Abstract
Pelvic carcinosarcomas (PCSs) are rare aggressive biphasic tumors that localize in the ovary, fallopian tube, or peritoneum and present frequently as bilateral disease. We undertook a morphological, p53 immunohistochemical and TP53 gene mutational analysis study in a single institution cohort of 16 PCSs in order to investigate the nature of bilateral tumors and to shed light on their origin and pathogenesis. Of the 16 patients, 10 presented with bilateral disease, 6 with a carcinosarcoma in both adnexa, and the remaining cases with a carcinosarcoma in one adnexum and a carcinoma in the opposite. The carcinoma component showed high-grade serous features in 13/16 of cases (81 %). In 10 patients (63 %), a serous tubal intraepithelial carcinoma (STIC) was found, in one case bilateral, making a total of 11 STICs. STIC was found only in cases with a carcinoma component with high-grade serous features. All 10 bilateral tumors and all 11 PCS-associated STICs showed a similar p53 immunostaining pattern. At mutation analysis of the TP53 gene, all five bilateral PCS contained an identical mutation in both localizations. Furthermore, a TP53 mutation was found in 8 of 10 STICs, with an identical mutation in the associated PCS. The finding of similar p53 immunostaining in all bilateral cases and identical TP53 mutations in most PCS-associated STIC provides evidence for a clonal relation between these neoplastic lesions, supporting a metastatic nature of bilateral PCS and suggesting that they have an extraovarian origin in a STIC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinosarcomas (malignant mixed Müllerian tumors) are biphasic tumors composed by two malignant high-grade components respectively of epithelial and mesenchymal nature, according to the recent World Health Organization Classification of Tumors of Female Reproductive Organs [1]. The carcinomatous component may show high-grade serous, endometrioid, clear cells, or undifferentiated features [2]. The sarcomatous component may be homologous or heterologous, the latter most commonly exhibiting chondroid or rhabdomyoblastic differentiation [3].
Pelvic extrauterine carcinosarcomas (PCSs) can arise in the ovary, fallopian tube, or peritoneum and are bilateral in approximately half of the cases [4]. In the ovary, they account approximately for 1–4 % of all ovarian malignancies [5]. They behave more aggressively than high-grade serous carcinomas (HGSC) [6].
While it has been suggested that the most common histotypes of ovarian carcinoma have an extraovarian origin, from serous tubal intraepithelial carcinoma (STIC) for HGSC [7] and endometriosis for endometrioid (EC) [8] and clear cell carcinomas (CCC) [9], pathogenesis and origin of PCS are still poorly understood. Moreover, whether bilateral synchronous PCSs are independent primary tumors or reflect metastatic disease is yet to be elucidated.
In this study, we undertook a comprehensive morphological, p53 immunohistochemical and TP53 mutational analysis of 16 PCSs, to elucidate whether PCSs might be derived from extraovarian neoplastic precursor lesions and to clarify the relationship between bilateral tumors.
Materials and methods
Case selection
PCSs cases were searched retrospectively in the archives of the Department of Pathology, Spedali Civili of Brescia. All patients were treated and followed at the Division of Gynecological Oncology at the same hospital institution. Surgical stage was assigned based on the criteria of the International Federation of Gynecology and Obstetrics (FIGO) [10]. Original reports and tumor slides were reviewed in order to confirm the diagnosis of carcinosarcoma, to define the primary tumor site based on the localization of the largest tumor mass and to assess morphological features of the carcinomatous and sarcomatous components. Fallopian tube sampling had been performed for each patient; in 11 of 16 patients, fimbrial ends had been sampled including two recently diagnosed cases in which the SEE-FIM protocol was applied. Fallopian tubes were carefully evaluated in order to identify the presence of STIC following previously reported morphological and immunohistochemical criteria [11]. The study was performed in accordance with the guidelines of the Institutional Research and Brescia Hospital Ethical Board protocols.
Immunohistochemical staining of p53
Immunohistochemical analysis was performed using a mouse monoclonal anti-p53 antibody (clone DO-7 Mouse IgG2b/kappa, Thermo Fisher Scientific Fremont, USA) on a Bond Max System (Leica Biosystem, New Castle Ltd., UK). Briefly, antigen retrieval using citrate buffer at pH 6.0 was followed by incubation of sections with primary antibody (1:1000 dilution) for 30 min. Immunoreactivity was revealed by Bond Polymer Refine Detection Kit (Leica Biosystem) and diaminobenzidine as chromogen and with hematoxylin as nuclear counterstain.
Immunostaining results were assessed blinded to TP53 gene mutational data, applying a previously reported scoring system [12], the results of which correlate with the TP53 gene status. An overexpressed pattern was attributed to cases with ≥60 % of tumor cells showing intense nuclear p53 staining. Tumors completely negative for p53 in the presence of positive internal controls were defined as having a null pattern. Weak/focal pattern was attributed to cases showing weak and focal p53.
Tumor dissection and DNA extraction
Tumor dissection was performed manually using an 18-gauge needle under microscopic visualization, separately for carcinomatous and sarcomatous components and by laser microdissection for STICs. For laser microdissection serial, 10-μm thick sections were mounted onto polyethylene naphthalate slides (Leica Microsystem, Wetzlar, Germany) and treated according to protocol. A consecutive hematoxylin and eosin-stained section was used as a guide to identify areas of interest. An average of 500–1000 cells for each STIC were laser microdissected using Leica LMD 6000 laser microdissection system and collected into tubes. After 24 h of proteinase digestion, DNA extraction was performed automatically on a MagCore Nucleic Acid Extractor (RBC Bioscience, New Taipei City, Taiwan) or with a QIAamp DNA Micro Kit (Qiagen, Valencia, CA, USA) for microdissected samples, following the manufacturer’s protocols.
TP53 gene mutation analysis
TP53 gene mutation analysis was performed only on tumor specimens of patients with a STIC (10 patients). For each patient, non-neoplastic control tissue was also analyzed. We first searched TP53 mutations in DNA extracted from the carcinoma components using next-generation sequencing (NGS). Subsequently, DNA was extracted from other tumor components (homolateral sarcomatous component; all tumor components from eventual contralateral tumor; STIC) and analyzed with Sanger sequencing to confirm the variants identified with NGS.
TP53 gene NGS was performed on an Ion Torrent PGM platform (LifeTechnologies, Carlsbad, CA, USA). Barcoded libraries were constructed with the Ion AmpliSeq Library Kit 2.0 (Life Technologies) following the manufacturer’s protocol. Briefly, 20 ng of somatic DNA was amplified using the validated TP53 Ion AmpliSeq™ Panel (LifeTechnologies) that consists of 24 amplicons distributed in two pools (Supplementary Table 1). The final target of 1.280 bp includes all coding and flanking regions of TP53. After dilution to 15 ng/ml, the libraries were sequenced on Ion314 Chips using Ion PGM 200 Sequencing Kit (LifeTechnologies). The mean read length was 97 bp and the average reads were approximately 14,944 bp of sequence per sample with 24/24 (100 %) amplicons covered at least 50-fold.
Initial sequences from the PGM runs were aligned with Torrent Suite software and the Torrent Suite variant caller v4.2 performed variant identification. Further, the files generated from Torrent Suite software (BAM and BAI file) were used in order to visualize the sequence in the Integrative Genomics Viewer (IGV, Broad Institute) [13] and to confirm the mutations identified in the variant caller file (VCF). Additionally, detected mutations were confirmed by Sanger sequencing. The variants observed were compared to those present in the “1000 Genomes Project,” “Exome Variant Server,” “Human Gene Mutation Database (HGMD),” “Exac Browser,” and “COSMIC database” to assess recurrent mutations in ovarian and breast cancer.
For Sanger confirmation, DNA was amplified and sequenced with primers corresponding to the amplicon of the TP53 gene panel for the mutation detected with NGS (Supplementary Table 1).
Results
Clinicopathological features of patients are summarized in Table 1. A total of 15 patients affected by PCS were identified between 2000 and 2014. We included in the study one patient with bilateral ovarian HGSC and omental metastasis with carcinosarcoma histology (pt ID 15), as an example of the rare occurrence of a primary ovarian carcinoma metastasizing as carcinosarcoma [14]. Patient age at presentation ranged between 52 and 86 years (mean 65.5 years; median 63.5 years). Thirteen patients (81 %) presented at stage III–IV and 3 at stage II. Follow-up was available for 15 patients, with a mean and median duration of 42.8 and 24.0 months (range 4–171 months) respectively. At last follow-up, nine patients had died of disease (60 %), two where alive with disease (13 %), and the remaining four patients (27 %) were alive without disease (Table 1).
We defined the ovary as primary tumor site in 14 cases and the fallopian tube in 2 cases. Bilateral tumors were found in ten patients: six with a carcinosarcoma in both adnexa, while in three patients, carcinosarcoma was found in one side and carcinoma in the other. The remaining case was the bilateral ovarian HGSC with omental metastasis of carcinosarcomatous histology mentioned above.
In 13 cases, the carcinomatous component showed high-grade serous features, in 2 cases endometrioid features, and in a single case, this was undifferentiated. The sarcomatous component was homologous in seven and heterologous in nine cases, the latter including rhabdomyoblastic and/or chondroid differentiation.
Details regarding fallopian tube sampling and tumor involvement are reported in Supplementary Table 2. STICs were identified in ten patients and one of them (ID 13) had bilateral STIC. STICs were found only when the carcinoma component showed high-grade serous features, and they were mostly localized in fimbriae, followed by the isthmus and the tuboperitoneal junction. In addition to STICs, we detected foci of invasive carcinoma in fimbrial ends in five cases with a dominant tumor mass in an ovary. In the two cases with primary fallopian tube carcinosarcoma, the ovaries were not involved while in one of them, minimal tumor deposits were found on the surface of the ovary. In one patient (ID 11), multiple foci of ovarian endometriosis were found, not contiguous with carcinosarcoma.
p53 immunohistochemistry
P53 immunostaining was performed in all cases on sections containing carcinoma and sarcoma components, and bilateral tumors as well as STICs were included. P53 immunostaining was evaluated separately for the carcinoma and sarcoma components of each tumor, and the staining patterns were compared. Results are summarized in Table 2.
Of the 22 pairs of carcinoma-sarcoma components obtained from bilateral and unilateral tumors, 21 (96 %) showed concordant p53 immunostaining in both components classified as overexpressed in 16, null in 5, and weak/focal in 1. In the single case showing discordant p53 staining between the components, overexpression was found in the sarcoma component but weak staining in the carcinoma component. Overexpressed and null p53 staining patterns were detected only in cases showing serous and undifferentiated carcinomatous components, while weak/focal p53 immunostaining was found exclusively in cases exhibiting endometrioid features.
All ten bilateral tumors showed concordant p53 immunostaining in the lesions on both sides. Intense diffuse staining for p53 was found in 8 of 11 STICs, while the remaining 3 cases were completely negative. Normal fallopian tube epithelium adjacent to STIC contained scattered nuclei weakly positive for p53, a finding consistent with wild-type p53 protein. In all of the 11 pairs with STIC and concomitant PCS, both lesions showed a concordant p53 immunostaining pattern.
TP53 gene mutation analysis
TP53 gene mutation analysis was performed on tumor specimens from the 10 patients with a STIC and included 16 carcinoma components, 12 sarcoma components, and 10 STICs (Table 3). All TP53 gene mutations detected by NGS were confirmed by Sanger sequencing. TP53 mutations were identified in 14/16 carcinoma components, in 12/12 sarcoma components, and in 8/10 STICs. They were located in exons 5–8, most often in exon 5 and of missense type. TP53 mutations were not detected in normal-tissue controls indicating their somatic character.
All 12 paired carcinoma-sarcoma component samples, obtained from monolateral and bilateral tumors, contained an identical TP53 mutation in both tumor components. In five bilateral tumors, the same TP53 mutation was found in all tumor components in both adnexa. Finally, in paired PCS-STIC samples, an identical TP53 mutation was detected in both lesions in 8/10 cases, while in the two remaining cases, a wild-type TP53 gene was found in the STIC and a missense TP53 mutation in the corresponding PCS (Table 3, case ID 13–14).
Correlation of TP53 mutation type with p53 immunostaining pattern
In keeping with previous observations [7, 12], TP53 mutation type and p53 immunostaining pattern were significantly correlated (p < 0.001, two-tailed Fisher’s exact test), missense mutations corresponding with p53 overexpression in 24 (Fig. 1) and non-sense TP53 mutations reflected in null p53 immunostaining in 10 neoplastic samples (Fig. 2) (Table 4). Of note, in four lesions, p53 immunostaining suggested the presence of a TP53 mutation while mutation analysis was wild type. Two of these cases (Table 4, pt. ID 13 and 14) concerned a STIC with p53 overexpression and the wild-type TP53 gene status might have resulted from contamination with normal tubal epithelial cells. The remaining two lesions consisted of bilateral PCS in which the carcinoma component in both adnexa showed a null p53 immunostaining pattern, without a TP53 anomaly by NGS (Table 4, pt. ID 5). Conceivably, in this case, alterations in the TP53 gene regulatory regions or complex molecular aberrations not detected by NGS might have been responsible for the discrepancy.
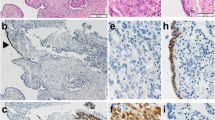
p53 immunostaining in carcinosarcoma and STIC and TP53 mutation of case ID 8. Carcinomatous component with high-grade serous features (a) with overexpressed p53 immunostaining (b). Sarcomatous component with chondroid elements (c) with p53 overexpressed immunostaining (d). Section of the fallopian tube showing a STIC in the left side and normal tubal epithelium in the right side (e); while STIC shows intense and diffuse p53 immunostaining, the normal tubal epithelium shows scattered nuclei weakly positive for p53 (f) (×20 magnification). TP53 missense mutation detected in all tumors components (g)
p53 immunostaining in carcinosarcoma and STIC and TP53 mutation of case ID 16. Carcinomatous component with high-grade serous features surrounding a central area composed by non-neoplastic stromal cells (a); carcinomatous cells are completely negative for p53 while non-neoplastic stromal cells show weak nuclear positivity for p53 (b). Sarcomatous component with chondroid elements (c) with p53 null immunostaining (d). Section of the fallopian tube showing a STIC in the lower part of the picture and normal tubal epithelium in the upper one (e); while STIC cells are completely negative for p53, the normal tubal epithelium shows occasional nuclei weakly positive for p53 (f) (×40 magnification). TP53 non-sense mutation detected in all tumors components (g)
Discussion
We studied 16 cases of PCS and found that they are frequently bilateral, most often composed of a high-grade serous carcinoma component, and in a high proportion of cases (10/16), associated with STIC in the fallopian tubes. By immunohistochemistry and mutation analysis, we found that in the carcinoma and sarcoma components of PCS as well as in bilateral tumors and in associated STICs, p53 staining pattern is often similar, along with identical TP53 mutations, suggesting a clonal relationship between all these neoplastic lesions. Previous ultrastructural, immunohistochemical, in vitro, and molecular studies have provided evidence in support of the derivation of the carcinoma and sarcoma components of both uterine and pelvic extrauterine carcinosarcomas from a single stem cell precursor [15–19]. This included p53 immunohistochemical and TP53 gene mutation analysis [20–22].
The issue of unilateral versus bilateral PCS has been overlooked in most previous reports [23–25], with the exception of a series of 64 cases from Taiwan, in which bilateral ovarian tumors were found in half of the cases [4]. In keeping with this observation, we found the majority of the cases in our study (10/16, 63 %) to be bilateral. The occurrence of similar p53 immunostaining patterns and identical TP53 mutation status in both adnexa of all studied PCSs is convincing evidence of their clonal relationship and supports the notion that one of the tumor sites is metastatic, rather than a synchronous independently developed malignancy.
The carcinoma component showed high-grade serous features in the vast majority of our cases, while endometrioid or undifferentiated features were observed only in two and one cases respectively. In three out of ten bilateral cases, a carcinosarcoma was present in one adnexum and a carcinoma in the other. In a single case (pt ID 15), carcinosarcoma histology was recognized in an omental metastasis of a primary bilateral ovarian HGSC.
The universal presence of a carcinoma component and its high-grade serous nature, which represents the most common ovarian cancer histotype in Western countries [26], suggests that pelvic carcinosarcomas, similar to uterine carcinosarcomas, may arise primarily as a high grade carcinoma. Some of them have been described to arise as ovarian HGSC or poorly differentiated ovarian endometrioid carcinoma and subsequently presented as carcinosarcoma only in the recurrent tumor [14, 27]. This hypothesis is further supported by the observation that STIC, which is considered the precursor of pelvic HGSC, occurs in fallopian tubes removed from patients affected by pelvic carcinosarcoma [28, 29]. In addition, PCSs have been reported to arise in patients with germline mutations of BRCA1/2 and RAD51C genes [30–32], a condition typically associated with STIC and HGSC.
The occurring of STIC in PCS has been described in a case report [28] and, more recently, in 8 of 21 cases of ovarian carcinosarcoma [29]. We found STICs to be even more frequent as they were identified in 10 out of 16 cases, all as PCS with a high-grade serous carcinomatous component. Furthermore, using laser microdissection, we provided evidence that STIC and the associated invasive carcinoma and sarcoma components harbor identical p53/TP53 abnormalities in most cases. Taken together, these observations suggest that a large proportion of PCSs, especially those with a high-grade serous carcinoma component, derive from STIC, which transforms into invasive HGSC that subsequently gives rise to the sarcoma component. This provides further support to the conversion theory, which suggests that ovarian carcinosarcomas arise through dedifferentiation of a preexisting carcinoma, similar to uterine carcinosarcoma which is considered a metaplastic tumor [33, 34]. This implies that the primary tumor site in PCS should be determined according to the criteria recently suggested in the literature [35]. For PCS with a high-grade serous carcinomatous component, it should not be based exclusively on the dominant tumor mass but additional features, in particular, the presence of STIC or invasive carcinoma in the fallopian tube, should be taken into consideration.
Traditionally, PCSs are considered very aggressive tumors [36]. Similar to previous studies, most patients of our series presented at advanced FIGO stage (III–IV stage in 13/16 cases) and with a median duration of follow up (24.0 months), which is in the range described in the literature [37–39]. In addition, as other authors also have pointed out [20, 37], some of our patients had a favorable outcome with a long survival time. Further studies are needed to improve our understanding of these differences in clinical behavior.
In conclusion, by combining morphological, p53 immunohistochemical, and TP53 mutation analysis, we provide evidence that PCSs are monoclonal tumors, that bilateral cases most likely represent metastatic tumors rather than independent primary malignancies, and that those with a high-grade serous carcinoma component derive from a STIC. These findings shed light on PCS carcinogenesis and might have implications for treatment and early detection and prevention of these highly aggressive tumors.
References
Kurman RJ, Carcangiu ML, Herrington CS, et al. (2014) World Health Organization classification of tumours classification of tumours of female reproductive organs, 4th edn. International Agency for Research on Cancer, Lyon
D’Angelo E, Prat J (2011) Pathology of mixed Mullerian tumours. Best Pract Res Clin Obstet Gynaecol 25:705–718
Silverberg SG, Major FJ, Blessing JA, et al. (1990) Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol 9:1–19
Lu CH, Chen IH, Chen YJ, et al. (2014) Primary treatment and prognostic factors of carcinosarcoma of the ovary, fallopian tube, and peritoneum: a Taiwanese Gynecologic Oncology Group study. Int J Gynecol Cancer 24:506–512
Mano MS, Rosa DD, Azambuja E, et al. (2007) Current management of ovarian carcinosarcoma. Int J Gynecol Cancer 17:316–324
Rauh-Hain JA, Diver RJ, Clemmer JT, et al. (2013) Carcinosarcoma of the ovary: a case-control study. Gynecol Oncol 121:477–481
Kuhn E, Kurman RJ, Vang R, et al. (2012) TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma-evidence supporting the clonal relationship of the two lesions. J Pathol 226:421–426
Chene G, Ouellet V, Rahimi K, et al. (2015) The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynaecol Obstet 130:27–30
Ayhan A, Mao TL, Seckin T, et al. (2012) Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer 22:1310–1315
Prat J (2014) Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 124:1–5
Visvanathan K, Vang R, Shaw P, et al. (2011) Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol 35:1766–1775
Yemelyanova A, Vang R, Kshirsagar M, et al. (2011) Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol 24:1248–1253
Robinson JT, Thorvaldsdóttir H, Winckler W, et al. (2011) Integrative Genomics Viewer. Nat Biotechnol 29:24–26
Gallardo A, Matias-Guiu X, Lagarda H, et al. (2002) Malignant Mullerian mixed tumor arising from ovarian serous carcinoma: a clinicopathologic and molecular study of two cases. Int J Gynecol Pathol 21:268–272
Geisinger KR, Dabbs DJ, Marshall RB, et al. (1987) Malignant mixed Mullerian tumors. An ultrastructural and immunohistochemical analysis with histogenetic considerations. Cancer 59:1781–1790
De Brito PA, Silverberg SG, Orenstein JM, et al. (1993) Carcinosarcoma (malignant mixed mullerian (mesodermal) tumor) of the female genital tract: immunohistochemical and ultrastructural analysis of 28 cases. Hum Pathol 24:132–142
Emoto M, Iwasaki H, Kikuchi M, et al. (1992) Two cell lines established from mixed Mullerian tumors of the uterus. morphologic, immunocytochemical, and cytogenetic analyses. Cancer 69:1759–1768
Wada H, Enomoto T, Fujita M, et al. (1997) Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors. Cancer Res 57:5379–5385
Fujii H, Yoshida M, Gong ZX, et al. (2000) Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer 60:114–120
Zorzou MP, Markaki S, Rodolakis A, et al. (2005) Clinicopathological features of ovarian carcinosarcomas: a single institution experience. Gynecol Oncol 96:136–142
Costa MJ, Vogelsan J, Young LJ, et al. (1994) p53 gene mutation in female genital tract carcinosarcomas (malignant mixed Mullerian tumors): a clinicopathologic study of 74 cases. Mod Pathol 7:619–627
Kounelis S, Jones MW, Papadaki H, et al. (1998) Carcinosarcomas (malignant mixed Mullerian tumors) of the female genital tract: comparative molecular analysis of epithelial and mesenchymal components. Hum Pathol 29:82–87
Del Carmen MG, Birrer M, Schorge JO (2012) Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol 25:271–277
Brown E, Stewart M, Rye T, et al. (2004) Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer 100:2148–2153
Guy JB, Trone JT, Casteillo F, et al. (2014) Carcinosarcomas in female genital tracts: general review. Bull Cancer 101:760–764
Goodman MT, Shvetsov YB (2009) Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomark Prev 18:132–139
Moritani S, Moriya T, Kushima R, et al. (2001) Ovarian carcinoma recurring as carcinosarcoma. Pathol Int 51:380–384
Brustmann H (2013) Ovarian carcinosarcoma associated with bilateral tubal intraepithelial carcinoma: a case report. Int J Gynecol Pathol 32:384–389
Seidman JD (2015) Serous tubal intraepithelial carcinoma localizes to the tubal-peritoneal junction: a pivotal clue to the site of origin of extrauterine high-grade serous carcinoma (ovarian cancer). Int J Gynecol Pathol 34:112–120
Ko ML, Jeng CH, Haung SH, et al. (2005) Primary peritoneal carcinosarcoma (malignant mixed Mullerian tumor): report of a case with five-year disease free survival after surgery and chemoradiation and a review of literature. Acta Oncol 44:756–760
Sonoda Y, Saigo PE, Federici MG, et al. (2000) Carcinosarcoma of the ovary in a patient with a germline BRCA2 mutation: evidence for monoclonal origin. Gynecol Oncol 76:226–229
Walsh T, Casadei S, Lee MK, et al. (2011) Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 108:18032–18037
McCluggage WG (2002) Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol 55:321–325
Sreenan JJ, Hart WR (1995) Carcinosarcomas of the female genital tract. A pathologic study of 29 metastatic tumors: further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am J Surg Pathol 19:666–674
McCluggage WG, Judge MJ, Clarke BA, et al. (2015) Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Mod Pathol 28:1101–1122
Loizzi V, Cormio G, Camporeale A, et al. (2011) Carcinosarcoma of the ovary: analysis of 13 cases and review of the literature. Oncology 80:102–106
Rutledge TL, Gold MA, McMeekinn DS, et al. (2006) Carcinosarcoma of the ovary-a case series. Gynecol Oncol 100:128–132
Harris MA, Delap LM, Sengupta PS, et al. (2003) Carcinosarcoma of the ovary. Br J Cancer 88:654–657
Jernigan AM, Fader AN, Nutter B, et al. (2013) Ovarian carcinosarcoma: effects of cytoreductive status and platinum-based chemotherapy on survival. Obstet Gynecol Int 2013:490508. doi:10.1155/2013/490508
Acknowledgments
LA was supported by Fondazione Beretta (Brescia, Italy). We wish to thank Dr. Domenico Allegra (Fondazione Filarete, Viale Ortles 22, Milano, Italy) for help in laser microdissection and Laura Fappani, Paola Bossini, and Chiara Barisani for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have read and approved the final version of the manuscript and declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 311 kb)
Rights and permissions
About this article
Cite this article
Ardighieri, L., Mori, L., Conzadori, S. et al. Identical TP53 mutations in pelvic carcinosarcomas and associated serous tubal intraepithelial carcinomas provide evidence of their clonal relationship. Virchows Arch 469, 61–69 (2016). https://doi.org/10.1007/s00428-016-1933-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1933-x