Abstract
Pancreatic cancer remains one of the deadliest malignancies in the world. Inflammatory response and tumor environment are thought to play a major role in its pathogenesis. Knowledge on TLR expression and impact on patient survival in pancreatic cancer is limited. The study’s aim was to clarify the role of different TLRs in pancreatic cancer. TLR2, TLR4, and TLR9 expression was investigated in 65 surgically resected pancreatic ductal adenocarcinoma specimens by immunohistochemistry. The association between TLR expression, clinical parameters, and local inflammatory response to the tumor was assessed using chi-square test. Relation between patient survival and TLR expression was calculated with multivariable Cox regression, adjusted for age, sex, and tumor stage. We found TLR2, TLR4, and TLR9 to be expressed in pancreatic cancer. There was no association between TLR expression and tumor stage, tumor size, lymph node metastasis, or tumor necrosis. Contrary to our initial hypothesis, high cytoplasmic TLR9 expression was associated with longer patient survival, and multivariate analysis identified low TLR9 expression as an independent risk factor for cancer-specific death (HR 3.090, 95% CI 1.673–5.706). The results suggest that high TLR9 expression in pancreatic ductal adenocarcinoma indicates improved prognosis. The prognostic effect of TLR9 might be associated with bacterial exposure, but this needs further evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite efforts to develop effective treatment strategies, pancreatic adenocarcinoma remains one of the most lethal cancers with a 5-year survival rate of only 5% [1, 2]. Known risk factors for the development of pancreatic cancer include smoking and age and various genetic disorders [1]. Currently available treatment strategies consist of surgery, chemotherapy, and radiation [3].
Toll-like receptors (TLR) are pattern-recognition receptors of the innate immune system [4]. In a normally functioning organism, they recognize unique proteins derived from microbes, called pathogen-associated molecular patterns (PAMPs) [5]. The recognition of PAMPs activates multiple pro-inflammatory responses through downstream signaling, ultimately leading to antigen-specific acquired immunity [5]. TLR2 and TLR4 recognize lipopolysaccharides derived from exogenous microbes and are located mostly on the cell membrane [6], whereas TLR9 recognizes viral and bacterial unmethylated CpG-DNA and is typically located intracellularly [7].
Involvement of TLRs in carcinogenesis is becoming undisputed. TLRs recognize damage-associated molecular patterns (DAMPs), released into the system by tissue damage [8]. Chronic activation of TLRs creates a favorable environment for cancer development through pro-inflammatory responses [9]. This leads to enhancement of anti-apoptotic and proliferative properties of the microenvironment and the emergence of cancer cells [10].
TLR involvement has previously been reported in gastrointestinal malignancies such as colon and esophageal cancers [11, 12], as well as prostate and tongue cancers [13, 14]. There is also evidence of TLR involvement in pancreatic cancer [15]. Single studies have shown that TLR2 is expressed in pancreatic cancer tissue and has been suggested as potential target for immunotherapy [16]. There is also some evidence of TLR4 expression in pancreatic cancer [17–19]. TLR9 has previously been linked to acute pancreatitis, its expression has been reported in pancreatic cancer, and it has been suggested to participate in tumorigenesis [15, 20, 21]. In a recent study, our group showed that germ-free mice had significantly higher levels of TLR9 in the exocrine pancreas compared to conventional mice [22]. There is recent evidence that bacteria may affect pancreatic carcinogenesis, and oral pathogens as well as Helicobacter Pylori have been suggested to participate in pancreatic cancer progression [23, 24]. In this study, we aimed to investigate associations between expression of bacteria-sensing TLR2, TLR4, and TLR9 in pancreatic cancer cells and patient survival and correlation with local inflammatory cell response and clinical parameters.
Materials and methods
Patients
The material consisted of paraffin-embedded archival specimens (n = 69) of patients with surgically resected pancreatic cancer. In 65 cases, representative carcinoma tissue was available in the tissue blocks. In the four remaining patients, either only normal pancreas or premalignant ductal alterations were found (Table 1). The specimens were collected from Oulu University Hospital Pathology Archive between the years 1993 and 2011. Patient clinical and laboratory data were extracted from patient records. The time and cause of death were obtained from Statistics Finland. All had been diagnosed as pancreatic adenocarcinoma, confirmed by an experienced gastrointestinal pathologist (TJK). The patients underwent pancreaticoduodenectomy according to Whipple (n = 54), subtotal pancreatic resection (n = 2), total pancreatic resection (n = 11), or total pancreaticoduodenectomy (n = 2). During the operation, in six of the 69 patients, macroscopic intravenous tumor invasion was found. Median age of the patients at diagnosis was 66 years (range 36–77 years). Median follow-up time of the patients was 21 months (range 1–60 months). Use of the samples and patient data were approved by the Oulu University Hospital Ethics Committee and by the National Authority for Medicolegal Affairs (VALVIRA).
Immunohistochemistry
Immunohistochemistry was performed on tissue blocks, which were selected on the basis of hematoxylin and eosin staining as representative for the tumor mass in the resected specimen. Antigen retrieval was performed by exposure to high temperature in Tris-EDTA buffer for 15 min (pH 9.0). Immunostaining was performed in a Dako Autostainer (Dako) with mouse monoclonal antibodies against TLR2 (MAB0066, IgG1, clone 1030A5.138, Abnova, Taipei, Taiwan) at a dilution of 1:50, TLR4 (H00007099-M02, IgG2a, clone 3B6, Abnova, Taipei, Taiwan) at a dilution of 1:1000, and TLR9 (IMG-305A, IgG1, clone 26C593.2, Imgenex, San Diego, CA, USA) at a dilution of 1:150. For detection of the first antibody binding, we used Dako Envision flex kit (Dako, Copenhagen, Denmark). Diaminiobenzidine (Dako basic DAB-kit) was used as a chromogen. As negative control, we used omission of the primary antibody and replacement of the primary antibody with non-specific mouse primary antibody isotype.
Histological analysis
Immunohistochemical staining of TLR2, TLR4, and TLR9 was evaluated by two observers independently (J. L, O. H), blinded to patient clinical data as described earlier [25, 26]. Staining intensity and the percentage of positive epithelial cells were assessed separately in pancreatic ductal adenocarcinoma (n = 65) and adjacent normal pancreatic tissue, when available (for TLR2 in 37 cases, for TLR4 in 38 cases, and for TLR9 in 38 cases. Tissue specimen containing cancer tissue was unavailable for four patients. Membranous, cytoplasmic, and nuclear staining was evaluated independently, and staining intensity was given a score from 0 (absent) to 3 (strong intensity). In addition, the percentage of cells positive for each subcellular localization was estimated (from 0 to 100%). When inter-observer difference was less than one point in intensity or less than 30% in the proportion of positive cells, the mean value of the independent estimates was used for statistical analysis. Cases with higher differences were re-evaluated, and a single score was given by consensus between the two observers. Only one case stained for TLR9 needed re-evaluation. Mean intensity and mean percentage were then multiplied to obtain a histoscore (0–300). For statistical evaluation, histoscore for each stain (TLR2, TLR4, and TLR9) was then dichotomized by median value into two equal sized groups (low and high).
The inflammatory cell response in the invasive front of the cancer and in the bulk of the cancer was evaluated as described earlier [27] by two independent observers (J. L, O. H). The response was given a score ranging from absent (score 0) to strong increase (score 3). The mean value of two independent estimates was then used to divide the cohort into three equal groups (low, moderate, and high).
Statistical analysis
Statistical analysis was done using IBM SPSS statistics for Windows, version 22.0. Armonk, NY: IBM Corp. Wilcoxon paired test was used to assess differences in TLR expression between cancer tissue and adjacent normal pancreatic cancer. Pearson’s chi-square test was used to calculate statistically significant correlations between clinicopathological variables (TNM-staging, tumor grade, tumor size, BMI, gender, age at diagnosis) and TLR histoscore. Kaplan-Meier method was used to calculate life tables, and survival curves were compared with the log-rank test. Cox proportional hazards model was calculated for the following covariates: age at diagnosis (<65 or ≥65), gender (male or female), tumor stage (I, II, and III–IV), and cytoplasmic TLR9 histoscore (low or high). The hazard ratios (HRs) and 95% confidence intervals (CIs) are provided for each covariate in the model after the adjustment. p values less than 0.05 were accepted as statistically significant.
Results
Expression and localization of TLR2, TLR4, and TLR9 in pancreatic cancer and adjacent normal pancreatic tissue
TLR2 staining was found in adjacent normal pancreatic tissue on cell membranes in only 1/37 cases and in the cytoplasm in 34/37 cases (mean histoscore 75, SD 47, range 0–200) (Fig. 1b). In 24/65 cancers, TLR2 expression was found on the cell membrane (mean histoscore 27, median histoscore 0, SD 48, range 0–180) and in all cancers in the cytoplasm (mean histoscore 223, median histoscore 250, SD 70, range 85–300). (Fig. 1a, Fig. 2a, b). Both membranous and cytoplasmic TLR2 staining was more intense in cancer tissue compared to adjacent normal pancreatic tissue (p = 0.001, p < 0.001, respectively).
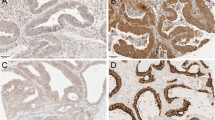
High magnification images (×20) illustrating typical subcellular localization patterns of the staining in pancreatic cancer tissue (a, c, and e) and examples of typical positive staining of adjacent normal pancreatic tissue (b, d, and f). Moderate intensity cytoplasmic TLR2 expression, without nuclear or membranous staining (a). High membranous and cytoplasmic TLR4 expression without nuclear staining (c). Moderate cytoplasmic TLR9 staining without membranous or nuclear staining (e). Examples of typical staining of adjacent normal pancreatic tissue for TLR2 (b), TLR4 (d), and TLR9 (f)
Examples of variation of TLR staining intensity in pancreatic cancer visualized with low magnification (×10). TLR2 expression in pancreatic cancer in samples with low (a) and high (b) intensity cytoplasmic expression. TLR4 expression in pancreatic cancer samples with low cytoplasmic (c) and high cytoplasmic as well as membranous (d) staining pattern. TLR9 expression in pancreatic cancer tissue with low (e) and high (f) cytoplasmic expression
In all the studied samples, TLR4 staining was present in the cytoplasm but not on the membrane of adjacent normal cells (mean histoscore 141, SD 53, range 100–250), (Fig. 1d). In cancer tissue, membranous TLR4 staining was found in 25/65 cases (mean histoscore 53, median histoscore 0, SD 76, range 0–270) and cytoplasmic staining in all cases (mean histoscore 211, median histoscore 200, SD 74, range 90–300). (Fig. 1c, Fig. 2c, d). Cytoplasmic TLR4 staining was significantly higher in cancer tissue compared to adjacent normal pancreatic tissue (p < 0.001).
In all studied samples, in adjacent normal pancreatic tissue, TLR9 staining was present in the cytoplasm (mean histoscore 150, SD 94, range 0–300), but not on the membrane (Fig. 1f). In cancer tissue, TLR9 staining was found on the membrane in 20/65 cases (mean histoscore 31, median histoscore 0, SD 60, range 0–270) and in the cytoplasm of 61/65 cases (mean histoscore 130, median histoscore 113, SD 84, range 0–300). (Fig. 1e, Fig. 2e, f). There was no statistically significant difference in cytoplasmic TLR9 expression between cancer and adjacent normal pancreatic tissue.
Nuclear TLR2 staining in cancer cells was observed in 42/65 cases. Similarly, nuclear TLR4 was seen in 5/65 cases and nuclear TLR9 in 19/65 cases.
TLR expression and correlation with clinicopathological variables
Pearson’s chi-square test was used to investigate associations between TLR expression and clinicopathological variables (TNM-stage, tumor grade, tumor size, BMI, gender, age at diagnosis). High cytoplasmic TLR4 expression is associated with high tumor grade (p = 0.010) and low cell membrane TLR9 expression with low BMI (p = 0.031). There were no other significant correlations between TLR expression and clinicopathological variables (Table 2).
Correlation between TLR expression and local inflammatory response
Pearson’s chi-square test was applied to assess associations between TLR expression and the inflammatory response. Low cell membrane TLR2 expression was associated with low local inflammatory response in the tumor bulk (p = 0.019; Table 2). We found no other associations between local inflammatory response and TLR expression.
Local inflammatory response and survival
To test associations between local inflammatory response and 5-year patient survival, the cohort was subdivided according to the density of inflammatory cells in three equal groups (low, moderate, and high). Inflammatory response was assessed separately in the bulk of the cancer and in the invasive front. Only a high density of inflammatory cells in the invasive front showed a trend towards association with longer survival (p = 0.087, log rank test).
Correlation between TLR expression and systemic inflammatory response
We assessed correlations between preoperative peripheral blood leukocyte counts and TLR expression. The only significant correlation found was between higher than median (>7.3 × 109/l) blood leukocyte count and low cytoplasmic TLR9 expression (p = 0.031). We found no correlation between blood leukocyte count and local inflammatory response to the tumor.
TLR expression and patient survival
We assessed correlations between expression of TLR2, 4, and 9 and 5-year survival. Survival time for patients with high cytoplasmic TLR9 expression (mean = 36 months; 95% CI 29–42) was significantly longer compared to patients with low cytoplasmic TLR9 expression (mean = 21 months; 95% CI 15–26); p = 0.001 (log-rank; Fig. 3).
Multivariate analysis identified low cytoplasmic TLR9 expression as independent prognostic factor (HR 3.090, 95% CI 1.673–5.706; p = 0.000) for pancreatic cancer-specific death. Age, gender, or tumor stage were not significantly associated with survival as shown in Table 3. Expression of TLR2 or TLR4 was not associated with survival, neither in uni- nor in multivariate analysis (data not shown).
Discussion
We report here an association between TLR9 expression and patient survival in pancreatic cancer. High cytoplasmic TLR9 expression was associated with a mean survival advantage of 15 months (p = 0.001). Multivariate analysis identified low cytoplasmic TLR9 expression as an independent factor predicting cancer-specific death. Abundant inflammatory response was not related with TLR expression. In agreement with previous studies, expression of TLR2 and TLR4 was higher in pancreatic carcinoma cells as compared to adjacent normal pancreatic tissue [18, 28]. TLR2 expression was not associated with patient survival. TLR4 expression has been previously associated with poor survival, which was not observed in our material [18]. This difference might be due to different geographical locations and populations.
It is noteworthy that we studied histologically normal pancreatic tissue adjacent to cancer tissue, which might not be functionally identical to more distant pancreatic tissue or tissue from a normal pancreas. However, we minimized this bias by carefully assessing normal histology. Additionally, a previous study suggested that adjacent carcinoma has only limited or no impact on TLR expression in normal epithelium, suggesting that there is no field effect in pancreatic cancer in terms of TLR expression [22].
Associations between TLR9 expression and patient survival appear to be related to tumor type. Previously, high TLR9 expression has been associated with adverse prognosis in esophageal adenocarcinoma, squamous cell carcinoma of the tongue, and prostate cancer [29–31]. In contrast and in agreement with our observations, in triple-negative breast cancer, renal cell carcinoma and mucoepidermoid salivary gland carcinoma high TLR9 expression are associated with better prognosis [32–34]. The authors hypothesized that TLR9 expression in triple-negative breast cancer and renal cell carcinoma might be explained by hypoxia, but this purported biological link needs further evidence [34]. Of note, high expression of TLR is linked to better survival in organs without physiological exposure to bacteria. In contrast, in organs where bacteria are present in abundance such as gastric and colorectal cancers and squamous cell carcinoma of the mobile tongue, TLR9 ligation induces cell invasion and increased viability and migration of esophageal cancer cells [30, 35, 36]. In pancreatic cancer, however, TLR9 ligation has been shown to have mostly inhibitory effects on cancer cells [21]. Studies in mice suggest that TLR9 ligation with synthetic CPG ODNs, in combination with vaccines based on immune stimulatory complexes (ISCOM) or gemcitabine, may inhibit pancreatic cancer cell growth and invasive properties, enhance immunological response to cancer, and lead to tumor regression and prolonged survival [21, 37–39]. On the other hand, TLR9 ligation induced epithelial cell proliferation in pancreatic stellate cells, suggesting that TLR9 may also have tumor-promoting effects on pancreatic cancer [20]. Taken together, the role of TLR9 in pancreatic cancer remains controversial.
Pancreatic cancer risk is elevated in carriers of oral pathogens such as Fusobacterium species and Porphyromonas gingivalis [23, 40, 41]. Bacteria may affect pancreatic cancer progression, reaching the pancreas through the bloodstream or biliary tract [23, 24, 42]. Local presence of oral Fusobacterium species might associate with poor outcome in pancreatic cancer, as well as in colorectal and esophageal cancers [43–45]. This has been explained by reactive oxygen species (ROS) and inflammatory cytokines of TLR signaling pathways such as IL-6 and TNF [45]. There is evidence that bacteria might have a downregulating effect on TLR levels [46]. TLR9 expression in the normal pancreas might be also downregulated by exposure to bacteria, as suggested by a study comparing pancreatic TLR expression in germ-free and conventional mice [22]. We also found patients with low TLR9 expression to have higher blood leukocyte count, suggesting the possibility of activation of a systemic inflammatory response. There might be differences in the regulation of innate immunity receptors in the presence of bacteria. High TLR9 expression leading to improved survival in pancreatic cancer might be related to lack of bacterial exposure in this group of patients. This explanation, however, remains hypothetical [20, 47]. Blood leukocyte count is a highly unspecific clinical marker elevated in various inflammatory states and, therefore, does not unequivocally equate to bacterial exposure.
In conclusion, we found high TLR9 expression to be associated with improved survival of patients with pancreatic ductal adenocarcinoma. Expression of TLR2 and TLR4 is not associated with pancreatic cancer survival. The prognostic effect of TLR9 might be associated with bacterial exposure, but this needs further evidence.
References
Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363:1049–1057
Falasca M, Kim M, Casari I (2016) Pancreatic cancer: current research and future directions. Biochim. Biophys. Acta
Silvestris N, Longo V, Cellini F, Reni M, Bittoni A, Cataldo I, Partelli S, Falconi M, Scarpa A, Brunetti O, Lorusso V, Santini D, Morganti A, Valentini V, Cascinu S (2016) Neoadjuvant multimodal treatment of pancreatic ductal adenocarcinoma. Crit Rev OncolHematol 98:309–324
Takeda K and Akira S (2007) Toll-like receptors. Curr. Protoc. Immunol; Chapter 14:Unit 14.12
Cook DN, Pisetsky DS, Schwartz DA (2004) Toll-like receptors in the pathogenesis of human disease. Nat Immunol 5:975–979
Botos I, Segal DM, Davies DR (2011) The structural biology of toll-like receptors. Structure 19:447–459
Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK (2003) Toll-like receptors in health and disease: complex questions remain. J Immunol 171:1630–1635
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Pandey S, Singh S, Anang V, Bhatt AN, Natarajan K, Dwarakanath BS (2015) Pattern recognition receptors in cancer progression and metastasis. Cancer Growth Metastasis 8:25–34
Castano-Rodriguez N, Kaakoush NO, Mitchell HM (2014) Pattern-recognition receptors and gastric cancer. Front Immunol 5:336
Kauppila JH, Selander KS (2014) Toll-like receptors in esophageal cancer. Front Immunol 5:200
Cammarota R, Bertolini V, Pennesi G, Bucci EO, Gottardi O, Garlanda C, Laghi L, Barberis MC, Sessa F, Noonan DM, Albini A (2010) The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. J Transl Med 8:112 5876-8-112
Kauppila JH, Mattila AE, Karttunen TJ, Salo T (2013) Toll-like receptor 5 (TLR5) expression is a novel predictive marker for recurrence and survival in squamous cell carcinoma of the tongue. Br J Cancer 108:638–643
Gonzalez-Reyes S, Fernandez JM, Gonzalez LO, Aguirre A, Suarez A, Gonzalez JM, Escaff S, Vizoso FJ (2011) Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother 60:217–226
Vaz J, Akbarshahi H, Andersson R (2013) Controversial role of toll-like receptors in acute pancreatitis. World J Gastroenterol 19:616–630
Huynh AS, Chung WJ, Cho HI, Moberg VE, Celis E, Morse DL, Vagner J (2012) Novel toll-like receptor 2 ligands for targeted pancreatic cancer imaging and immunotherapy. J Med Chem 55:9751–9762
Fan P, Zhang JJ, Wang B, Wu HQ, Zhou SX, Wang CY, Zhang JH, Tian Y, Wu HS (2012) Hypoxia-inducible factor-1 up-regulates the expression of toll-like receptor 4 in pancreatic cancer cells under hypoxic conditions. Pancreatology 12:170–178
Zhang JJ, Wu HS, Wang L, Tian Y, Zhang JH, Wu HL (2010) Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J Gastroenterol 16:2881–2888
Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, Badar S, Hajdu CH, Frey AB, Bar-Sagi D, Miller G (2012) MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med 209:1671–1687
Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, Jonnadula S, Torres-Hernandez A, Tippens D, Pushalkar S, Eisenthal A, Saxena D, Ahn J, Hajdu C, Engle DD, Tuveson D, Miller G (2015) TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med 212:2077–2094
Wu HQ, Wang B, Zhu SK, Tian Y, Zhang JH, Wu HS (2011) Effects of CPG ODN on biological behavior of PANC-1 and expression of TLR9 in pancreatic cancer. World J Gastroenterol 17:996–1003
Huhta H, Helminen O, Kauppila JH, Salo T, Porvari K, Saarnio J, Lehenkari PP, Karttunen TJ (2016) The expression of toll-like receptors in normal human and murine gastrointestinal organs and the effect of microbiome and cancer. J Histochem Cytochem 64:470–482
Michaud DS, Izard J (2014) Microbiota, oral microbiome, and pancreatic cancer. Cancer J 20:203–206
Wang C, Li J (2015) Pathogenic microorganisms and pancreatic cancer. Gastrointest Tumors 2:41–47
Huhta H, Helminen O, Kauppila JH, Takala H, Metsikko K, Lehenkari P, Saarnio J, Karttunen T (2015) Toll-like receptor 9 expression in the natural history of Barrett mucosa. Virchows Arch 467:9–18
Helminen O, Huhta H, Takala H, Lehenkari PP, Saarnio J, Kauppila JH, Karttunen TJ (2014) Increased toll-like receptor 5 expression indicates esophageal columnar dysplasia. Virchows Arch 464:11–18
Laurila JJ, Ala-Kokko TI, Laurila PA, Saarnio J, Koivukangas V, Syrjala H, Karttunen TJ (2005) Histopathology of acute acalculous cholecystitis in critically ill patients. Histopathology 47:485–492
Morse DL, Balagurunathan Y, Hostetter G, Trissal M, Tafreshi NK, Burke N, Lloyd M, Enkemann S, Coppola D, Hruby VJ, Gillies RJ, Han H (2010) Identification of novel pancreatic adenocarcinoma cell-surface targets by gene expression profiling and tissue microarray. Biochem Pharmacol 80:748–754
Vaisanen MR, Jukkola-Vuorinen A, Vuopala KS, Selander KS, Vaarala MH (2013) Expression of toll-like receptor-9 is associated with poor progression-free survival in prostate cancer. Oncol Lett 5:1659–1663
Kauppila JH, Korvala J, Siirila K, Manni M, Makinen LK, Hagstrom J, Atula T, Haglund C, Selander KS, Saarnio J, Karttunen TJ, Lehenkari PP, Salo T (2015) Toll-like receptor 9 mediates invasion and predicts prognosis in squamous cell carcinoma of the mobile tongue. J Oral Pathol Med 44:571–577
Kauppila JH, Takala H, Selander KS, Lehenkari PP, Saarnio J, Karttunen TJ (2011) Increased toll-like receptor 9 expression indicates adverse prognosis in oesophageal adenocarcinoma. Histopathology 59:643–649
Korvala J, Harjula T, Siirila K, Almangush A, Aro K, Makitie AA, Grenman R, Karttunen TJ, Leivo I, Kauppila JH, Salo T (2014) Toll-like receptor 9 expression in mucoepidermoid salivary gland carcinoma may associate with good prognosis. J. Oral Pathol. Med. 43:530–537
Ronkainen H, Hirvikoski P, Kauppila S, Vuopala KS, Paavonen TK, Selander KS, Vaarala MH (2011) Absent toll-like receptor-9 expression predicts poor prognosis in renal cell carcinoma. J Exp Clin Cancer Res 30:84 9966-30-84
Tuomela J, Sandholm J, Karihtala P, Ilvesaro J, Vuopala KS, Kauppila JH, Kauppila S, Chen D, Pressey C, Harkonen P, Harris KW, Graves D, Auvinen PK, Soini Y, Jukkola-Vuorinen A, Selander KS (2012) Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res Treat 135:481–493
Zhang Y, Wang Q, Ma A, Li Y, Li R, Wang Y (2014) Functional expression of TLR9 in esophageal cancer. Oncol Rep 31:2298–2304
Kauppila JH, Karttunen TJ, Saarnio J, Nyberg P, Salo T, Graves DE, Lehenkari PP, Selander KS (2013) Short DNA sequences and bacterial DNA induce esophageal, gastric, and colorectal cancer cell invasion. APMIS 121:511–522
Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, Kisser U, Bauer CA, Dauer M, Eigler A, Maraskovsky E, Endres S, Schnurr M (2011) An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer 128:897–907
Silva A, Mount A, Krstevska K, Pejoski D, Hardy MP, Owczarek C, Scotney P, Maraskovsky E, Baz MA (2015) The combination of ISCOMATRIX adjuvant and TLR agonists induces regression of established solid tumors in vivo. J Immunol 194:2199–2207
Pratesi G, Petrangolini G, Tortoreto M, Addis A, Belluco S, Rossini A, Selleri S, Rumio C, Menard S, Balsari A (2005) Therapeutic synergism of gemcitabine and CpG-oligodeoxynucleotides in an orthotopic human pancreatic carcinoma xenograft. Cancer Res 65:6388–6393
Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, Ravel J, Hayes RB, Ahn J (2016) Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut
Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjonneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Kaaks R, Boeing H, Foerster J, Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Sieri S, Palli D, Tumino R, Panico S, Siersema PD, Peeters PH, Lund E, Barricarte A, Huerta JM, Molina-Montes E, Dorronsoro M, Quiros JR, Duell EJ, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Travis RC, Vineis P, Bueno-de-Mesquita HB, Riboli E (2013) Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 62:1764–1770
Zambirinis CP, Pushalkar S, Saxena D, Miller G (2014) Pancreatic cancer, inflammation, and microbiome. Cancer J 20:195–202
Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki M, Sakamoto Y, Yamashita Y, Yoshida N, Watanabe M, Baba H (2016) Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res
Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, Kostic AD, Giannakis M, Bullman S, Milner DA, Baba H, Giovannucci EL, Garraway LA, Freeman GJ, Dranoff G, Garrett WS, Huttenhower C, Meyerson M, Meyerhardt JA, Chan AT, Fuchs CS, Ogino S (2015) Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut
Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, Tachibana M, Tanuma T, Maguchi H, Shinohara T, Hasegawa T, Imamura M, Kimura Y, Hirata K, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y (2015) Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6:7209–7220
Otte JM, Cario E, Podolsky DK (2004) Mechanisms of cross hyporesponsiveness to toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126:1054–1070
Vaz J, Andersson R (2014) Intervention on toll-like receptors in pancreatic cancer. World J Gastroenterol 20:5808–5817
Acknowledgements
We thank Erja Tomperi and Riitta Vuento for expertise in preparation of the immunohistochemical stainings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by grants from the Finnish Cultural Foundation (J. L), Finnish Medical Foundation (J. L), Sigrid Jusélius Foundation (J.H.K.), Mary and Georg C. Ehrnroot Foundation (J.H.K.), Thelma Mäkikyrö Foundation (J.H.K.), Orion Research Foundation (J.H.K.), and Medical Research Center Oulu Focus Group Funding (T.J.K.).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Leppänen, J., Helminen, O., Huhta, H. et al. High toll-like receptor (TLR) 9 expression is associated with better prognosis in surgically treated pancreatic cancer patients. Virchows Arch 470, 401–410 (2017). https://doi.org/10.1007/s00428-017-2087-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2087-1