Abstract
β-Catenin exerts multiple functions in several neoplasms, playing a major role in cell signaling and tumor progression. This study analyzed possible CTNNB1 mutations in salivary gland pleomorphic adenomas (PAs) and adenoid cystic carcinomas (ACCs), and determined possible differences in β-catenin immunoexpression in relation to these mutations, as well as histopathological aspects of these tumors. Twenty-four PAs (15 cell-rich and 9 cell-poor tumors) and 24 ACCs (10 tubular, 8 cribriform, and 6 solid tumors) were selected for the analysis of β-catenin distribution and cellular localization. Furthermore, β-catenin expression was evaluated using the H-score scoring system. Mutations in CTNNB1 exon 3 were investigated by the single-strand conformational polymorphism test. Diffuse β-catenin expression was more frequently observed in ACCs compared to PAs (P = 0.008). No significant difference in β-catenin cellular localization was observed between these tumors (P = 0.098). Comparisons between PA and ACC cases revealed a higher median H-score in the latter (P = 0.036). Cell-rich PAs exhibited a trend for higher H-score than cell-poor tumors (P = 0.060), whereas lower H-scores were observed in cribriform ACCs when compared to tubular and solid ACCs (P = 0.042). Mutations in CTNNB1 were observed in 6 PAs and 7 ACCs, with no significant difference in H-scores for β-catenin according to mutation status (P = 0.135). β-Catenin is important in the pathogenesis of salivary gland PAs and ACCs. In addition, CTNNB1 exon 3 mutations do not seem to significantly influence β-catenin cytoplasmic/membranous expression or nuclear translocation in these tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivary gland tumors are a special group among head and neck neoplasms, due to their relatively rare occurrence and high variation in histological subtypes [1, 2]. Pleomorphic adenomas (PAs) are the most frequent benign salivary neoplasm, while adenoid cystic carcinomas (ACCs) account for the second most common salivary gland malignancy [3]. Although these lesions are considered neoplasms that display different biological behavior, they present similar origin and cell components [4].
Malignant transformation and tumor progression are closely related to the adhesive properties of the cells. In this way, β-catenin is particularly interesting, because it functions both as a component of the cadherin–β-catenin adhesion complex and as a signaling molecule [5,6,7]. β-Catenin is a key protein of the Armadillo family that is encoded by the CTNNB1 gene in the Wnt signaling pathway [8, 9]. In the cell membrane, it plays a main physiological role in the maintenance of cell–cell adhesion, while cytoplasmic β-catenin is essential for signal transduction from the membrane to the nucleus, and its over-accumulation, secondary to its lack of degradation, is essential for it to function as a transcription factor [5, 6]. Furthermore, investigations indicate that high membrane β-catenin expressions are linked to better outcomes in salivary gland neoplasms [6, 10].
Mutations of the β-catenin gene have been reported in various types of malignancies, such as hepatocellular carcinomas [11] and lymphomas [12]. In these cases, exon 3 of the CTNNB1 gene has been investigated, since this is a crucial regulatory site that results in cytoplasmic β-catenin accumulation [11,12,13]. Thus, further delineation of this molecular alteration in salivary gland neoplasms is of great interest.
Based on this background and given the scarcity of studies evaluating mutations of the CTNNB1 gene in salivary gland neoplasms, this study aimed to investigate possible mutations in CTNNB1 exon 3 in PAs and ACCs and determine possible differences in β-catenin immunoexpression in relation to these mutations, as well as investigate histopathological aspects of these neoplasms.
Material and methods
After approval by the Institutional Ethics Committee on Research Involving Humans (Protocol 081/2005), tissue samples from 24 PAs and 24 ACCs were randomly selected from the archives of the Department of Oral Pathology, Federal University of Rio Grande do Norte (UFRN), and from the archives of the Department of Pathology and Legal Medicine, Federal University of Ceará (UFC). PAs were divided into two categories according to the amount and cellular composition of the stroma: cell-rich tumors (predominance of epithelial cells) (Fig. 1a–b) and cell-poor tumors (predominance of myxoid and chondroid areas) (Fig. 1c) [14]. ACCs were classified into the following histological subtypes: cribriform (pure cribriform tumors or mixed with < 30% of solid areas) (Fig. 1d), tubular (tumors with both tubular and cribriform areas without solid components) (Fig. 1e), and solid (predominantly solid pattern) (Fig. 1f) [15]. The cases were not matched for age, gender, or type of salivary gland (major or minor).
DNA extraction
Genomic DNA was extracted from formalin-fixed paraffin-embedded tissue blocks by preparing six 10-μm-thick sections collected in sterile microtubes. The paraffin was removed by incubation in xylene and the specimens were subsequently rehydrated in ethanol. DNA was extracted with a proteinase K and Chelex-100 resin solution (BioRad Laboratories, Hercules, CA, USA). The integrity of the extracted DNA was tested by electrophoresis, followed by amplification of the methylenetetrahydrofolate reductase gene through a polymerase chain reaction (PCR).
Analysis of the CTNNB1 gene
To detect mutations in the CTNNB1 gene, the single-strand conformational polymorphism (SSCP) test was performed, under the premise that abnormal electrophoretic migration results from genetic mutation, as demonstrated previously [16]. Exon 3 of the CTNNB1 gene was amplified by PCR using the following primers: sense 5′-GCTGATTTGATGGAGTTGGA-3′ and anti-sense 5′-GGCCACAGCCAATCAGCA-3′. Each PCR reaction for a total of 25 μL final volume consisted of 0.2 mmol L−1 of four deoxynucleotide triphosphates, 1.5 mmol L−1 MgCl2, 0.4 μmol L−1 of each primer, and 0.5 units of Taq DNA polymerase (Invitrogen®, Carlsbad, CA, USA). Amplification consisted of 40 denaturation cycles at 95 °C for 60 s, annealing at 56 °C for 60 s, and extension at 72 °C for 60 s, followed by a final extension cycle at 72 °C for 5 min. For the SSCP analysis, 5 or 6 μL of the PCR product was mixed with an equal volume of stop solution (95% of formamide, 20 mmol L−1 EDTA, and 0.05% of xylene cyanol and bromophenol blue), heated at 95 °C for 5 min and immediately placed on ice and loaded onto a 12.5% acrylamide gel (GenePhor™, Amersham Biosciences, Uppsala, Sweden). Electrophoresis was performed using an Electrophoresis GenePhor Unit (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) at 4 °C for 3 h. The band patterns were then visualized by a DNA Silver Staining Kit (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Immunohistochemistry
Immunohistochemistry was performed by the EnVision peroxidase procedure (Dako, Carpinteria, CA, USA). Briefly, dewaxed and rehydrated tumor sections (3-μm-thick) were subjected to antigen retrieval in a steamer with citrate buffer, pH 6.0. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide. After treatment with normal serum, the sections were incubated for 18 h with the primary anti-β-catenin antibody diluted at a 1:150 ratio (clone 14, BD Biosciences Pharmingen, San Jose, CA, USA). The reaction was developed with diaminobenzidine (Liquid DAB+, Dako, Carpinteria, CA, USA) as the chromogen, and the sections were counterstained with Mayer’s hematoxylin. Healthy salivary glands and oral epithelial lining included in the specimens were used as positive controls. As negative controls, the specimens were treated as described above, with replacement of the primary antibody with bovine serum albumin in phosphate-buffered saline solution.
Immunostaining evaluation
Under light microscopy, β-catenin immunoexpression was evaluated in terms of pattern of distribution, according to the following scores: 0 = no staining, 1 = focal staining, and 2 = diffuse staining. Immunoexpression was also analyzed in terms of cellular localization (membrane, cytoplasm, nucleus). Analysis of β-catenin expression according to pattern of distribution and cellular localization was performed irrespectively of the intensity of staining.
In addition, 1000 neoplastic cells were counted in a series of fields randomly chosen in PA and ACC specimens at × 400 magnification. Immunostaining intensity was scored as follows: 0 (no staining), 1+ (weak), 2+ (moderate), and 3+ (strong). Using an adaptation of a previously detailed method [17], an H-score was calculated as the sum of the percentages of cells that stained at each intensity multiplied by the weighted intensity of the staining: H-score = (i + 1)π, where i = 1, 2, and 3 and π ranges from 0 to 100%.
Statistical analyses
The results were submitted to statistical analyses using the Statistical Package for the Social Sciences (version 17.0; SPSS Inc., Chicago, IL, USA). Fisher’s exact test and Pearson’s chi-square test were applied for the analysis of β-catenin distribution patterns. The distribution of the H-score data was evaluated by the Kolmogorov–Smirnov test, which indicated the absence of a normal distribution. Thus, the median H-scores were compared between PAs and ACCs by the non-parametric Mann–Whitney test. Comparison of H-scores between the histological ACC and PA subtypes was performed by the non-parametric Kruskal–Wallis test and non-parametric Mann–Whitney test, respectively. The non-parametric Mann–Whitney test was used to analyze H-scores according to the presence or absence of CTNNB1 gene mutations. The level of significance was set at 5% for all tests (P < 0.05).
Results
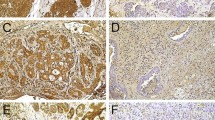
β-Catenin was expressed in 22 PA cases (91.6%), with a focal pattern in most cases (59.1%). Cytoplasmic immunoreactivity was observed in all 22 cases, whereas membrane staining was observed in only 10 cases (Fig. 2a–b) (45.5%). Nuclear positivity for β-catenin was observed in only 1 case (4.5%) of PA. Areas of squamous differentiation revealed strong β-catenin immunoexpression, while myoepithelial cells trapped in tumor stroma were mostly negative for this protein.
All ACC cases were positive for β-catenin, with the predominance of the diffuse pattern (79.1%). Cytoplasmic expression was detected in all cases, whereas membrane immunostaining was observed in 11 tumors (45.8%) (Fig. 3a–c). Only 6 cases (25.0%) of ACC exhibited nuclear β-catenin expression.
Statistical analyses indicated significant differences in the β-catenin distribution pattern between PAs and ACCs, with a higher frequency of the diffuse pattern in the latter (P = 0.008). However, no significant difference in β-catenin cellular localization was observed between these tumors (P = 0.098).
The median H-score for β-catenin was 87.6 (range 0.0–203.3) in PA and 138.8 (range 59.9–226.7) in ACC, with a statistically significant difference between groups (P = 0.002) (Table 1). Cell-rich PAs (median 111.4, range 9.0–203.3) exhibited a trend for higher β-catenin H-scores when compared to cell-poor PAs (median 59.0, range 0.0–111.6) (P = 0.060) (Table 1). A statistically significant lower H-score was observed in cribriform ACCs (median 89.4, range 58.9–190.8) when compared to tubular (median 150.5, range 126.6–226.7) and solid (median 145.0, range 92.9–198.3) ACCs (P = 0.042) (Table 1).
Mutations in CTNNB1 exon 3 were observed in 13 cases (27.1%) (6 PAs and 7 ACCs) (Table 2). Of these, only 2 showed nuclear positivity for β-catenin, both ACCs. The median H-score was of 90.7 (range 23.1–167.0) in tumors presenting the CTNNB1 gene mutation and 129.60 (range: 0.0–226.7) in tumors without the CTNNB1 gene mutation. No statistically significant differences were found between these median H-scores regarding the mutation status of the CTNNB1 gene (P = 0.135).
Discussion
The pathophysiology and behavior of salivary gland neoplasms are still not completely understood, due to the variety of histopathological phenotypes and the paucity of previous molecular studies [10, 18, 19]. The CTNNB1 gene codifies β-catenin, which plays an essential role in the Wnt/β-catenin signaling pathway and is intricately involved in the pathogenesis of several types of cancer. However, its role in benign and malignant salivary gland neoplasms has not yet been completely elucidated [7, 10, 20]. In the present study, we sought to investigate mutations in CTNNB1 exon 3 in PAs and ACCs and determine possible differences in the immunoexpression of β-catenin in relation to these mutations, as well as assess some morphological aspects of these salivary gland neoplasms.
In our study, cytoplasmic expression of β-catenin was detected in all positive cases of PA and ACC, whereas membrane immunostaining was found in less than 46% of these tumors. The predominance of non-membranous expression of β-catenin in salivary gland PAs and ACCs has been reported in previous studies [19, 21]. In our sample, ACCs exhibited higher H-scores for β-catenin (P = 0.002) and a higher percentage of cases with nuclear positivity for this protein (25 versus 4.5%) when compared to PAs. Together, these findings highlight the importance of β-catenin in the pathogenesis of salivary gland PAs and ACCs. In addition, it could be suggested that differences in the biological behavior of these tumors may not be related to reduced membranous expression of β-catenin, but in part to differences in the cytoplasmic accumulation and subsequent nuclear translocation of this protein.
It is generally accepted that β-catenin exerts distinct functions, depending on its subcellular localization [5, 6, 10]. In this context, membrane-bound β-catenin mediates cell–cell adhesion, whereas elevation of the cytoplasmic and nuclear pool of this protein is associated with oncogenic functions [5, 6]. In malignant salivary gland neoplasms, it has been reported that reduced and cytoplasmic localization of β-catenin could indicate lack of differentiation, invasive potential, and aggressive behavior [21, 22]. Coherently, high membranous expression of β-catenin has been associated with better overall survival for patients with minor salivary gland carcinomas, including ACCs [10]. Nevertheless, Furuse et al. [23] suggest that simple loss of β-catenin expression is not necessarily implicated in the malignant transformation of salivary gland neoplasms. According to these authors, PAs and ACCs do not exhibit a consistent change in the pattern of β-catenin immunoexpression, which could reflect different patterns of tumorigenesis in these salivary gland tumors [23].
Despite relatively intense investigation, the biological significance of aberrant expression of β-catenin and/or loss of its membranous expression in salivary gland tumors is not fully understood [19]. In the present study, solid ACCs, which are considered the most invasive histological subtype, exhibited a high H-score for β-catenin. These findings are similar to those reported by Wang et al. [20] and may indicate a possible role of this protein in the aggressive clinical behavior of ACCs. On the other hand, the varying expression of β-catenin in PAs could be related to their remarkable histomorphological heterogeneity [21]. As observed in the present study, cell-rich PAs showed a trend for higher β-catenin H-scores when compared to cell-poor PAs. In line with these findings, Fonseca et al. [24] reported that alterations in β-catenin localization in salivary PAs occur mainly in non-epithelial cell types. Thus, the expression of β-catenin in PAs may be related to cellular composition (epithelial cells versus myoepithelial cells), as well as to the degree of differentiation of myoepithelial cells.
Interestingly, nuclear staining for β-catenin was found in 1 PA of the present sample, similarly to the results reported by Chandrashekar et al. [21] and Genelhu et al. [25]. In contrast, Hakata et al. [19] verified a high percentage (71.4%) of salivary gland PAs with nuclear expression of β-catenin. These authors also observed higher Ki-67 labeling indexes in PAs with non-membranous expression of β-catenin when compared to PAs with negative or membranous expression of this protein. Taken together, these findings suggest an oncogenic function for the Wnt/β-catenin signaling pathway in a subset of salivary gland PAs, which could be relevant in the behavior of some tumors regarding recurrence and malignant transformation [25].
Nevertheless, it should be emphasized that the Wnt/β-catenin signaling pathway can promote inversely correlated processes, such as cell proliferation and differentiation. Therefore, it has been demonstrated that the β-catenin transcriptional complex has the ability to choose and transactivate specific gene sets depending on the context [5, 26]. These observations highlight the need for further investigations, with larger sample sizes and clinical data, to better establish the biological significance and the clinical importance of β-catenin in salivary gland PAs and ACCs.
Most of the mutations in the CTNNB1 gene in neoplasms occur in exon 3, so this was the chosen molecular alteration in the present study [12, 18, 27]. Point mutations in this exon stabilize the protein and make it insensitive to degradation by the APC multiprotein complex [6, 28]. This results in the accumulation of β-catenin and activation of target gene expression, upregulating the transcriptional activity of CCND1, C-MYC, and C-JUN, among other genes [28].
Some studies have investigated the presence of mutations in the CTNNB1 gene in salivary gland tumors [18, 29,30,31,32]. Daa et al. [18] demonstrated that 35% of 20 cases of ACC exhibited mutations in, at least, one of the three following genes: CTNNB1, AXIN1, or APC. In the present study, mutations in CTNNB1 exon 3 were observed in 13 cases (27.1%) (6 PAs and 7 ACCs), with a trend for lower β-catenin expression in tumors presenting mutations. Furthermore, these mutations did not result in aberrant β-catenin nuclear translocation in PAs and ACCs. Similarly, Kawahara et al. [33] found no statistically significant association between CTNNB1 exon 3 mutations and nuclear β-catenin expression in basal cell adenomas of salivary glands. Together, these findings suggest that differences in β-catenin expression between salivary gland ACCs and PAs may not be related to the mutation status of CTNNB1 exon 3.
Emerging data suggest that CTNNB1 mutations are relatively frequent in basal cell adenomas of salivary glands, where 33.3 to 80% of cases harbor exon 3 mutations, but not in ACCs [30,31,32]. Some studies also highlight that nuclear expression of β-catenin is characteristic of basal cell adenomas, suggesting that it could be a useful marker in the differential diagnosis between these lesions and other salivary gland basaloid tumors, including ACCs [30, 32]. Nonetheless, in agreement with our results, nuclear expression of β-catenin in salivary gland ACCs has been observed in other investigations, with variable percentages of positivity [20, 22, 34].
Considering these contradictory findings, analysis of MYB-NFIB fusion, which has been regarded as a key oncogenic event of ACCs [35, 36], could help in the differential diagnosis between these tumors and basal cell adenomas. This fusion leads to elevated expression levels of MYB, which can be demonstrated by immunohistochemistry [35, 36]. Despite being relatively frequent, MYB immunoexpression and MYB-NFIB translocation have not been observed in all cases of ACC [35]. Furthermore, some authors argue that MYB expression may not help separate basal cell adenomas from ACCs [37]. Taken together, these findings highlight the importance of association of ancillary tests to establish the definitive diagnosis in difficult cases, such as minimally sized histologic biopsies.
In conclusion, β-catenin is important in the pathogenesis of salivary gland PAs and ACCs. The highly invasive behavior of ACCs could be partially related to high β-catenin expressions. In addition, CTNNB1 exon 3 mutations do not seem to significantly influence β-catenin cytoplasmic/membranous expression or nuclear translocation in salivary gland PAs and ACCs. Further studies are suggested to evaluate the relationship between β-catenin expression and clinical parameters in a larger sample universe of PAs and ACCs.
References
Namboodiripad PC (2014) A review: immunological markers for malignant salivary gland tumors. J Oral Biol Craniofac Res 4:127–134
de Ridder M, Balm AJ, Smeele LE, Wouters MW, van Dijk BA (2015) An epidemiological evaluation of salivary gland cancer in the Netherlands (1989-2010). Cancer Epidemiol 39:14–20
Wang XD, Meng LJ, Hou TT, Huang SH (2015) Tumours of the salivary glands in northeastern China: a retrospective study of 2508 patients. Br J Oral Maxillofac Surg 53:132–137
Cavalcante RB, Nonaka CF, Rabenhorst SH, da Costa Miguel MC, Pinto LP, de Souza LB (2017) Pleomorphic adenoma and adenoid cystic carcinoma of salivary glands: E-cadherin immunoexpression and analysis of the CDH1 -160C/A polymorphism. Arch Oral Biol 73:48–54
González-Moles MA, Ruiz-Ávila I, Gil-Montoya JA, Plaza-Campillo J, Scully C (2014) β-Catenin in oral cancer: an update on current knowledge. Oral Oncol 50:818–824
Psyrri A, Kotoula V, Fountzilas E, Alexopoulou Z, Bobos M, Televantou D, Karayannopoulou G, Krikelis D, Markou K, Karasmanis I, Angouridakis N, Kalogeras KT, Nikolaou A, Fountzilas G (2014) Prognostic significance of the Wnt pathway in squamous cell laryngeal cancer. Oral Oncol 50:298–305
Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ (2017) Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol 10:101
Bánkfalvi A, Krassort M, Végh A, Felszeghy E, Piffkó J (2002) Deranged expression of the E-cadherin/beta-catenin complex and the epidermal growth factor receptor in the clinical evolution and progression of oral squamous cell carcinomas. J Oral Pathol Med 31(450):7
Zhang Q, Meng XK, Wang WX, Zhang RM, Zhang T, Ren JJ (2016) The Wnt/β-catenin signaling pathway mechanism for pancreatic cancer chemoresistance in a three-dimensional cancer microenvironment. Am J Transl Res 8:4490–4498
Schneider S, Thurnher D, Seemann R, Brunner M, Kadletz L, Ghanim B, Aumayr K, Heiduschka G, Lill C (2016) The prognostic significance of β-catenin, cyclin D1 and PIN1 in minor salivary gland carcinoma: β-catenin predicts overall survival. Eur Arch Otorhinolaryngol 273:1283–1292
Tornesello ML, Buonaguro L, Tatangelo F, Botti G, Izzo F, Buonaguro FM (2013) Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics 102:74–83
Takahara M, Kishibe K, Bandoh N, Nonaka S, Harabuchi Y (2004) P53, N- and K-Ras, and beta-catenin gene mutations and prognostic factors in nasal NK/T-cell lymphoma from Hokkaido, Japan. Hum Pathol 35:86–95
Garcia-Rostan G, Camp RL, Herrero A, Carcangiu ML, Rimm DL, Tallini G (2001) Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 158:987–996
Soares AB, de Araújo VC, Juliano PB, Altemani A (2009) Angiogenic and lymphangiogenic microvessel density in recurrent pleomorphic adenoma. J Oral Pathol Med 38:623–629
Ellis GL, Auclair PL (2008) Tumors of the salivary glands. AFIP atlas of tumor pathology. ARP Press, Silver Spring MD
Neves Filho EH, Cordeiro DE, Vieira AP, Rabenhorst SH (2012) TP53 codon 72 and intron 3 polymorphisms and mutational status in gastric cancer: an association with tumor onset and prognosis. Pathobiology 79:323–328
McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721
Daa T, Kashima K, Kaku N, Suzuki M, Yokoyama S (2004) Mutations in components of the Wnt signaling pathway in adenoid cystic carcinoma. Mod Pathol 17:1475–1482
Hakata Y, Fukui H, Sekikawa A, Yamagishi H, Ichikawa K, Tomita S, Imura J, Kawamata H, Imai Y, Fujimori T (2010) Expression of β-catenin and REG Iα in relation to cell proliferative ability in salivary gland tumors. Exp Ther Med 1:437–443
Wang R, Geng N, Zhou Y, Zhang D, Li L, Li J, Ji N, Zhou M, Chen Y, Chen Q (2015) Aberrant Wnt-1/beta-catenin signaling and WIF-1 deficiency are important events which promote tumor cell invasion and metastasis in salivary gland adenoid cystic carcinoma. Biomed Mater Eng 26:S2145–S2153
Chandrashekar C, Angadi PV, Krishnapillai R (2011) β-Catenin expression in benign and malignant salivary gland tumors. Int J Surg Pathol 19:433–440
Zhou CX, Gao Y (2006) Aberrant expression of beta-catenin, Pin1 and cylin D1 in salivary adenoid cystic carcinoma: relation to tumor proliferation and metastasis. Oncol Rep 16:505–511
Furuse C, Cury PR, Altemani A, dos Santos Pinto D Jr, de Araújo NS, de Araújo VC (2006) Beta-catenin and E-cadherin expression in salivary gland tumors. Int J Surg Pathol 14:212–217
Fonseca I, Fonseca R, Martins C, Soares J (2008) Alteration of beta-catenin localization in salivary pleomorphic adenomas is not related to t(3;8)(p21;q12) and is mainly present in non-epithelial cell types. Histopathology 52:244–247
Genelhu MC, Gobbi H, Arantes DC, Cardoso SV, Cassali GD (2007) Immunolocalization of beta-catenin in pleomorphic adenomas and carcinomas ex-pleomorphic adenomas of salivary glands. Appl Immunohistochem Mol Morphol 15:273–278
Masuda T, Ishitani T (2017) Context-dependent regulation of the β-catenin transcriptional complex supports diverse functions of Wnt/β-catenin signaling. J Biochem 161:9–17
Liu Y, Patel L, Mills GB, Lu KH, Sood AK, Ding L, Kucherlapati R, Mardis ER, Levine DA, Shmulevich I, Broaddus RR, Zhang W (2014) Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst 106
Jamieson C, Sharma M, Henderson BR (2014) Targeting the β-catenin nuclear transport pathway in cancer. Semin Cancer Biol 27:20–29
Shiratsuchi H, Nakashima T, Hirakawa N, Toh S, Nakagawa T, Saito T, Tsuneyoshi M, Komune S (2007) Beta-catenin nuclear accumulation in head and neck mucoepidermoid carcinoma: its role in cyclin D1 overexpression and tumor progression. Head Neck 29:577–584
Jo VY, Sholl LM, Krane JF (2016) Distinctive patterns of CTNNB1 (β-catenin) alterations in salivary gland basal cell adenoma and basal cell adenocarcinoma. Am J Surg Pathol 40:1143–1150
Wilson TC, Ma D, Tilak A, Tesdahl B, Robinson RA (2016) Next-generation sequencing in salivary gland basal cell adenocarcinoma and basal cell adenoma. Head Neck Pathol 10:494–500
Lee YH, Huang WC, Hsieh MS (2017) CTNNB1 mutations in basal cell adenoma of the salivary gland. J Formos Med Assoc. https://doi.org/10.1016/j.jfma.2017.11.011
Kawahara A, Harada H, Abe H, Yamaguchi T, Taira T, Nakashima K, Mihashi H, Akiba J, Kage M (2011) Nuclear β-catenin expression in basal cell adenomas of salivary gland. J Oral Pathol Med 40:460–466
Ferrazzo KL, Neto MM, dos Santos E, dos Santos PD, de Sousa SO (2009) Differential expression of galectin-3, beta-catenin, and cyclin D1 in adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma of salivary glands. J Oral Pathol Med 38:701–707
Xu B, Drill E, Ho A, Ho A, Dunn L, Prieto-Granada CN, Chan T, Ganly I, Ghossein R, Katabi N (2017) Predictors of outcome in adenoid cystic carcinoma of salivary glands: a clinicopathologic study with correlation between MYB fusion and protein expression. Am J Surg Pathol 41:1422–1432
West RB, Kong C, Clarke N, Gilks T, Lipsick JS, Cao H, Kwok S, Montgomery KD, Varma S, Le QT (2011) MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol 35:92–99
Rooney SL, Robinson RA (2017) Immunohistochemical expression of MYB in salivary gland basal cell adenocarcinoma and basal cell adenoma. J Oral Pathol Med 46:798–802
Funding
This study was supported by grant number 479935/04-1 from the National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from each patient after approval by the Institutional Ethics Committee on Research Involving Humans.
Rights and permissions
About this article
Cite this article
Cavalcante, R.B., Nonaka, C.F.W., Santos, H.B.d. et al. Assessment of CTNNB1 gene mutations and β-catenin immunoexpression in salivary gland pleomorphic adenomas and adenoid cystic carcinomas. Virchows Arch 472, 999–1005 (2018). https://doi.org/10.1007/s00428-018-2335-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2335-z