, respectively, and the ratio of BCL2 positive tumors was also comparable (47 vs. 48%). These genetic abnormalities were mutually exclusive, while in NFL, 1p36 deletion was the most common secondary cytogenetic lesion besides the t(14;18) translocation. Based on this observation, FISH examination of the BCL2 translocation and 1p36 deletion may be applied as a differential diagnostic tool to distinguish primary cutaneous and nodal FL involving the skin. Frequent 1p36 deletions and copy number neutral loss of heterozygosity were also identified in special types of NFL patients negative for t(14;18): localized FL in the inguinal region first described by Katzenberger T et al., and pediatric FL (PFL), both with localized disease and excellent prognosis [20, 21].
Cytogenetic abnormalities in our series did not show any association with morphological features and disease outcome, although, relapse tended to occur in shorter time for cases with cytogenetic aberrations and the only case with systemic dissemination also carried a deletion of 1p36. Our two cases with BCL2 break responded to therapy with CR or PR and one case showed relapse during the 12 and 19 months follow-up. Multiple lesions were found significantly more often at diagnosis in cases with BCL2 break or 1p36 deletion. Vernadet et al. found comparable results with no significant effect of cytogenetic aberrations on disease outcome; however, CR was achieved less frequently in cases of PCFCL with cytogenetic aberrations compared to those without chromosomal abnormalitites [10]. In their study, disease stage at diagnosis was not defined. BCL2 break itself did not show association with clinical outcome in most studies on PCFCL; however, Ledard et al. observed association of extranodal dissemination with BCL2 translocation [22]. Data on the prognostic significance of cytogenetic alterations in NFL are controversial: Cheng et al. described NFL cases with 1p36 deletions associated with poor prognosis, but others found no association with clinical outcome [11, 18].
Immunohistochemistry revealed BCL2 expression in 48% of our cases, with a tendency to correlate with low proliferation rate and small cell morphology. This can be explained by the fact that, FLs with high BCL2 expression gain resistance to apoptosis and can expand with low proliferation rate, while BCL2 negative FL need high proliferation rates to expand. BCL2 positivity ranged from 12 to 75% of the reported cases in the literature [7, 8, 10, 17, 23]. The discrepancy might be caused by using different antibodies or by the method evaluating the BCL2 immunostaining. High frequency of BCL2 protein expression was also observed in t(14;18) negative NFL, indicating other mechanisms than BCL2 gene translocation for protein overexpression in the germinal center derived tumor cells. BCL2 amplification could be one, but this abnormality has been seldom reported and we did not observe BCL2 amplification in our cases [8].
Next, we investigated the possibility of TNFRSF14 gene inactivation by somatic mutations in PCFCL and found 4 cases out of 17 harboring two types of mutations in the TNFRSF14 gene. Three patients had c.35G > A; p.Trp12* mutation that was previously described by Cheung et al. in NFL [18]. We are the first to report a mutation occurring in exon 2 (c.157 T > G; p. Cys53Gly) possibly affecting the protein function. Two of the four mutant cases had concomitant 1p36 deletion leading to complete loss of functional TNFRSF14. The tumor suppressor role of TNFRSF14 gene was suggested based on earlier observations on lymphoma and adenocarcinoma cell lines and was confirmed by recent studies on FL [24, 25]. High rate of partial or complete loss of functional TNFRSF14 gene by deletion and/or somatic mutations was described in classical NFL by several authors, and TNFRSF14 mutations were accompanied by copy number neutral loss of heterozygosity of the 1p36 locus in over 70% of mutated cases in pediatric-type FL, that is a t(14;18) negative variant of FL with low genetic complexity and excellent clinical outcome [11, 18, 21]. Significant impact of mutations on disease outcome of our four patients with TNFRSF14 gene was not observed.
We performed mutation analysis of the histone methyltransferase EZH2 at three known mutation hotspots and assessed the EZH2 protein expression using immunohistochemistry. While all cases were wild type for the hotspots analyzed, about half of the cases (52%) expressed high levels of EZH2 protein, and this was strongly associated with BCL2 negativity. However, we did not find correlation between EZH2 and Ki67 expression. In contrast, EZH2 was coexpressed with Ki67 in the neoplastic centroblasts in systemic follicular lymphoma like the non-neoplastic centroblasts in the germinal centers [26]. High EZH2 expression was found to correlate with high proliferation rate in DLBCL and also in T-cell neoplasms, but not with BCL2 expression [27, 28]. High EZH2 expression was shown to be a good prognostic factor for ABC-type DLBCL in another study [29]. We did not find any correlation between the EZH2 expression and clinical outcome in our PCFCL cohort.
In summary, findings of this study extend our knowledge on the genetic background of PCFCL, that seems to be heterogeneous. We identified for the first time somatic mutations of the TNFRSF14 gene and/or 1p36 loss in the minority of the cases, a small minority showed BCL2 rearrangement and other cases exist in which the genetic abnormalities have yet to be identified. FISH examination may help in the diagnostic process to identify primary cutaneous cases with 1p36 deletion negative for BCL2 rearrangement, but this should be confirmed in a larger study group. A large subset of our cohort negative for BCL2 protein expressed high level of EZH2 protein, might be a target for future treatment.
References
Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, Diaz-Perez JL, Geerts ML, Goos M, Knobler R, Ralfkiaer E, Santucci M, Smith N, Wechsler J, van Vloten WA, Meijer CJ (1997) EORTC classification for primary cutaneous lymphomas: a proposal from the cutaneous lymphoma study Group of the European Organization for Research and Treatment of Cancer. Blood 90(1):354–371
Senff NJ, Hoefnagel JJ, Neelis KJ, Vermeer MH, Noordijk EM, Willemze R (2007) Results of radiotherapy in 153 primary cutaneous B-cell lymphomas classified according to the WHO-EORTC classification. Arch Dermatol 143(12):1520–1526. https://doi.org/10.1001/archderm.143.12.1520
Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ (2005) WHO-EORTC classification for cutaneous lymphomas. Blood 105(10):3768–3785. https://doi.org/10.1182/blood-2004-09-3502
Goodlad JR, Krajewski AS, Batstone PJ, McKay P, White JM, Benton EC, Kavanagh GM, Lucraft HH (2002) Primary cutaneous follicular lymphoma: a clinicopathologic and molecular study of 16 cases in support of a distinct entity. Am J Surg Pathol 26(6):733–741. https://doi.org/10.1097/00000478-200206000-00006
Vergier B, Belaud-Rotureau MA, Benassy MN, Beylot-Barry M, Dubus P, Delaunay M, Garroste JC, Taine L, Merlio JP (2004) Neoplastic cells do not carry bcl2-JH rearrangements detected in a subset of primary cutaneous follicle center B-cell lymphomas. Am J Surg Pathol 28(6):748–755. https://doi.org/10.1097/01.pas.0000126775.27698.6e
Kim BK, Surti U, Pandya A, Cohen J, Rabkin MS, Swerdlow SH (2005) Clinicopathologic, immunophenotypic, and molecular cytogenetic fluorescence in situ hybridization analysis of primary and secondary cutaneous follicular lymphomas. Am J Surg Pathol 29(1):69–82. https://doi.org/10.1097/01.pas.0000146015.22624.c7
Streubel B, Scheucher B, Valencak J, Huber D, Petzelbauer P, Trautinger F, Weihsengruber F, Mannhalter C, Cerroni L, Chott A (2006) Molecular cytogenetic evidence of t(14;18)(IGH;BCL2) in a substantial proportion of primary cutaneous follicle center lymphomas. Am J Surg Pathol 30(4):529–536. https://doi.org/10.1097/00000478-200604000-00015
Abdul-Wahab A, Tang SY, Robson A, Morris S, Agar N, Wain EM, Child F, Scarisbrick J, Neat M, Whittaker S (2014) Chromosomal anomalies in primary cutaneous follicle center cell lymphoma do not portend a poor prognosis. J Am Acad Dermatol 70(6):1010–1020. https://doi.org/10.1016/j.jaad.2014.01.862
Lucioni M, Berti E, Arcaini L, Croci GA, Maffi A, Klersy C, Goteri G, Tomasini C, Quaglino P, Riboni R, Arra M, Dallera E, Grandi V, Alaibac M, Ramponi A, Rattotti S, Cabras MG, Franceschetti S, Fraternali-Orcioni G, Zerbinati N, Onida F, Ascani S, Fierro MT, Rupoli S, Gambacorta M, Zinzani PL, Pimpinelli N, Santucci M, Paulli M (2016) Primary cutaneous B-cell lymphoma other than marginal zone: clinicopathologic analysis of 161 cases: comparison with current classification and definition of prognostic markers. Cancer Med 5(10):2740–2755. https://doi.org/10.1002/cam4.865
Verdanet E, Dereure O, Rene C, Tempier A, Benammar-Hafidi A, Gallo M, Frouin E, Durand L, Gazagne I, Costes-Martineau V, Cacheux V, Szablewski V (2017) Diagnostic value of STMN1, LMO2, HGAL, AID expression and 1p36 chromosomal abnormalities in primary cutaneous B cell lymphomas. Histopathology 71(4):648–660. https://doi.org/10.1111/his.13279
Launay E, Pangault C, Bertrand P, Jardin F, Lamy T, Tilly H, Tarte K, Bastard C, Fest T (2012) High rate of TNFRSF14 gene alterations related to 1p36 region in de novo follicular lymphoma and impact on prognosis. Leukemia 26(3):559–562. https://doi.org/10.1038/leu.2011.266
Bouska A, McKeithan TW, Deffenbacher KE, Lachel C, Wright GW, Iqbal J, Smith LM, Zhang W, Kucuk C, Rinaldi A, Bertoni F, Fitzgibbon J, Fu K, Weisenburger DD, Greiner TC, Dave BJ, Gascoyne RD, Rosenwald A, Ott G, Campo E, Rimsza LM, Delabie J, Jaffe ES, Braziel RM, Connors JM, Staudt LM, Chan WC (2014) Genome-wide copy-number analyses reveal genomic abnormalities involved in transformation of follicular lymphoma. Blood 123(11):1681–1690. https://doi.org/10.1182/blood-2013-05-500595
Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA (2010) Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42(2):181–185. https://doi.org/10.1038/ng.518
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (2017) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4h Edition, vol 2, 4th edn. IARC press, Lyon
Langerak AW, Groenen P, Brüggemann M, Beldjord K, Bellan C, Bonello L, Boone E, Carter GI, Catherwood M, Davi F, Delfau-Larue M, Diss T, Evans PAS, Gameiro P, Garcia Sanz R, Gonzalez D, Grand D, Håkansson Å, Hummel M, Liu H, Lombardia L, Macintyre EA, Milner BJ, Montes-Moreno S, Schuuring E, Spaargaren M, Hodges E, van Dongen JJM (2012) EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 26(10):2159–2171. https://doi.org/10.1038/leu.2012.246
Papp G, Mihaly D, Sapi Z (2017) Unusual signal patterns of break-apart FISH probes used in the diagnosis of soft tissue sarcomas. Pathology oncology research : POR 23(4):863–871. https://doi.org/10.1007/s12253-017-0200-z
Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, Dummer R, Hoppe RT (2007) TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 110(2):479–484. https://doi.org/10.1182/blood-2006-10-054601
Cheung KJ, Johnson NA, Affleck JG, Severson T, Steidl C, Ben-Neriah S, Schein J, Morin RD, Moore R, Shah SP, Qian H, Paul JE, Telenius A, Relander T, Lam W, Savage K, Connors JM, Brown C, Marra MA, Gascoyne RD, Horsman DE (2010) Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res 70(22):9166–9174. https://doi.org/10.1158/0008-5472.CAN-10-2460
Szablewski V, Ingen-Housz-Oro S, Baia M, Delfau-Larue MH, Copie-Bergman C, Ortonne N (2016) Primary cutaneous follicle center lymphomas expressing BCL2 protein frequently harbor BCL2 gene break and may present 1p36 deletion: a study of 20 cases. Am J Surg Pathol 40(1):127–136. https://doi.org/10.1097/pas.0000000000000567
Katzenberger T, Kalla J, Leich E, Stocklein H, Hartmann E, Barnickel S, Wessendorf S, Ott MM, Muller-Hermelink HK, Rosenwald A, Ott G (2009) A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood 113(5):1053–1061. https://doi.org/10.1182/blood-2008-07-168682
Schmidt J, Gong S, Marafioti T, Mankel B, Gonzalez-Farre B, Balague O, Mozos A, Cabecadas J, van der Walt J, Hoehn D, Rosenwald A, Ott G, Dojcinov S, Egan C, Nadeu F, Ramis-Zaldivar JE, Clot G, Barcena C, Perez-Alonso V, Endris V, Penzel R, Lome-Maldonado C, Bonzheim I, Fend F, Campo E, Jaffe ES, Salaverria I, Quintanilla-Martinez L (2016) Genome-wide analysis of pediatric-type follicular lymphoma reveals low genetic complexity and recurrent alterations of TNFRSF14 gene. Blood 128(8):1101–1111. https://doi.org/10.1182/blood-2016-03-703819
Pham-Ledard A, Cowppli-Bony A, Doussau A, Prochazkova-Carlotti M, Laharanne E, Jouary T, Belaud-Rotureau MA, Vergier B, Merlio JP, Beylot-Barry M (2015) Diagnostic and prognostic value of BCL2 rearrangement in 53 patients with follicular lymphoma presenting as primary skin lesions. Am J Clin Pathol 143(3):362–373. https://doi.org/10.1309/ajcp4subr4npsptn
Lawnicki LC, Weisenburger DD, Aoun P, Chan WC, Wickert RS, Greiner TC (2002) The t(14;18) and bcl-2 expression are present in a subset of primary cutaneous follicular lymphoma: association with lower grade. Am J Clin Pathol 118(5):765–772. https://doi.org/10.1309/2tju-dnlq-5jba-ab4t
Costello RT, Mallet F, Barbarat B, Schiano De Colella JM, Sainty D, Sweet RW, Truneh A, Olive D (2003) Stimulation of non-Hodgkin's lymphoma via HVEM: an alternate and safe way to increase Fas-induced apoptosis and improve tumor immunogenicity. Leukemia 17(12):2500–2507. https://doi.org/10.1038/sj.leu.2403175
Harrop JA, McDonnell PC, Brigham-Burke M, Lyn SD, Minton J, Tan KB, Dede K, Spampanato J, Silverman C, Hensley P, DiPrinzio R, Emery JG, Deen K, Eichman C, Chabot-Fletcher M, Truneh A, Young PR (1998) Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem 273(42):27548–27556. https://doi.org/10.1074/jbc.273.42.27548
van Kemenade FJ, Raaphorst FM, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Otte AP, Meijer CJ (2001) Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B-cell non-Hodgkin lymphoma. Blood 97(12):3896–3901. https://doi.org/10.1182/blood.V97.12.3896
Zhou Z, Gao J, Popovic R, Wolniak K, Parimi V, Winter JN, Licht JD, Chen YH (2015) Strong expression of EZH2 and accumulation of trimethylated H3K27 in diffuse large B-cell lymphoma independent of cell of origin and EZH2 codon 641 mutation. Leuk Lymphoma 56(10):2895–2901. https://doi.org/10.3109/10428194.2015.1006220
Shi M, Shahsafaei A, Liu C, Yu H, Dorfman DM (2015) Enhancer of zeste homolog 2 is widely expressed in T-cell neoplasms, is associated with high proliferation rate and correlates with MYC and pSTAT3 expression in a subset of cases. Leuk Lymphoma 56(7):2087–2091. https://doi.org/10.3109/10428194.2014.968780
Lee HJ, Shin DH, Kim KB, Shin N, Park WY, Lee JH, Choi KU, Kim JY, Lee CH, Sol MY (2014) Polycomb protein EZH2 expression in diffuse large B-cell lymphoma is associated with better prognosis in patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 55(9):2056–2063. https://doi.org/10.3109/10428194.2013.858816
Acknowledgements
This work was funded by the Momentum grant (LP-95021) of the Hungarian Academy of Sciences and the NVKP_16-1-2016-0004 and KH17-126718 grants of the Hungarian National Research, Development and Innovation Office (NKFIH).
Funding
This work was funded by the Momentum grant (LP-95021) of the Hungarian Academy of Sciences and the NVKP_16-1-2016-0004 KH17-126718 grants of the Hungarian National Research, Development and Innovation Office (NKFIH).
Author information
Authors and Affiliations
Contributions
MM, EK, TS, and MV have made substantial contributions to acquisition of the data. AG, BB, MV, DK, GP, and JC have made substantial contributions to analysis and interpretation of data. ÁSZ, CB, and AM made substantial contribution to conception and design of the manuscript, and ÁSZ has written the manuscript. AG, BB, MV, DK, GP, JC, and CB conducted cytogenetic, phenotypic, and biologic analyses. All authors have been involved in revising the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
All protocols were approved by the Institutional Ethical Review Board (TUKEB no. 7/2006).
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Gángó, A., Bátai, B., Varga, M. et al. Concomitant 1p36 deletion and TNFRSF14 mutations in primary cutaneous follicle center lymphoma frequently expressing high levels of EZH2 protein. Virchows Arch 473, 453–462 (2018). https://doi.org/10.1007/s00428-018-2384-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2384-3